Abstract
Hemophilia B is a bleeding disorder resulting from factor IX (FIX) deficiency that might be treated with gene therapy. Neonatal delivery would correct the disease sooner than would transfer into adults, and could reduce immunological responses. Neonatal mice were injected intravenously with a Moloney murine leukemia virus–based retroviral vector (RV) expressing canine FIX (cFIX). They achieved 150% to 280% of normal cFIX antigen levels in plasma (100% is 5 μg/mL), which was functional in vitro and in vivo. Three newborn hemophilia B dogs that were injected intravenously with RV achieved 12% to 36% of normal cFIX antigen levels, which improved coagulation tests. Only one mild bleed has occurred during 14 total months of evaluation. This is the first demonstration of prolonged expression after neonatal gene therapy for hemophilia B in mice or dogs. Most animals failed to make antibodies to cFIX, demonstrating that neonatal gene transfer may induce tolerance. Although hepatocytes from newborns replicate, those from adults do not. Adult mice therefore received hepatocyte growth factor to induce hepatocyte replication prior to intravenous injection of RV. This resulted in expression of 35% of normal cFIX antigen levels for 11 months, although all mice produced anti-cFIX antibodies. This is the first demonstration that high levels of FIX activity can be achieved with an RV in adults without a partial hepatectomy to induce hepatocyte replication. We conclude that RV-mediated hepatic gene therapy is effective for treating hemophilia B in mice and dogs, although the immune system may complicate gene transfer in adults.
Introduction
Hemophilia B is a sex-linked disorder due to deficiency of factor IX (FIX) activity that affects 1 in 30 000 males.1 Hepatic gene therapy could result in the continuous secretion of FIX into the blood and improve bleeding symptoms. Achieving more than 10% of normal activity should prevent most spontaneous bleeds, whereas 1% of normal activity should reduce, but not prevent, bleeding. There has been recent success in using hepatic gene therapy to express therapeutic levels of FIX in mice.2 For example, lentiviral vectors resulted in FIX antigen levels that were 2% to 7% of normal3,4; adeno-associated virus (AAV) vectors resulted in levels that were 10% to 40% of normal5-9 or 400% of normal10; adenoviral vectors resulted in levels that were 8% of normal11 or 60% to 4100% of normal12-16; and plasmids resulted in levels that were 7% to 80% of normal.17-19 In addition, intramuscular injection of AAV vectors resulted in 3% to 7% of normal5,20,21 or 1000% of normal levels,22whereas other nonhepatic approaches generally result in lower long-term expression.2
Gene therapy appears to be less efficacious in large-animal models than in mice. In some cases, lower expression was obtained despite the use of a dose of vector per body weight similar to that used in mice, although in other cases lower expression could be attributed at least in part to the use of a lower relative dose of vector. For example, delivery of AAV vectors to the liver of hemophilia B dogs resulted in expression of 1%,7 1% to 4%,23 or 5% to 12%24 of normal canine FIX (cFIX) antigen levels, whereas intramuscular injection of an AAV vector resulted in expression of 0.5% to 2% of normal levels.25-27 Antigen levels were 0.1% of normal after delivery of a Moloney murine leukemia virus (MLV)–based retroviral vector (RV) after partial hepatectomy28 or 1% of normal after electroporation of muscle.29 Although expression from an adenoviral vector was high in hemophilia B dogs at 270% of normal, it was transient owing to immune responses.12 In primates, 3 of 5 animals expressed human FIX (hFIX) antigen levels that were 3% to 8% of normal after portal vein or hepatic artery injection of an AAV vector, although 2 animals had undetectable expression.30 Expression was 80% of normal after intravenous injection of an adenoviral vector into primates, but was transient and associated with the development of inhibitory antibodies.31
Results from these animal studies have led to 2 phase 1 clinical trials of gene therapy for hemophilia B in humans. Intramuscular injection of an AAV vector expressing hFIX may have caused a slight (less than 1%) increase in hFIX functional activity and a modest (less than 50%) decrease in factor usage, but these may represent assay variation and a placebo effect, respectively.32 Data are not yet available from the second trial, which involves hepatic artery injection of an AAV vector.33 Thus, it is unclear if AAV vectors will achieve a full therapeutic effect in patients, and it remains possible that other approaches might be as, or more, efficient.
Recent advances in MLV-based RV technology have led us to re-evaluate its efficacy for gene therapy for hemophilia B. Although MLV-based RVs transduce only dividing cells,34 it was recently shown that they transduce hepatocytes from newborn mice35,36 and dogs37 without a stimulus for replication, or hepatocytes from adults after the administration of hepatocyte growth factor (HGF) to induce replication.38-42In addition, use of the human α1-antitrypsin (hAAT) promoter43,44 and the woodchuck hepatitis virus posttranscriptional regulatory element (WPRE)45 have improved the expression per copy of RV. These advances led us to investigate the ability of this vector to correct the bleeding diathesis in hemophilia B. We demonstrate here therapeutic expression of cFIX after transfer of an MLV-based RV into newborn and adult mice and into newborn dogs.
Materials and methods
Reagents
Reagents were obtained from Sigma Chemical (St Louis, MO) unless otherwise stated. Recombinant human HGF was purified from 293-N3S cells as detailed previously46 and stored at −70°C in phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 10.1 mM Na2HPO4, and 1.8 mM KH2PO4, pH 7.4).
Retroviral vector construction and production
The 1.5-kb canine factor IX (cFIX) cDNA47containing 42 and 146 nucleotides (nt's) of 5′ and 3′ untranslated sequence, respectively, was ligated as a NotI fragment into the NotI site of hAAT–WPRE-76737 to generate hAAT-cFIX–WPRE. This was cotransfected into ecotropic gag-pol (GP) plus E86 cells (GP + E86)48 with a neomycin-resistance vector, and the supernatant from a pool resistant to 800 μg/mL G418 (Invitrogen, Carlsbad, CA) was used to infect the amphotropic GP plus AM12 packaging cells (GP + AM12)49 as described.37 Screening of GP + AM12 colonies was as described previously for a vector expressing factor X.44 Large-scale production of RV was performed as described previously.37 RV titer was determined after freezing once. At 2 days after NIH 3T3 cells were infected with dilutions of the RV, immunostaining was performed to identify cFIX-expressing cells. Cells were fixed for 20 minutes at 4°C with 10% PBS-buffered formalin followed by 10 minutes with methanol. A rabbit anti-cFIX antibody (RACIX; Enzyme Research Laboratories, South Bend, IN) diluted 1:2000 in PBS with 3% bovine serum albumin was incubated for 1.5 hours at room temperature (RT). Cells were washed, and a 1:100 dilution of a horseradish peroxidase (HRP)–coupled donkey anti-rabbit immunoglobulin G (IgG) antibody (Amersham Life Science, Piscataway, NJ) was incubated for 1.5 hours at RT, and the staining developed with 3,3′-diamobenzidine. The RV was tested for the presence of replication-competent retrovirus (RCR) by a vector rescue assay50 as detailed previously.37 The titer of concentrated RV was 1.4 to 2.4 × 108 transducing units (TUs) per milliliter, and there were fewer than 10 copies of RCR per milliliter. Polybrene was added to a final concentration of 8 μg/mL just prior to injection. The injectate contained 570 ± 193 ng/mL cFIX protein.
Animal procedures
National Institutes of Health (NIH) and US Department of Agriculature (USDA) guidelines for the care and use of animals in research were followed. Neonatal mice were obtained after breeding BALB/c mice (Jackson Laboratory, Bar Harbor, ME), heterozygous mucopolysaccharidosis VII (MPS VII) mice from a C57BL/6 (B6.C-H-2bm1) background,51 or hemophilia B mice from a mixed 129 × C57BL/6 background,52 and RVs (1 × 1010 TU/kg) were injected intravenously at 2 to 3 days after birth via the temporal vein. Six-week-old mice were used for adult transduction. For BALB/c mice, approximately 400 μL PBS solution containing 0.29 mg/mL HGF and 0.46 mg/mL dextran sulfate with average molecular weight 5000 kD was injected intraperitoneally at 5.8 mg/kg HGF every 3 hours from 0 to 12 hours. Control BALB/c mice received PBS at the same times. Mice received approximately 400 μL of 3.3 × 109 TU/kg per dose hAAT-cFIX–WPRE intravenously via the tail vein at 30, 36, and 48 hours after the first dose of HGF or PBS. For adult hemophilia B mice, only RV was injected. For analysis of antigen levels, blood was drawn from the retro-orbital plexus with a capillary tube, and diluted with porcine heparin (Elkins-Sinn, Cherry Hill, NJ) to 250 U/mL. For analysis of coagulation activity, blood was mixed with a 1/10 vol 3.2% sodium citrate.
Hemophilia B puppies from the Chapel Hill colony53 were produced by mating hemophilia B dogs, and their phenotype was confirmed by a prolonged whole blood clotting time.12 At 2 days after birth, 4 doses, at 5 mL RV each, were injected intravenously over 5 minutes at 4-hour intervals via an external jugular catheter. G08 and G42 were male and weighed 0.378 and 0.561 kg, respectively. G18 was female and weighed 0.35 kg.
Immunoassay for cFIX
For mouse samples, the first antibody was the rabbit anti-cFIX antibody used for immunostaining, diluted 1:200 in 0.1 M NaHCO3, pH 9.5. For dog samples, the first antibody was a 1:500 dilution of a mouse monoclonal anti-hFIX antibody (Roche, Indianapolis, IN). Wells were blocked with Tris (tris(hydroxymethyl)aminomethane)–buffered saline (TBS) (40 mM Tris-HCl, 150 mM NaCl, pH 7.4) with 5% nonfat dry milk (Schnucks Grocery, St Louis, MO) (TBS-milk) and washed 6 times with TBS with 0.05% Tween 20 (TBS-Tween) between each step. Samples were diluted in TBS-milk and incubated for 2 hours at 37°C. For the mouse and canine assays, a 1:500 or 1:200 dilution, respectively, of an HRP-conjugated sheep anti-cFIX IgG (SACIX-HRP; Affinity Biologicals, Ancaster, ON, Canada) was incubated for 2 hours at 37°C, and the assay was developed with 3, 3′, 5′, 5′-tetramethylbenzidine. The cFIX was undetectable in plasma from mice or hemophilia B dogs. Standards were dilutions of pooled plasma from normal Chapel Hill dogs, which was assumed to have 5 μg/mL cFIX.
Anti-cFIX IgG antibody assay
Enzyme-linked immunosorbent assay (ELISA) plates were coated with 5 μg/mL purified cFIX in PBS, then blocked with TBS-milk. Control wells for nonspecific binding were coated with PBS. Plasma was diluted 1:100 or higher in TBS-milk, and incubated overnight at 4°C. For samples from mice, an HRP-coupled antimouse IgG (Roche) was diluted 1:200 and incubated with the samples at 37°C for 2 hours, followed by development for 3 minutes with tetramethylbenzidine. For dog samples, an HRP-coupled sheep anticanine IgG antibody (Serotec, Raleigh, NC) was used as the second antibody. If the optical density (OD) exceeded 0.5 for samples that were captured with cFIX, the sample was diluted further. The background increased with age for samples from BALB/c mice, but remained low for other samples. The signal was determined by subtracting the OD of the PBS-coated well from the OD obtained with the cFIX-coated well, and multiplying by the dilution factor used for the plasma sample.
Coagulation assays
The activated partial thromboplastin time (aPTT) assay incubated 100 μL aPTT reagent (Organon Teknika, Durham, NC) with 100 μL plasma at 37°C for 5 minutes; then 100 μL of 25 mM CaCl2 was added and the time to clot formation measured with a Fibrosystem BBL fibrometer (Becton Dickinson, Cockeysville, MD). For samples from RV-treated normal mice, assays used 75 μL hFIX-deficient human, 5 μL RV-treated mouse, and 20 μL cFIX-deficient dog plasma. The times were compared with a standard curve obtained with the use of 75 μL hFIX-deficient human, 5 μL normal mouse, 0 to 20 μL normal dog, and 20 to 0 μL cFIX-deficient dog plasma. If necessary, samples from RV-treated mice were diluted with normal mouse plasma. For samples from RV-treated hemophilia B mice, assays used 75 μL hFIX-deficient human, 10 μL RV-treated hemophilia B mouse, and 15 μL cFIX-deficient dog plasma; and the standards used 75 μL hFIX-deficient human, 10 μL hemophilia B mouse, 0 to 15 μL normal dog, and 15 to 0 μL cFIX-deficient dog plasma. The aPTT from RV-treated dogs used 100 μL plasma, and standards were created by mixing plasma from normal and hemophilia B dogs from the Chapel Hill colony. The tail-clip bleeding assay was performed on 4-month-old mice after cutting the tail where the diameter was 2 mm as previously described10 except the end point was bleeding from the tail at 15 minutes after clip was performed.
DNA analysis for RV DNA sequences
DNA was isolated from organs and 100 ng was analyzed by real-time polymerase chain reaction (PCR) with the use of Taqman technology (Applied Biosystems, Rockville, MD) as described previously.36 DNA from nontransduced mice was isolated and amplified at the same time as DNA from the experimental mice to demonstrate a lack of contamination. Standards contained 0.5 copies or fewer of a WPRE-containing RV per diploid genome.36 The sensitivity of the assay was 0.002 copies per cell.
Results
Generation of an RV expressing cFIX
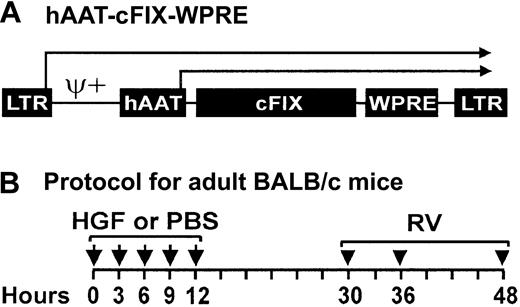
The cFIX cDNA was cloned into an MLV-based RV vector downstream of the hAAT promoter and upstream of the WPRE (Figure1A). This vector does not contain a selectable marker to avoid the expression of unnecessary sequences that might provoke an immune response. This was used to generate an amphotropic RV.
Retroviral vector and time course for administration of HGF and RV to adult mice.
(A) Retroviral vector hAAT-cFIX–WPRE. The MLV-based RV contains intact long terminal repeats (LTRs) at the 5′ and 3′ ends, an extended packaging signal (ψ+), the 403-nt human α1-antitrypsin promoter (hAAT), the 1.5-kb cFIX, and the 591-nt woodchuck hepatitis posttranscriptional regulatory element (WPRE). Transcription can initiate from the LTR or the hAAT promoters as indicated by the arrows. (B) Time course for administration of HGF and RV to adult BALB/c mice. Six-week-old BALB/c mice were injected intraperitoneally with 5 doses of HGF every 3 hours between 0 and 12 hours (arrows) for a cumulative dose of 29 mg/kg. Controls received PBS at the same times. Equal doses of RV were then injected intravenously into both groups at 30, 36, and 48 hours (arrowheads) after the first dose of HGF or PBS, for a cumulative dose of 1 × 1010 TU/kg.
Retroviral vector and time course for administration of HGF and RV to adult mice.
(A) Retroviral vector hAAT-cFIX–WPRE. The MLV-based RV contains intact long terminal repeats (LTRs) at the 5′ and 3′ ends, an extended packaging signal (ψ+), the 403-nt human α1-antitrypsin promoter (hAAT), the 1.5-kb cFIX, and the 591-nt woodchuck hepatitis posttranscriptional regulatory element (WPRE). Transcription can initiate from the LTR or the hAAT promoters as indicated by the arrows. (B) Time course for administration of HGF and RV to adult BALB/c mice. Six-week-old BALB/c mice were injected intraperitoneally with 5 doses of HGF every 3 hours between 0 and 12 hours (arrows) for a cumulative dose of 29 mg/kg. Controls received PBS at the same times. Equal doses of RV were then injected intravenously into both groups at 30, 36, and 48 hours (arrowheads) after the first dose of HGF or PBS, for a cumulative dose of 1 × 1010 TU/kg.
cFIX expression and antibody response in BALB/c mice
We first tested whether hAAT-cFIX–WPRE could result in therapeutic levels of cFIX in plasma after transfer into neonatal or adult BALB/c mice. Because neonatal mice have sufficient hepatocyte replication to achieve gene transfer with an MLV-based vector without a stimulus for replication, a single dose of 1 × 107 TU (1 × 1010 TU/kg) was injected intravenously at 2 to 3 days after birth without preceding it with HGF. For transfer into adult mice, HGF was given prior to the injection of RV to stimulate the hepatocyte replication that is needed for transduction, as diagrammed in Figure 1B. Control adult mice received PBS prior to the RV to assess the level of expression that could be achieved without induction of hepatocyte replication. Beginning at 30 hours after the first dose of HGF (or PBS), 3 equal doses of RV were injected intravenously for these adult animals, for a cumulative dose that was identical to the dose used in neonatal mice per body weight (1 × 1010 TU/kg). Preliminary experiments demonstrated that hepatocyte replication occurred at these times after the initiation of HGF administration in mice (C.G., K.P.P., data not shown).
BALB/c mice that were transduced as newborns achieved 14.3 ± 4.1 μg/mL cFIX (2.9-fold normal) in blood at 1 month after transduction, which was maintained at stable levels at 3 months (Figure2A). The BALB/c mice that were transduced as adults after HGF administration achieved 2.8 ± 0.6 μg/mL cFIX in plasma at 1 month, which was maintained at 1.75 ± 0.3 μg/mL (35% of normal) at 11 months (Figure 2B). The cFIX level in the adults that received PBS prior to RV administration was only 0.033 ± 0.015 μg/mL at 1 month after transduction, and declined thereafter (Figure2C). We conclude that intravenous injection of RV into newborns results in 8-fold higher antigen levels than in adults that were treated with HGF prior to intravenous injection of RV, but that both approaches achieved therapeutic levels. Prior administration of HGF to adults increased cFIX levels by 86-fold over levels in adult animals that received the same dose of RV without preceding HGF (P = .0002).
Levels of cFIX expression and anti-cFIX IgG antibody after transduction of normal BALB/c mice.
(A) Levels of cFIX in mice transduced as neonates. Neonatal normal BALB/c mice were injected intravenously with a single dose of 1 × 1010 TU/kg hAAT-cFIX–WPRE at 2 or 3 days after birth. Plasma cFIX levels were determined by immunoassay at the indicated time after transduction, and the average at each point ± standard error of the mean (SEM) is shown. The number of animals evaluated (N) is shown. The normal range of cFIX (3 to 8 μg/mL) is indicated by a gray bar. (B) Levels of cFIX in mice transduced as adults with preceding HGF. Adult BALB/c mice were injected intravenously with RV after administration of HGF as diagrammed in Figure 1B, and plasma cFIX antigen levels were determined. Values for individual mice are shown. (C) Levels of cFIX in mice transduced as adults without preceding HGF. Adult BALB/c mice were injected with RV after administration of PBS as diagrammed in Figure 1B, and plasma cFIX antigen levels were determined. Values for individual mice are shown. (D) Anti-cFIX IgG antibody levels in mice transduced as neonates. Plasma from the mice whose cFIX levels are shown in panel A was tested for anti-cFIX–specific antibodies at the indicated times after transduction as detailed in “Materials and methods.” The antibody levels are presented as the specific OD observed for a sample that was captured with cFIX minus the nonspecific signal for that sample, times the dilution of the plasma sample that was used. None of the animals had detectable antibodies. (E) Anti-cFIX IgG antibodies in mice transduced as adults after preceding HGF. Plasma from the mice whose cFIX levels are shown in panel B was tested for anti-cFIX–specific antibodies. Individual animals have the same symbol in both panels. (F) Anti-cFIX IgG antibodies in mice transduced as adults without preceding HGF. Plasma from the mice whose cFIX levels are shown in panel C was tested for anti-cFIX–specific antibodies. Individual animals have the same symbol in both panels.
Levels of cFIX expression and anti-cFIX IgG antibody after transduction of normal BALB/c mice.
(A) Levels of cFIX in mice transduced as neonates. Neonatal normal BALB/c mice were injected intravenously with a single dose of 1 × 1010 TU/kg hAAT-cFIX–WPRE at 2 or 3 days after birth. Plasma cFIX levels were determined by immunoassay at the indicated time after transduction, and the average at each point ± standard error of the mean (SEM) is shown. The number of animals evaluated (N) is shown. The normal range of cFIX (3 to 8 μg/mL) is indicated by a gray bar. (B) Levels of cFIX in mice transduced as adults with preceding HGF. Adult BALB/c mice were injected intravenously with RV after administration of HGF as diagrammed in Figure 1B, and plasma cFIX antigen levels were determined. Values for individual mice are shown. (C) Levels of cFIX in mice transduced as adults without preceding HGF. Adult BALB/c mice were injected with RV after administration of PBS as diagrammed in Figure 1B, and plasma cFIX antigen levels were determined. Values for individual mice are shown. (D) Anti-cFIX IgG antibody levels in mice transduced as neonates. Plasma from the mice whose cFIX levels are shown in panel A was tested for anti-cFIX–specific antibodies at the indicated times after transduction as detailed in “Materials and methods.” The antibody levels are presented as the specific OD observed for a sample that was captured with cFIX minus the nonspecific signal for that sample, times the dilution of the plasma sample that was used. None of the animals had detectable antibodies. (E) Anti-cFIX IgG antibodies in mice transduced as adults after preceding HGF. Plasma from the mice whose cFIX levels are shown in panel B was tested for anti-cFIX–specific antibodies. Individual animals have the same symbol in both panels. (F) Anti-cFIX IgG antibodies in mice transduced as adults without preceding HGF. Plasma from the mice whose cFIX levels are shown in panel C was tested for anti-cFIX–specific antibodies. Individual animals have the same symbol in both panels.
Although anti-FIX antibodies can develop after repeated injections of FIX protein or with many gene therapy approaches, 0 of 7 BALB/c mice that were transduced as newborns developed detectable anti-cFIX IgG antibodies (Figure 2D). In contrast, 3 of 3 BALB/c mice that were treated as adults with RV after HGF administration (Figure 2E) and 6 of 6 BALB/c mice that received gene transfer as adults without preceding HGF administration (Figure 2F) developed antibodies within 1 week after transduction. Although these antibodies did not appear to affect antigen levels for most mice, the 2 animals with the highest levels of antibody had a rapid drop in their antigen levels (Figure 2C,F filled-in symbols). Fisher exact test demonstrated that detectable antibody formation was statistically less common in BALB/c mice that were transduced as newborns than in mice that were transduced as adults after HGF administration (P = .008) or in mice that were transduced as adults without HGF (P = .0006).
cFIX expression and antibody response in hemophilia B mice
The effect of gene transfer upon bleeding manifestations was assessed in hemophilia B mice that were present on a mixed 129 × C57BL/6 background. These mice were generated by homologous recombination into the region that contains the promoter through exon 3, and do not express detectable murine FIX. Neonatal transduction resulted in 8.1 ± 1.9 μg/mL cFIX in plasma (Figure3A; 162% of normal), which was stable for 11 months after transduction. Levels of cFIX antigen were undetectable at all times after injection into adult hemophilia B mice that did not receive preceding HGF (Figure 3B). An attempt to transduce adult hemophilia B mice after HGF administration was unsuccessful owing to the peritoneal bleeding induced by multiple intraperitoneal injections (not shown).
Levels of cFIX expression and anti-cFIX IgG antibody after transduction of 129 × C57BL/6 hemophilia B mice.
(A) Levels of cFIX in mice transduced as neonates. Neonatal 129 × C57BL/6 hemophilia B mice were injected intravenously with a single dose of 1 × 1010 TU/kg hAAT-cFIX–WPRE at 2 or 3 days after birth. The average plasma cFIX antigen levels ± SEM is shown for the indicated number of animals (N). (B) Levels of cFIX in mice transduced as adults without preceding HGF. Adult (6-week-old) 129 × C57BL/6 hemophilia B mice were injected intravenously with a single dose of 3.3 × 109 TU/kg hAAT-cFIX–WPRE, and plasma cFIX antigen levels were determined. Values for individual mice are shown. (C) Anti-cFIX IgG antibody levels in mice transduced as neonates. Plasma from the mice whose cFIX levels are shown in panel A was tested for anti-cFIX–specific antibodies at the indicated times after transduction as detailed in “Materials and methods.” Individual values are shown for the 2 mice that developed a transient and low-titer antibody. Fifteen mice did not develop detectable antibodies at any time point, which is indicated as a single line at the bottom of the graph. (D) Anti-cFIX IgG antibodies in mice transduced as adults without preceding HGF. Plasma from the mice whose cFIX levels are shown in panel B was tested for anti-cFIX–specific antibodies. Individual animals have the same symbol in both panels.
Levels of cFIX expression and anti-cFIX IgG antibody after transduction of 129 × C57BL/6 hemophilia B mice.
(A) Levels of cFIX in mice transduced as neonates. Neonatal 129 × C57BL/6 hemophilia B mice were injected intravenously with a single dose of 1 × 1010 TU/kg hAAT-cFIX–WPRE at 2 or 3 days after birth. The average plasma cFIX antigen levels ± SEM is shown for the indicated number of animals (N). (B) Levels of cFIX in mice transduced as adults without preceding HGF. Adult (6-week-old) 129 × C57BL/6 hemophilia B mice were injected intravenously with a single dose of 3.3 × 109 TU/kg hAAT-cFIX–WPRE, and plasma cFIX antigen levels were determined. Values for individual mice are shown. (C) Anti-cFIX IgG antibody levels in mice transduced as neonates. Plasma from the mice whose cFIX levels are shown in panel A was tested for anti-cFIX–specific antibodies at the indicated times after transduction as detailed in “Materials and methods.” Individual values are shown for the 2 mice that developed a transient and low-titer antibody. Fifteen mice did not develop detectable antibodies at any time point, which is indicated as a single line at the bottom of the graph. (D) Anti-cFIX IgG antibodies in mice transduced as adults without preceding HGF. Plasma from the mice whose cFIX levels are shown in panel B was tested for anti-cFIX–specific antibodies. Individual animals have the same symbol in both panels.
Hemophilia B mice were tested to determine if they developed antibodies to cFIX. Fifteen of 17 mice that were transduced as newborns had no detectable antibody at any time of evaluation, whereas 2 of these mice had a low level of antibody that resolved spontaneously and never affected the antigen levels or the coagulation activity (Figure 3C and data not shown). In contrast, 6 of 6 hemophilia B mice that received RV as adults without preceding HGF developed antibodies (Figure3D). Development of antibodies at any time of evaluation was statistically less likely in mice that were treated as newborns than in adult mice that received RV without preceding HGF (P = .0003 with Fisher exact test).
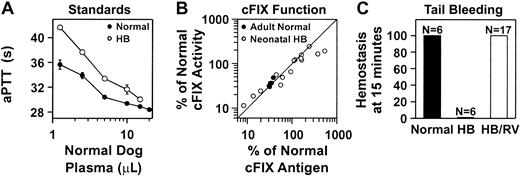
Functional activity of cFIX in mice
We also tested if the cFIX present in RV-treated mice was functional. Two aPTT assays specific for cFIX functional activity were developed for analysis of plasma from normal or hemophilia B mice as detailed in “Materials and methods” and shown in Figure4A. The cFIX functional activity correlated well with the antigen levels for normal BALB/c mice that were transduced as adults after HGF administration, and for most hemophilia B mice that were treated with RV as neonates (Figure 4B). However, a few animals with antigen levels that exceeded 300% of normal had approximately 50% of the expected functional activity. All hemophilia B mice that were treated with RV as newborns rapidly achieved hemostasis after tail clip, although untreated hemophilia B mice did not (Figure 4C). We conclude that the cFIX produced after neonatal or adult gene therapy was functional, although some animals with very high antigen levels had somewhat lower activity than expected.
Functional activity of cFIX in RV-treated mice.
(A) Standard curve for samples from normal or hemophilia B mice. The aPTT coagulation assays were performed with the use of plasma from hFIX-deficient human plasma, plasma from normal or hemophilia B mice, and varying amounts of plasma from normal and cFIX-deficient dogs as detailed in “Materials and methods.” The average time to clot ± SEM is plotted versus the amount, in microliters, of normal dog plasma added for assays using plasma from normal mice (normal; ●) or hemophilia B mice (HB; ○). (B) In vitro coagulation activity for plasma from RV-treated mice. Normal BALB/c mice whose expression levels are shown in Figure 2B were treated with RV after HGF administration as young adults (adult normal; ●). To determine the amount of functional cFIX in the plasma, an aPTT assay was performed as detailed in “Materials and methods” and compared with the times obtained with the standards in panel A that used normal mouse plasma. The percentage of normal functional activity was plotted versus the percentage of normal antigen levels from the same animal. The line represents values where the antigenic and functional activity is the same. The hemophilia B mice whose expression levels are shown in Figure 3A were treated with RV shortly after birth (neonatal HB; ○). An aPTT assay was performed as detailed in “Materials and methods” and compared with the standards in panel A that used plasma from hemophilia B mice. (C) In vivo hemostasis assay in hemophilia B mice after neonatal transduction. The hemophilia B 129 × C57BL/6 mice whose expression levels are shown in Figure 3A were transduced with RV as newborns. Tail clip was performed at 4 months after transduction, and the percentage of the animals that achieved hemostasis within 15 minutes was determined. Tail clip was also performed on age-matched normal C57BL/6 and untreated hemophilia B 129 × C57BL/6 mice. The number of animals (N) in each group is indicated.
Functional activity of cFIX in RV-treated mice.
(A) Standard curve for samples from normal or hemophilia B mice. The aPTT coagulation assays were performed with the use of plasma from hFIX-deficient human plasma, plasma from normal or hemophilia B mice, and varying amounts of plasma from normal and cFIX-deficient dogs as detailed in “Materials and methods.” The average time to clot ± SEM is plotted versus the amount, in microliters, of normal dog plasma added for assays using plasma from normal mice (normal; ●) or hemophilia B mice (HB; ○). (B) In vitro coagulation activity for plasma from RV-treated mice. Normal BALB/c mice whose expression levels are shown in Figure 2B were treated with RV after HGF administration as young adults (adult normal; ●). To determine the amount of functional cFIX in the plasma, an aPTT assay was performed as detailed in “Materials and methods” and compared with the times obtained with the standards in panel A that used normal mouse plasma. The percentage of normal functional activity was plotted versus the percentage of normal antigen levels from the same animal. The line represents values where the antigenic and functional activity is the same. The hemophilia B mice whose expression levels are shown in Figure 3A were treated with RV shortly after birth (neonatal HB; ○). An aPTT assay was performed as detailed in “Materials and methods” and compared with the standards in panel A that used plasma from hemophilia B mice. (C) In vivo hemostasis assay in hemophilia B mice after neonatal transduction. The hemophilia B 129 × C57BL/6 mice whose expression levels are shown in Figure 3A were transduced with RV as newborns. Tail clip was performed at 4 months after transduction, and the percentage of the animals that achieved hemostasis within 15 minutes was determined. Tail clip was also performed on age-matched normal C57BL/6 and untreated hemophilia B 129 × C57BL/6 mice. The number of animals (N) in each group is indicated.
Organ distribution of vector
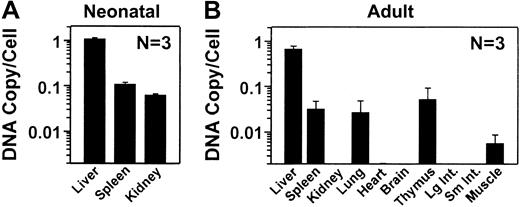
To evaluate the RV distribution, real-time PCR was performed on DNA isolated from liver and other organs with the use of the WPRE sequences to detect the RV, and mouse β-actin sequences to normalize for the amount of DNA. DNA was analyzed from 3 C57BL/6 normal mice that were transduced as neonates and that, at the time of being killed at 10 months, had levels of expression (5.9 ± 2.5 μg/mL) similar to those observed in the BALB/c and hemophilia B mice. After neonatal transduction, the liver contained an average of 1.09 ± 0.06 (SEM) copies of the RV per cell (Figure5A). The spleen and the kidney had 0.11 ± 0.010 and 0.06 ± 0.004 copies of RV per cell, which are 10% and 6%, respectively, of the values in liver. In mice that were treated as adults with HGF followed by RV, there were 0.67 ± 0.1 copies of the RV per cell in the liver at 11 months after transduction (Figure 5B). RV sequences were undetectable (fewer than 0.002 copies per cell) in kidney, heart, brain, large intestine, and small intestine. DNA was detectable in other organs, but the copy number was lower than 7% of that in liver. We conclude that the liver was the major target organ after transduction of adult or neonatal mice with an MLV-based RV. The higher cFIX expression in neonatal-transduced than in adult-transduced mice appeared to be due to a higher transduction efficiency.
Distribution of RV DNA in mice.
DNA was isolated from organs, and the number of copies of the RV per diploid genome was determined by real-time PCR and shown as the average ± SEM. Two nontransduced normal mice from each group had no detectable RV sequences (fewer than 0.002 copies per cell) in any organ (not shown). (A) Copy number after transduction of newborns. The RV DNA copy number was determined in the indicated organs at 10 months after neonatal transduction for 3 normal C57BL/6 mice. The average plasma cFIX levels for these animals was 5.9 ± 2.5 μg/mL (SEM). (B) Copy number after transduction of adult mice. The RV DNA copy number was determined at 11 months after transduction for the BALB/c mice, whose expression levels are shown in Figure 2B, that were transduced as adults with hAAT-cFIX–WPRE after preceding administration of HGF. Large and small intestines are labeled Lg Int and Sm Int, respectively.
Distribution of RV DNA in mice.
DNA was isolated from organs, and the number of copies of the RV per diploid genome was determined by real-time PCR and shown as the average ± SEM. Two nontransduced normal mice from each group had no detectable RV sequences (fewer than 0.002 copies per cell) in any organ (not shown). (A) Copy number after transduction of newborns. The RV DNA copy number was determined in the indicated organs at 10 months after neonatal transduction for 3 normal C57BL/6 mice. The average plasma cFIX levels for these animals was 5.9 ± 2.5 μg/mL (SEM). (B) Copy number after transduction of adult mice. The RV DNA copy number was determined at 11 months after transduction for the BALB/c mice, whose expression levels are shown in Figure 2B, that were transduced as adults with hAAT-cFIX–WPRE after preceding administration of HGF. Large and small intestines are labeled Lg Int and Sm Int, respectively.
Neonatal gene therapy in hemophilia B dogs
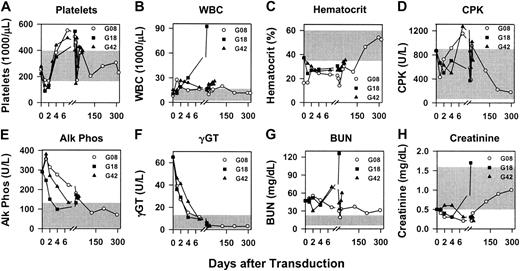
RV transduction was performed on neonatal hemophilia B dogs from the Chapel Hill colony, which have a missense mutation that changes amino acid 379 from glycine to glutamate, and results in less than 1% of normal antigenic and functional activity in plasma.53 G08 and G18 received 1.3 × 1010TU/kg, whereas G42 received 0.8 × 1010 TU/kg of hAAT-cFIX–WPRE at 2 to 3 days after birth as detailed in “Materials and methods.” The average platelet count fell from 250 ± 156 × 109/L prior to injection to 131 ± 24 × 109/L at 24 hours (P = .03 for 24 hours versus pre–gene transfer values) and 123 ±15.1 × 109/L at 48 hours (P = .01 for 48 hours versus pre–gene transfer values), but returned to normal by day 4 (Figure6A). The average white blood cell (WBC) count increased from 11.2 ± 2 × 109/L (11 200 ± 2000/μL) prior to gene therapy to 17.7 ± 5 × 109/L (17 700 ± 5000/μL) at 24 hours and 22.5 ± 2.6 × 109/L (22 500 ± 2600/μL) at 48 hours (Figure 6B), but these elevations were not statistically significant (P = .4 andP = .06 when the 24 hours and 48 hours were compared with the pre–gene therapy values, respectively), and the upper limit of normal for WBC count in dogs is higher than in humans. For G08, the WBC count has been normal for most samples at 4 days or later. For G18, the WBC count increased dramatically at 2 weeks after gene transfer when he became clinically ill and was humanely killed; at necropsy, G18 was found to have pyelonephritis that we believe is unrelated to the gene transfer. For G42, the WBC count has remained slightly elevated at most times for unclear reasons. The hematocrit was low for all dogs at early times (Figure 6C); this may be due to their age. CPK values were occasionally modestly elevated (Figure6D). The alkaline phosphatase, γGT, and BUN were elevated for 1 to 2 weeks after gene transfer (Figure 6E-G); these probably represent physiologically normal elevations in newborns, as the elevated levels were present prior to gene therapy, and analysis in 6 untreated normal neonatal dogs showed similar levels (data not shown). The creatinine was normal or below normal at most times (Figures 6H). The serum glutamic-oxaloacetic transaminase (SGOT), serum glutamic-pyruvic transaminase (SGPT), bilirubin, and amylase levels were within normal limits, and the albumin and total protein levels were either normal or below normal at all times for all dogs (data not shown).
Evaluation of blood tests after neonatal transduction of hemophilia B dogs.
Newborn hemophilia B dogs were injected intravenously with 0.8 to 1.3 × 1010 TU/kg hAAT-cFIX–WPRE at 2 days after birth as detailed in “Materials and methods.” Blood tests were evaluated at the indicated times after the first dose of RV. For all panels, the range of normal values for that test for adult dogs is indicated by the shaded region. (A) Platelets. (B) White blood cell (WBC) count. (C) Hematocrit. (D) Creatine phosphokinase (CPK). (E) Alkaline phosphatase (Alk Phos). (F) γ-glutamyl peptidase (γGT). (G) Blood urea nitrogen (BUN). (H) Creatinine.
Evaluation of blood tests after neonatal transduction of hemophilia B dogs.
Newborn hemophilia B dogs were injected intravenously with 0.8 to 1.3 × 1010 TU/kg hAAT-cFIX–WPRE at 2 days after birth as detailed in “Materials and methods.” Blood tests were evaluated at the indicated times after the first dose of RV. For all panels, the range of normal values for that test for adult dogs is indicated by the shaded region. (A) Platelets. (B) White blood cell (WBC) count. (C) Hematocrit. (D) Creatine phosphokinase (CPK). (E) Alkaline phosphatase (Alk Phos). (F) γ-glutamyl peptidase (γGT). (G) Blood urea nitrogen (BUN). (H) Creatinine.
RV-treated dogs were evaluated for cFIX functional activity and antigen levels and for correction of the bleeding phenotype. The whole blood clotting time was corrected to the normal range at 1 week or later after gene transfer for all dogs (Figure7A), and the aPTT was shortened but not normalized (Figure 7B). The relative amount of functional activity was determined by comparing the results of the aPTT with a standard curve created by mixing plasma from normal and hemophilia B dogs. The functional cFIX activity averaged 3.1% ± 0.3% for G08, 6.7% ± 2.5% for G18, and 2.5% ± 0.3% for G42 (Figure 7C). Antigen levels that were determined by immunoassay were 9.9% of normal (0.5 μg/mL) for G08 for 11 months, 36% of normal (1.8 μg/mL) for G18 at 1 week, and 9.7% of normal (0.5 μg/mL) for G42 at 10 weeks (Figure 7D). Although cFIX levels were higher at 2 weeks for G18, this may reflect an acute-phase response from the pyelonephritis inducing the hAAT promoter in the RV. The percentage of cFIX that was functional was determined by dividing the percentage of normal cFIX activity by the percentage of normal antigen levels for the same sample, and was 32.4% ± 2.8% for G08, 9.7% ± 1.8% for G18, and 25.5% ± 2.1% for G42. Thus, the cFIX was not fully functional even with below-normal antigen levels. One mild episode of bleeding between the shoulder blades in G08 at 4 months after transduction resolved with 2 doses of plasma containing 5 U/kg cFIX at 24-hour intervals, whereas the other dogs have never bled. No anti-cFIX antibodies were detected in any dogs at any time (Figure 7E).
Evaluation of expression and clinical effect after neonatal transduction of hemophilia B dogs.
Neonatal hemophilia B dogs were treated with gene therapy as described in the legend to Figure 6. The arrow for G08 at 4 months after transduction indicates a minor bleed that was treated with 2 doses of canine plasma. G18 died of pyelonephritis at 18 days after gene transfer, whereas G08 and G42 are still being followed. (A) Whole blood clotting time (WBCT). The whole blood clotting time is 8 to 13 minutes for normal dogs (gray shaded region), and longer than 60 minutes for hemophilia B dogs (hatched region). (B) aPTT coagulation assay. The range of aPTT for normal dogs from the same colony (average ± 2 standard deviations) is 11.6 to 12.6 seconds (gray shaded region). The range of aPTT for 5 untreated hemophilia B dogs from the same colony was 37.5 to 59.1 seconds (hatched region). (C) Percentage of normal cFIX activity. The percentage of normal cFIX activity in the plasma samples from the RV-treated dogs was determined by comparing the aPTT with a standard curve generated by means of mixtures of normal and cFIX-deficient dog plasma. The normal range (normal) of cFIX (60% to 160%) is indicated by the gray shaded region. Untreated hemophilia B dogs have less than 0.1% of normal activity (hatched region). (D) Percentage of normal cFIX antigen levels. The cFIX antigen levels were determined by immunoassay and compared with the level present in a pool of normal dog plasma from the Chapel Hill colony to determine the percentage of normal antigen levels. The normal range (normal) of cFIX is 60% to 160% (3 to 8 μg/mL) and is indicated by the gray shaded region. Untreated hemophilia B dogs (HB) have less than 0.02% of normal cFIX antigen levels, as indicated by the hatched region. (E) Anti-cFIX IgG antibody levels. Dogs were tested for anti-cFIX IgG antibodies as detailed in “Materials and methods.”
Evaluation of expression and clinical effect after neonatal transduction of hemophilia B dogs.
Neonatal hemophilia B dogs were treated with gene therapy as described in the legend to Figure 6. The arrow for G08 at 4 months after transduction indicates a minor bleed that was treated with 2 doses of canine plasma. G18 died of pyelonephritis at 18 days after gene transfer, whereas G08 and G42 are still being followed. (A) Whole blood clotting time (WBCT). The whole blood clotting time is 8 to 13 minutes for normal dogs (gray shaded region), and longer than 60 minutes for hemophilia B dogs (hatched region). (B) aPTT coagulation assay. The range of aPTT for normal dogs from the same colony (average ± 2 standard deviations) is 11.6 to 12.6 seconds (gray shaded region). The range of aPTT for 5 untreated hemophilia B dogs from the same colony was 37.5 to 59.1 seconds (hatched region). (C) Percentage of normal cFIX activity. The percentage of normal cFIX activity in the plasma samples from the RV-treated dogs was determined by comparing the aPTT with a standard curve generated by means of mixtures of normal and cFIX-deficient dog plasma. The normal range (normal) of cFIX (60% to 160%) is indicated by the gray shaded region. Untreated hemophilia B dogs have less than 0.1% of normal activity (hatched region). (D) Percentage of normal cFIX antigen levels. The cFIX antigen levels were determined by immunoassay and compared with the level present in a pool of normal dog plasma from the Chapel Hill colony to determine the percentage of normal antigen levels. The normal range (normal) of cFIX is 60% to 160% (3 to 8 μg/mL) and is indicated by the gray shaded region. Untreated hemophilia B dogs (HB) have less than 0.02% of normal cFIX antigen levels, as indicated by the hatched region. (E) Anti-cFIX IgG antibody levels. Dogs were tested for anti-cFIX IgG antibodies as detailed in “Materials and methods.”
Discussion
Neonatal RV gene therapy corrects the bleeding phenotype in mice and dogs
Neonatal injection of an RV expressing cFIX resulted in plasma cFIX antigen levels that were 2.8-fold (BALB/c), 1.6-fold (hemophilia B 129 × C57BL/6), and 1.5-fold (C57BL/6 mice; data not shown) the level found in normal dogs. These levels compare favorably with those achieved with most other gene therapy approaches in mice (reviewed in “Introduction”), although they are lower than in some studies after intramuscular injection of an AAV1 vector22 or intravenous injection of an adenoviral vector.14-16 The average cFIX antigen level in the 3 hemophilia B dogs was considerably lower at 18.5% of normal, although this still exceeds that observed in most other gene therapy studies in hemophilia B dogs (reviewed in “Introduction”), and is similar to that achieved with liver-directed gene therapy with an AAV vector with a similar promoter.24 It is unclear why expression after neonatal transduction was only 9% as high in dogs when compared with mice that received a similar dose of RV per kilogram of body weight. Similarly, expression was lower in neonatal MPS VII dogs37 than in mice.36 The higher cFIX expression here than in a previous study that used an MLV-based RV in dogs28 is probably due at least in part to higher expression per copy owing to a stronger promoter and inclusion of the WPRE. Neonatal transduction is relatively specific for the liver, which contained in excess of 10-fold more copies of DNA than did the kidney and spleen in this study as well as in a previous study in which more organs were analyzed.36Although DNA distribution was not analyzed in dogs in this study, the copy number at 5 days after gene transfer was high in liver and spleen and was much lower in other organs in a previous study.37
There was a modest (less than 50%) decline in the percentage of cFIX that was functional for some mice with above-normal antigen levels, although the protein was fully functional when antigen levels were normal or lower. However, the RV-treated hemophilia B dogs had only 22.5% of the expected functional cFIX activity on the basis of their antigen levels even with below-normal antigen levels. A similar reduction in the percentage of cFIX that was functional was observed in mice when above-normal levels of FIX were achieved after intravenous injection of an adenoviral15 or intramuscular injection of an AAV122 vector in mice. This may represent reduced efficiency in an overexpressing cell of posttranslational modifications, such as γ-carboxylation, that are necessary for biological activity. Nevertheless, all RV-treated hemophilia B mice achieved hemostasis after a bleeding challenge, and only 1 bleed has occurred after a total of 14 months of evaluation in the RV-treated hemophilia B dogs, which is a markedly lower frequency than the average of 6 bleeds per year in untreated hemophilia B dogs from this colony.
This is the first report of using neonatal gene therapy to achieve long-term correction of hemophilia B in either mice or dogs. Adenoviral14 and AAV8 vectors resulted in only transient expression after neonatal delivery, which may be due to the instability of nonintegrated vectors in rapidly dividing cells, or to other causes such as promoter attenuation. The ability of an RV to integrate into the host cell chromosome and the longevity of hepatocytes54 are probably responsible for the durability of this neonatal approach. Similarly, neonatal gene therapy with an RV achieved long-term expression of factor VIII in hemophilia A mice.35
Neonatal gene transfer has the potential to reduce immune responses, as newborn immune systems are immature.55 Indeed, in this study, antibody responses were much less likely after neonatal gene transfer to BALB/c or hemophilia B 129 × C57BL/6 mice than they were after transfer into adult mice of the same strains. Similarly, no antibodies were detected in the neonatal transduced dogs, although a caveat here is that the Chapel Hill colony of hemophilia B dogs rarely produced antibodies after injection of cFIX protein or gene therapy with cFIX-expressing vectors.
Although the neonatal RV gene therapy approach is promising, there are 5 concerns about using this approach in humans. First, the dogs had a modest decrease in their platelet counts at 24 and 48 hours after gene transfer. A similar decrease in the number of platelets from 238 ×109/L to 86 ×109/L occurred at 24 hours after neonatal intravenous injection of an RV into 5 MPS VII dogs, although there were no other complete blood count (CBC) abnormalities (L.X., Mark Haskins, and K.P.P., unpublished data, 2002). Because schistocytes were rare or undetectable on the peripheral blood smear and the total bilirubin level was below 5.13 μM (0.3 mg/dL) for 8 dogs that were evaluated at 24 hours after gene transfer, the decrease in platelets was probably not due to severe disseminated intravascular coagulation, although an insufficient amount of blood was available to perform coagulation studies or test for fibrin degradation products. In contrast to results in dogs, platelets did not fall at 24 or 48 hours after intravenous injection of 1 × 1010 TU/kg hAAT-cFIX–WPRE at 3 days after birth in mice (data not shown). In addition, for normal dogs that received a lower dose (2.5 × 109 TU/kg) of an RV expressing human factor IX as newborns, the drop in platelets was more modest (183 ±26 × 109/L at 24 hours and 175 ±104 × 109/L at 48 hours), which suggests that the drop is dose related. Further studies will attempt to determine why platelets fall in dogs and if the RV induces inflammatory responses. A second concern of neonatal gene transfer is that insertional mutagenesis could result in the development of liver cancer. Although we believe that this risk is low, mice and dogs will be followed for the long term for this possible complication prior to applying this approach for hemophilia B. Third, the risk of antibody formation needs to be better defined in large animals, including primates. Fourth, sperm will be analyzed once males undergo puberty to determine if germ line transmission occurs, although newborn males should not have replicating germ cells, and RV DNA was not detected in testis after neonatal gene transfer in mice36or dogs37 in previous studies. Finally, the exact percentage of hepatocytes that are replicating in newborn humans is unclear, although their rapid growth makes it likely that it should be sufficient to achieve gene transfer.
HGF-potentiated RV transfer into adult mice achieves therapeutic levels of expression
This study demonstrated that 35% of normal cFIX levels were achieved for 11 months with transfer of an MLV-based RV into adults after replication was induced with HGF. This is the first demonstration that injection of purified HGF protein prior to an RV can result in long-term expression of a therapeutic gene, although others have previously demonstrated that HGF potentiates RV transduction,38-41 and we showed that intramuscular injection of an adenoviral vector expressing HGF increased RV transduction and improved clinical efficacy in MPS VII mice.42 The level of cFIX achieved at late times after transfer into adult BALB/c mice (1.75 μg/mL) was only 19% of the average value observed in 3 different stains after transfer into neonatal mice (9.3 μg/mL) despite use of the same dose of RV per kg body weight. Similarly, the DNA copy number was lower after adult than neonatal transduction. The higher transduction in neonates may reflect higher levels of hepatocyte replication in newborns than in HGF-treated adults, or may be due to other factors.
HGF-potentiated RV transfer into adults achieved therapeutic levels of expression that compare favorably with the results obtained with other vectors in adult mice (reviewed in “Introduction”). Although lentiviral vectors are believed by some,3 but not all,4 to efficiently transduce nonreplicating hepatocytes, the level of FIX achieved here is greater than 10-fold higher than that achieved with a lentiviral vector without a partial hepatectomy. It is unclear, however, if this is due to more efficient transfer of the MLV-based RV into the liver or to greater expression per copy. In addition, this system for delivering an MLV-based RV was relatively specific for the liver. In contrast, intravenous injection of a lentiviral vector resulted in a high RV DNA copy number in spleen and bone marrow in addition to liver,56 although other organs had very few copies, and the earlier time of analysis in the lentiviral vector study may have affected the result.
MLV-based RV-mediated gene therapy might be easier to implement for adults than newborns, as adults are more qualified to grant informed consent, and an MLV-based RV has already been injected into adult patients with hemophilia A without adverse effects.57However, most of the concerns noted above for neonatal gene transfer need to be addressed prior to implementing this approach in human adults. In addition, it would be necessary to demonstrate that transient administration of HGF is safe, although it has been efficacious and well tolerated in animal models of liver disease58 and no overt adverse effects were noted in this or previous38-42 studies. Finally, immune modulation may be necessary at the time of gene therapy, given that antibody responses occurred in all animals that were transduced as adults.
We thank Kathy High, Roland Herzog, and Valder Arruda for the cFIX cDNA; for providing purified cFIX and assistance with immunoassays; and for shipping mice to us. We thank Hui-Feng Lin and Darrel Stafford for allowing us to use the hemophilia B mice and Marie Roberts for breeding mice.
Prepublished online as Blood First Edition Paper, January 16, 2003; DOI 10.1182/blood- 2002-10-3050.
Supported by National Institutes of Health grants DK48028 (K.P.P.), R24 HL63098 (T.C.N.), and DK57586 (M.S.S.), and a Judith Graham Pool fellowship from the National Hemophilia Foundation (L.X.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Katherine P. Ponder, Department of Internal Medicine, Washington University School of Medicine, 660 S Euclid Ave, St Louis, MO 63110; e-mail: kponder@im.wustl.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal