Abstract
Mice that lack the matricellular angiogenesis inhibitor, thrombospondin-2 (TSP2), display a bleeding diathesis, despite normal blood coagulation and the lack of thrombocytopenia. Although platelets do not contain detectable levels of TSP2, TSP2-null platelets are compromised in their ability to aggregate in vivo in response to denudation of the carotid artery endothelium, and in vitro following exposure to adenosine diphosphate (ADP). Megakaryocytes (MKs) show high levels of TSP2 by immunohistochemical analysis of bone marrow. However, when cultured in vitro, MKs contain little TSP2 protein or mRNA. These findings suggest that most TSP2 is acquired from the bone marrow microenvironment. Consistent with this hypothesis, MKs take up recombinant TSP2 in an integrin-dependent manner when it is supplied in the culture medium. Furthermore, uptake of TSP2 in vitro affects MK differentiation and proplatelet formation. The functional significance of this process is supported by the presence of ultrastructural abnormalities in TSP2-null bone marrow, including extensive fragmentation of the peripheral zone in MKs and failure of this zone to form close associations with vascular sinuses. We conclude that the uptake of TSP2 by MKs from the marrow milieu is required for proper MK function and the release of functionally competent platelets.
Introduction
Thrombospondin-2 (TSP2) is a potent inhibitor of angiogenesis that participates in the processes of development and tissue repair.1 Specifically, TSP2 is expressed during embryonic development, and during healing of dermal wounds and the foreign body response to implanted biomaterials.2-5 In addition, TSP2 has been implicated in the process of tumor growth where it has been shown to inhibit blood vessel formation and limit the growth of squamous cell carcinomas.6 As a matricellular protein, TSP2 can interact with both cell-surface receptors and extracellular matrix (ECM) components and is believed to function as a modulator of cell-matrix interactions.7
We have previously shown that mice that lack TSP2 develop a pleiotropic phenotype characterized by connective tissue abnormalities that include abnormal collagen fibrillogenesis.8 In addition, these mice display increased angiogenesis, increased bone formation, reduced fibroblast adhesion, and an unexpected bleeding diathesis. We hypothesized that the latter could result from an intrinsic qualitative platelet defect, from a subendothelial defect, or both. While we have preliminary evidence for the contribution of a connective tissue abnormality to the bleeding diathesis (“Discussion”), we were surprised to find a reproducible defect in aggregation in the response of TSP2-null platelets to a mild agonist. Since TSP2, unlike TSP1, is not present in platelets we hypothesized that the abnormality might arise earlier, during megakaryocyte (MK) development.
Proplatelets are long processes that issue from the surface of fully mature MKs, migrate through the endothelial barrier of marrow sinusoids, and fragment into platelets. At the molecular level, little is understood regarding the regulation of these events, except that β1 tubulin and protein kinase Cα (PKCα) are essential.9,10 Analysis of platelet function on the other hand, has benefited from the generation of genetic disruptions in mice that lead to the development of platelet abnormalities. Such models have provided new insights into the functional significance of several platelet proteins. For example, deficiencies in μ-calpain,11αIIbβ3,12 platelet endothelial cell adhesion molecule-1 (PECAM-1),13 and von Willebrand factor (VWF)14 have led to the development of distinct platelet abnormalities and/or bleeding diatheses. Interestingly, platelet aggregation defects are not always associated with prolonged bleeding times, as was seen in μ-calpain–null mice.11 The opposite condition, in which a bleeding diathesis can develop without a platelet defect, has also been observed in PECAM-1–null mice.15
The mode of action of TSP2 in the regulation of cell adhesion is just beginning to be elucidated, and much of our current understanding originates from the analysis of the adhesive defect in TSP2-null fibroblasts.8 These cells have a 2-fold increase in matrix metalloproteinase 2 (MMP2) that is responsible for their compromised adhesion.16 TSP2 can bind MMP2 and direct it to the lysosomes by interacting with one of its receptors, low-density lipoprotein receptor-related protein.17 We have also shown that TSP2 can modulate the levels of MMP2 in vivo during the formation of sponge granulomas.18 Since MKs contain MMP219 it is possible that TSP2 may play a role in modulating its levels in these cells.
To our knowledge, studies indicating that deficiency of a nonplatelet protein can lead to the development of an intrinsic platelet defect have not been previously reported. The present study focuses on an examination of the origin of the intrinsic platelet abnormality in TSP2-null mice and provides evidence for the uptake of TSP2 by MKs in vitro as an important factor in normal MK and platelet function.
Materials and methods
Animals
The generation of TSP2-null animals has been described.8 In this study TSP2-null and littermate wild-type mice on a C57BL/6/129 SvJ background were used. All mice ranged in age from 4 to 6 months and the same number of male and female animals were used for each genotype.
Platelet studies
Platelet preparation was performed as described previously20 21 except that blood was collected from the inferior vena cava. Both platelet-rich plasma (PRP) and washed platelets were used in aggregometric assays. Washed platelets were prepared by centrifugation of PRP and resuspension of the platelet pellet in Tyrode buffer. Platelet aggregation in PRP was performed at 37°C with shaking in microtiter wells and monitored with the aid of a plate reader (Molecular Dynamics, Piscataway, NJ) at 490 nm. Aggregation of washed platelets was performed in the presence of fibrinogen (20 μg/mL) and monitored in a Helena Laboratories aggregometer (Allen Park, MI). Platelet agonists included adenosine diphosphate (ADP; 10-20 μm), thrombin (0.1-1 U/mL), and collagen (1-10 μg/mL). ADP and thrombin were purchased from Sigma (St Louis, MO) and type I equine tendon collagen from Chrono-log (Havertown, PA).
Adenosine triphosphate (ATP) secretion was measured in whole blood in a lumi-aggregometer (Chrono-log). Samples of whole blood (900 μL) were incubated with 100 μL luciferin/luciferase reagent at 37°C for 30 seconds and stirred at 1000 rpm. Subsequently, ADP (20 μm) was added, and the ATP secretion tracings were recorded for 5 minutes.
Denudation of the carotid artery
Complete removal of the endothelium from the left carotid artery was achieved with a nylon thread as described previously.22,23 Mice were perfuse-fixed 10 minutes following injury and the carotid artery was removed, fixed, and processed for either scanning electron microscope (SEM) or tranmission electron microscope (TEM), as described previously.24
Reverse transcriptase–polymerase chain reaction (RT-PCR)
Reverse-transcribed (Superscript II; GIBCO BRL, Carlsbad, CA) RNA, purified from MKs (next section) with an Rneasy kit (Qiagen, Valencia, CA), was a kind gift of Dr Yoshitaka Miyakawa (University of Washington). PCR amplification with TSP2-specific primers was performed as described previously.16 Amplification of ribosomal protein S6 cDNA, as described previously,25 served as internal control. The mRNA origin of the observed band was confirmed by its resistance to DNAse digestion of the RNA prior to amplification.
Studies of MKs in vitro
Mice were injected (2 μg/d; 5 days) with human thrombopoeitin (TPO; Zymogenetics, Seattle, WA). MKs were cultured at 37°C in Iscove modified Dulbecco medium/1% Nutridoma/50 ng/mL TPO and were then purified over a discontinuous bovine serum albumin (BSA) gradient, as previously described prior to use.10 Subsequently, MKs were cultured with or without 10% plasma.
To examine TSP2 uptake, 1 × 103 MKs/well were plated in 16-well glass chamber slides (Nunc, Rochester, NY) and recombinant TSP2 (rTSP2; 10 μg/mL) was added one hour after plating. To block rTSP2 uptake, MKs were plated and either the arginine-glycine-aspartic acid (RGD)–containing peptide (GRGDTP; 50 μm) or a control peptide (YVGVAPG; 50 μm) was added to the cultures 2 hours prior to the addition of rTSP2. Then, 24 hours later, the culture media were removed and the cells were washed with phosphate-buffered saline (PBS) and fixed with 2% paraformaldehyde for 30 minutes at RT. The wells were then washed with PBS, blocked with 1% BSA/0.1% Triton X-100/PBS for 30 minutes and then incubated with rabbit anti-TSP2 antibodies. Fluorescein isothiocyanate (FITC)–conjugated goat anti–rabbit immunoglobulin G (IgG) was used to detect TSP2 in MKs. Both rTSP2 and the rabbit anti-TSP2 antibodies have been described.2
For confocal microscopy, MKs were isolated, plated, and treated with rTSP2 (10 μg/mL) in the presence or absence of 10% plasma. Following a 48-hour incubation, MKs were washed, fixed in 3.7% paraformaldehyde, and permeabilized with 0.2% Triton X-100. TSP2 was detected with FITC-conjugated IgG. Nuclei were visualized with DAPI (4,6 diamidino-2-phenylindole). FITC was collected with an argon laser, and DAPI with 2 photon excitation on a Leica TCS SP/MP confocal microscope (Heidelberg, Germany).
To examine the effect of TSP2 on proplatelet formation, we added TSP2 to MKs cultured in the absence of plasma, as described above. Then, 24 hours later, the medium was removed and the cells were washed gently with PBS. Medium containing 10% plasma was then added for 48 hours to induce MK differentiation. Cell viability and proplatelet formation were assessed as described previously.10 The latter was determined by examination of cultures under phase contrast. The cells were then fixed and examined. All experiments were performed in triplicate.
Bone marrow immunohistochemistry and electron microscopy
Bone marrow plugs were flushed with saline from femurs of 3-month-old TSP2-null and wild-type mice that had been killed. Plugs were embedded in optimum cutting temperature (OCT) compound and used to generate cryosections. To immunolocalize TSP1 and TSP2, sections were fixed with acetone for 10 minutes at −20°C and then air-dried for one hour. Following washing, TSP1 and TSP2 were visualized as described in the previous section. The antimouse TSP1 antibody has been characterized3 and was a kind gift of Dr D. Mosher, University of Wisconsin. To visualize and quantify MKs in bone marrow, formalin-fixed decalcified femurs (Accumate RDO rapid decalcifying solution; Sigma) were embedded in paraffin, and sections were stained with an anti-VWF antibody (DAKO, Carpinteria, CA) according to the supplier's instructions. For electron microscope (EM) analysis, bone marrow plugs were placed in ice-cold Karnovsky fixative and processed for analysis as described previously.26 27
Results
TSP2-null platelets fail to form aggregates in vivo
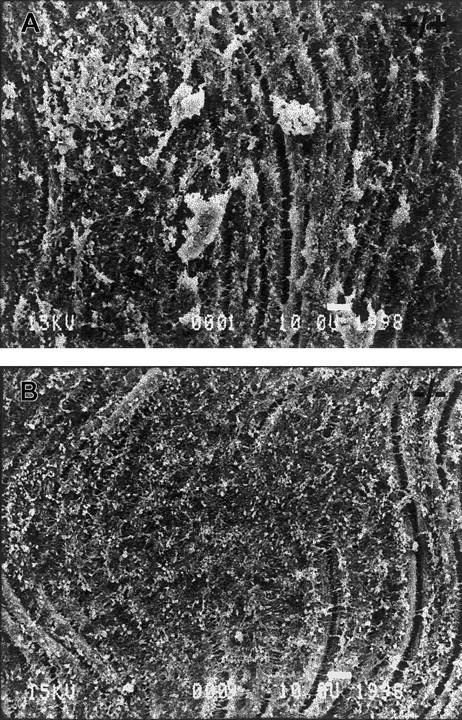
To examine the ability of TSP2-null platelets to form aggregates following vascular injury, the endothelium of the left common carotid artery of anesthetized mice was denuded by the insertion and rotation of a flexible nylon loop. Circulating platelets were allowed to interact with the exposed subendothelium for 10 minutes, and the arteries were then perfused-fixed in situ, harvested, processed, and analyzed by SEM and TEM. SEM analysis revealed the formation of numerous platelet aggregates on the exposed subendothelium of wild-type animals (Figure 1A). These aggregates ranged in size from 10 to 75 μm in diameter. On the contrary, large platelet aggregates, over 10 μm in diameter, were absent from the exposed subendothelium of TSP2-null mice (Figure 1B), although we observed many single platelets as well as small platelet aggregates. This observation suggests that the adhesion of platelets to the exposed subendothelium was not primarily compromised. In experiments in which the carotid arteries were harvested 15 minutes following denudation, we observed that larger platelet aggregates were beginning to form in TSP2-null mice, but these aggregates were still significantly smaller than those in wild-type mice (data not shown). Thus, we conclude that conditions in TSP2-null mice led to a delay in platelet aggregation.
TSP2-null mice fail to form platelet aggregates normally in vivo.
Representative SEM images of denuded carotid artery subendothelium 10 minutes following injury. Numerous platelet aggregates can be seen in wild-type mice (A), whereas none are observed in TSP2-null mice (B). A total of 5 mice per genotype were analyzed. Bar represents 10 μm.
TSP2-null mice fail to form platelet aggregates normally in vivo.
Representative SEM images of denuded carotid artery subendothelium 10 minutes following injury. Numerous platelet aggregates can be seen in wild-type mice (A), whereas none are observed in TSP2-null mice (B). A total of 5 mice per genotype were analyzed. Bar represents 10 μm.
To better examine the interaction of platelets with the exposed subendothelium we analyzed denuded arteries by TEM. By this method we observed numerous aggregates in wild-type mice that involved a layer of flattened and activated platelets adhering to the subendothelium, covered by layers of closely adherent platelets (Figure2A). TSP2-null subendothelia mostly lacked such aggregates, and only occasional small aggregates, consisting of loosely organized platelets and displaying minimal cell-cell interactions, were present (Figure 2B). The lack of intimacy in the interactions of TSP2-null platelets was in marked contrast to the extensive and close interactions displayed by wild-type cells. Consistent with our findings with SEM, numerous flattened platelets adhering to the subendothelium could be observed in TSP2-null mice. These findings suggest that platelet aggregation is compromised in TSP2-null mice and provide a basis for the bleeding diathesis in these animals. However, the denudation experiments do not distinguish between a subendothelial matrix abnormality and an intrinsic platelet defect. We proceeded to examine the latter possibility by analyzing platelet function ex vivo.
TEM images of platelet aggregates.
Representative TEM images of denuded carotid artery subendothelium 10 minutes following injury. A large platelet aggregate with several layers of intimately associated platelets can be seen in wild-type mice (A). A glancing section of the internal elastic lamina is seen as a white band. In contrast, a small aggregate of platelets displaying minimal interactions can be seen in TSP2-null mice (B). A total of 5 mice per genotype was analyzed. Bar represents 1 μm.
TEM images of platelet aggregates.
Representative TEM images of denuded carotid artery subendothelium 10 minutes following injury. A large platelet aggregate with several layers of intimately associated platelets can be seen in wild-type mice (A). A glancing section of the internal elastic lamina is seen as a white band. In contrast, a small aggregate of platelets displaying minimal interactions can be seen in TSP2-null mice (B). A total of 5 mice per genotype was analyzed. Bar represents 1 μm.
TSP2-null platelets are deficient in aggregation and secretion in vitro
PRP, prepared from wild-type and TSP2-null mice, was analyzed by aggregometry after exposure to the platelet agonists, thrombin, collagen, or ADP. Initial analysis was performed in microtiter wells to minimize the required amount of blood. Of the 3 agonists tested, only aggregation in response to ADP was compromised in TSP2-null platelets (Figure 3A). During the early (30-second) response to ADP, TSP2-null and wild-type platelets displayed similar aggregation, but the final level of aggregation of TSP2-null platelets was only 40% of normal. The response to thrombin and collagen was indistinguishable between TSP2-null and wild-type platelets (data not shown). In addition, similar levels of P-selectin on TSP2-null and wild-type platelets were observed by fluorescence-activated cell-sorter (FACS) analysis, following exposure of whole blood to thrombin (data not shown). These results demonstrate that TSP2-null platelets are compromised in their aggregative response to ADP and suggest the existence of an intrinsic platelet defect.
Effects of TSP2 deficiency on platelet aggregation.
(A) PRP (1 × 108 platelets/mL) from wild-type (●) and TSP2-null (○) mice was treated with ADP (10 μm), and the aggregation response was monitored by a change in transmittance. The aggregation of wild-type platelets was set at 90% to correct for the background signal obtained with platelet-poor plasma. The results are the average of 3 independent experiments. (B) Representative tracings of the aggregation responses of TSP2-null washed platelets (1 × 108/mL) to thrombin (1 U/mL), collagen (10 μg/mL), and ADP (10 μm) are shown. For the latter a representative tracing of the aggregation response of wild-type platelets (WT) is also shown. A total of 5 mice per genotype was used to generate washed platelets, and each experiment was repeated 4 times. (C) Representative tracings of ATP secretion from ADP-activated blood samples from wild-type (WT) and TSP2-null (KO) mice. The experiment was repeated twice.
Effects of TSP2 deficiency on platelet aggregation.
(A) PRP (1 × 108 platelets/mL) from wild-type (●) and TSP2-null (○) mice was treated with ADP (10 μm), and the aggregation response was monitored by a change in transmittance. The aggregation of wild-type platelets was set at 90% to correct for the background signal obtained with platelet-poor plasma. The results are the average of 3 independent experiments. (B) Representative tracings of the aggregation responses of TSP2-null washed platelets (1 × 108/mL) to thrombin (1 U/mL), collagen (10 μg/mL), and ADP (10 μm) are shown. For the latter a representative tracing of the aggregation response of wild-type platelets (WT) is also shown. A total of 5 mice per genotype was used to generate washed platelets, and each experiment was repeated 4 times. (C) Representative tracings of ATP secretion from ADP-activated blood samples from wild-type (WT) and TSP2-null (KO) mice. The experiment was repeated twice.
To exclude the possibility that TSP2-null plasma affected aggregation, we proceeded to analyze platelet function in washed platelets. We used standard aggregometric measurements instead of microtiter wells and a plate-reader, since the latter can provide measurements only at 30-second intervals. Analysis of TSP2-null platelets revealed that the response to ADP was again less than half maximal in comparison with wild-type platelets (Figure 3B). Furthermore, ATP secretion following exposure of whole blood to ADP was compromised in TSP2-null platelets (Figure 3C). TSP2-null platelets appeared to respond normally to thrombin and collagen, and in response to the latter showed the characteristic initial reduction in transmittance due to the induced change in cell shape. Dilution of collagen did not compromise their responses (data not shown).
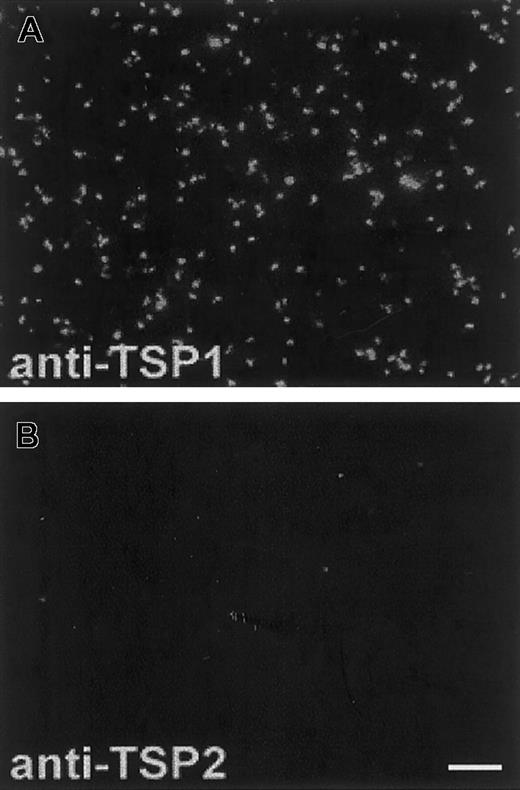
TSP2 is lacking in platelets
Since the presence of TSP2 in wild-type platelets had not been assessed previously, we examined this issue. Using anti-TSP1– and anti-TSP2–specific antibodies, we observed high levels of TSP1 (Figure 4A) but no TSP2 (Figure 4B) in wild-type platelets mildly activated with thrombin. The same anti-TSP2 antibody has been used successfully to detect TSP2 in bone marrow stroma cells (BMSCs),28 skin fibroblasts,16and in MKs (Figure 5A). We were also unable to detect TSP2 in platelet lysates by Western analysis (data not shown). Analysis of TSP2-null platelets by the same techniques revealed no changes in the levels of TSP1 (data not shown). This finding is consistent with previous observations that indicate the lack of compensatory up-regulation of TSP1 in TSP2-null mice. In addition, TSP2-null platelets, analyzed by FACS, displayed surface levels of α2 and αIIbβ3 integrins that were similar to those of wild-type platelets (data not shown).
TSP2 is lacking from platelets.
Thrombin-treated PRP from wild-type mice was immobilized on glass slides and analyzed with specific anti-TSP1 and anti-TSP2 antibodies. The presence of TSP1 (A) and the absence of TSP2 (B) from platelets are shown. Both antibodies were visualized with an FITC-conjugated antirabbit IgG. The fluorescence observed in (B) is not higher than background levels. Bar represents 10 μm (A-B).
TSP2 is lacking from platelets.
Thrombin-treated PRP from wild-type mice was immobilized on glass slides and analyzed with specific anti-TSP1 and anti-TSP2 antibodies. The presence of TSP1 (A) and the absence of TSP2 (B) from platelets are shown. Both antibodies were visualized with an FITC-conjugated antirabbit IgG. The fluorescence observed in (B) is not higher than background levels. Bar represents 10 μm (A-B).
Immunolocation of TSP2 in MKs in vivo.
Anti-TSP1 and anti-TSP2 antibodies were used to immunolocalize their respective antigens in cryosections of bone marrow. Reactions were visualized by FITC-conjugated IgG. TSP1 was abundantly distributed in the cytoplasm of all MKs (B), whereas TSP2 was present in a subset of MKs and was detected at the cell boundary (A). TSP2 was also present in the marrow stroma (A). Arrows indicate MKs. Bar represents 25 μm (A-B).
Immunolocation of TSP2 in MKs in vivo.
Anti-TSP1 and anti-TSP2 antibodies were used to immunolocalize their respective antigens in cryosections of bone marrow. Reactions were visualized by FITC-conjugated IgG. TSP1 was abundantly distributed in the cytoplasm of all MKs (B), whereas TSP2 was present in a subset of MKs and was detected at the cell boundary (A). TSP2 was also present in the marrow stroma (A). Arrows indicate MKs. Bar represents 25 μm (A-B).
TSP2 is present in MKs in vivo
Immunohistochemical staining of wild-type bone marrow cryosections with anti-TSP2 antibodies revealed the presence of strong immunoreactivity in MKs, and weaker immunoreactivity in the bone marrow stroma (Figure 5A). Consistent with previous demonstrations,29 30 MKs were also immunoreactive for TSP1 (Figure 5B). Similar observations were made in formalin-fixed, paraffin-embedded sections from wild-type, decalcified femurs (data not shown). No changes in TSP1 immunoreactivity and no TSP2 immunoreactivity were observed in TSP2-null bone marrow (data not shown). However, the distribution of TSP1 and TSP2 in MKs was different, with the former predominantly in the cytoplasm, presumably membrane-bound (Figure 5B), and the latter prominent in the periphery of the cell. In addition, contrary to TSP1, TSP2 was present only in a subset of MKs (approximately 30% of the MKs that exhibited immunoreactivity to TSP1 and VWF), suggesting that its presence in these cells may be dependent on their stage of maturation. We also quantified the number of MKs in TSP2-null and wild-type mice, following visualization of MKs by antibodies to VWF. The number of MKs in TSP2-null marrow (4.5 ± 1.8 MKs per 0.04 μm2; n = 5) was similar to that in control marrow (4.8 ± 2.1 MKs per 0.04 μm2; n = 5). To better understand the role of TSP2 in MK biology and platelet function we initiated studies of wild-type MKs in vitro.
Uptake of TSP2 by MKs in vitro
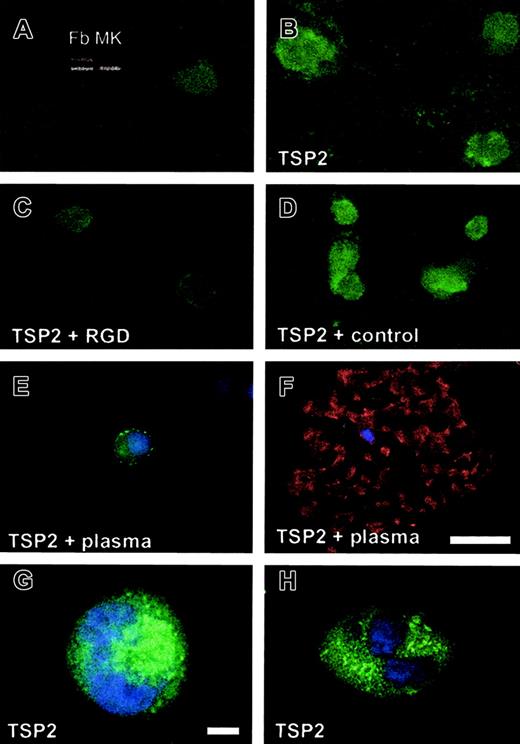
MKs, purified from the femurs of wild-type TPO-treated mice, were expanded in culture and analyzed for TSP2 expression by immunocytochemistry and RT-PCR. Very weak TSP2 staining, which was slightly higher than background, was observed in MKs cultured in the absence of plasma (Figure 6A). Analysis of MK RNA by RT-PCR with TSP2-specific primers revealed the presence of a minor band that migrated at the expected molecular weight of 539 bp (MK, inset in Figure 6A). A positive control was generated by amplifying dermal fibroblast-derived RNA (Fb, inset in Figure 6A). These observations were inconsistent with the high levels of TSP2 that were detected in MKs in vivo. Thus, we postulated that the source of TSP2 in MKs in vivo might be exogenous. To test this hypothesis we added rTSP2 to MKs grown in vitro and were able to detect a dramatic increase in their immunoreactivity by immunocytochemistry with anti-TSP2 antibodies (Figure 6B). This finding suggests that MKs are able to take up TSP2 from their extracellular environment. Uptake was dependent on integrin function and generation of ATP, since preincubation of MKs with an RGD-containing peptide inhibited the uptake (Figure 6C), and uptake was also inhibited by preincubation of MKs at 4°C (data not shown). A non-RGD control peptide had no effect on uptake (Figure 6D). Furthermore, culture of MKs in the presence of plasma, a condition shown to induce proplatelet formation and platelet release, also reduced the uptake of TSP2, and in this case TSP2 appeared to be associated with the cell membrane (Figure 6E). Proplatelet-forming MKs were observed in these cultures but they did not display immunoreactivity for TSP2 (Figure 6F). The latter finding suggests that either TSP2 is not taken up by MKs under conditions that induce their maturation or that mature MKs, those that can make proplatelets, have lost their TSP2.
Integrin-mediated uptake of TSP2 by MKs in vitro.
rTSP2 was added to MKs in panels B-F. MKs were grown in the absence (A-D) or presence (E-F) of plasma. The uptake of TSP2 was inhibited by an RGD-containing peptide (C) but not by a control peptide (D). The inset in panel A shows results of RT-PCR with TSP2-specific primers (top bands) and with ribosomal protein SP6-specific primers as control (bottom bands) from dermal fibroblasts (Fb) and MKs. Representative images, obtained by confocal microscopy, of rTSP2-treated MKs grown in the absence of plasma are shown (G-H). Uptake of TSP2 was detected by FITC-conjugated IgG. Nuclei in panels E-H were visualized with DAPI, and the cytoskeleton in panel F was visualized with phalloidin. Bars represent 25 μm (A-F) and 10 μm (G-H).
Integrin-mediated uptake of TSP2 by MKs in vitro.
rTSP2 was added to MKs in panels B-F. MKs were grown in the absence (A-D) or presence (E-F) of plasma. The uptake of TSP2 was inhibited by an RGD-containing peptide (C) but not by a control peptide (D). The inset in panel A shows results of RT-PCR with TSP2-specific primers (top bands) and with ribosomal protein SP6-specific primers as control (bottom bands) from dermal fibroblasts (Fb) and MKs. Representative images, obtained by confocal microscopy, of rTSP2-treated MKs grown in the absence of plasma are shown (G-H). Uptake of TSP2 was detected by FITC-conjugated IgG. Nuclei in panels E-H were visualized with DAPI, and the cytoskeleton in panel F was visualized with phalloidin. Bars represent 25 μm (A-F) and 10 μm (G-H).
To verify that addition of rTSP2 in vitro leads to intracellular uptake in MKs, we analyzed rTSP2-treated MKs by confocal microscopy. Permeabilized MKs, cultured in the absence of 10% plasma, were stained with anti-TSP2 antibodies and nuclei were visualized with DAPI. Analysis of images obtained from the plane of the nuclei confirmed the intracellular location of TSP2 (Figure 6G-H). The distribution of the protein was well within the cytoplasm of MKs and close to the nuclei, indicating that rTSP2 was internalized. On the contrary, the appearance of TSP2-specific immunoreactivity in MKs treated with rTSP2 in the presence of plasma was predominantly peripheral (not shown).
TSP2 limits MK differentiation and proplatelet formation
To investigate the significance of the uptake of TSP2 on MK differentiation and vice versa, we asked whether MKs that have taken up TSP2 could undergo proplatelet formation if they were placed in media containing 10% plasma. In addition, we determined whether addition of 10% plasma could reduce the number of MKs that contain TSP2. Immunocytochemical staining of these cultures with anti-TSP2 antibodies (to detect TSP2) and phalloidin (to detect the actin cytoskeleton) indicated that the presence of TSP2 correlated negatively with differentiation of MKs (Figure 7A-B). Thus, all cells that exhibited actin bundling, an event that precedes proplatelet formation, were devoid of TSP2 (Figure 7B). Similarly, all cells that exhibited TSP2 immunoreactivity did not have a bundled cytoskeleton (Figure 7A). Furthermore, none of the MKs that formed proplatelets were immunoreactive for TSP2 (Figure 7B). These results suggest that the uptake of TSP2 is limited to nonproplatelet-forming MKs. To determine whether TSP2 had a quantifiable, limiting effect on proplatelet formation, we determined the percentage of MKs that reached the proplatelet formation stage with or without exposure to TSP2. Because the detection of proplatelet-forming MKs from fixed and stained samples is not quantitatively reliable, we analyzed MK cultures by phase-contrast microscopy at 24 and 48 hours following treatment. As shown in Figure 7C, addition of TSP2 significantly reduced, or delayed, the number of MKs that were able to undergo proplatelet formation. It should be noted that this experiment could be followed only for a period of 72 hours, since MKs do not survive in culture beyond this time point. However, estimation of cell viability indicated that it was not affected by exposure to TSP2 (data not shown). Moreover, exposure of MKs containing TSP2 to 10% plasma caused a reduction in the number of these proplatelet-forming cells within 48 hours. Specifically, the number of MKs containing TSP2 was reduced from 74 ± 6.2% to 37.5 ± 12.9% (P ≤ .05) when exposed to plasma, suggesting that TSP2 is lost from cells undergoing differentiation (Figure 7D).
TSP2 inhibits MK differentiation.
MKs were allowed to take up TSP2 for a period of 24 hours and were then placed in media containing 10% plasma to induce differentiation. Uptake of TSP2 was detected by FITC-conjugated IgG. Nuclei and the actin cytoskeleton were visualized with DAPI and phalloidin, respectively. Panels A and B represent images of the same field showing a combination of phalloidin and DAPI stain (A) and FITC-conjugated IgG and DAPI (B). Small arrows in panels A and B indicate cells that contain bundled actin but lack TSP2, whereas arrowheads indicate cells that lack bundled actin but contain TSP2. Yellow arrow (A-B) shows proplatelet-forming MKs, with numerous pseudopodia, that are devoid of TSP2. The effect of the uptake of TSP2 by MKs on their differentiation was quantified at 24 and 48 hours following the replacement of plasma-containing media (C). At both time points, uptake of TSP2 resulted in a reduction in the number of proplatelet-forming MKs. Addition of 10% plasma also resulted in a reduction in the number of MKs that contain TSP2 (D). Bar represents 50 μm (A-B). *P ≤ .05
TSP2 inhibits MK differentiation.
MKs were allowed to take up TSP2 for a period of 24 hours and were then placed in media containing 10% plasma to induce differentiation. Uptake of TSP2 was detected by FITC-conjugated IgG. Nuclei and the actin cytoskeleton were visualized with DAPI and phalloidin, respectively. Panels A and B represent images of the same field showing a combination of phalloidin and DAPI stain (A) and FITC-conjugated IgG and DAPI (B). Small arrows in panels A and B indicate cells that contain bundled actin but lack TSP2, whereas arrowheads indicate cells that lack bundled actin but contain TSP2. Yellow arrow (A-B) shows proplatelet-forming MKs, with numerous pseudopodia, that are devoid of TSP2. The effect of the uptake of TSP2 by MKs on their differentiation was quantified at 24 and 48 hours following the replacement of plasma-containing media (C). At both time points, uptake of TSP2 resulted in a reduction in the number of proplatelet-forming MKs. Addition of 10% plasma also resulted in a reduction in the number of MKs that contain TSP2 (D). Bar represents 50 μm (A-B). *P ≤ .05
Ultrastructural abnormalities in TSP2-null MKs
Ultrastructural analysis of TSP2-null MKs in bone marrow plugs by TEM revealed 2 major abnormalities that are prominent in all the sections. First, many TSP2-null MKs displayed extensive fragmentation of their peripheral clear zones (Figure8B). Normally, the peripheral zones of MKs are continuous, as is observed in wild-type cells (Figure 8A). Furthermore, the peripheral zone of TSP2-null MKs, unlike that of wild-type MKs, failed to form an intimate association with the subendothelial component of vascular sinuses (Figure 9A-B). Instead, the interaction of MKs with the vascular wall was limited to selected sites and was not extensive. These observations suggest that the abnormalities detected in the peripheral zones of TSP2-null MKs may have a negative influence on the interaction of MKs with vascular sinuses. Interestingly, we were able to observe proplatelets in the vascular lumen of TSP2-null marrow, suggesting that ultimately their release is not compromised (data not shown). Furthermore, as noted originally,8 the number of platelets in the circulation of TSP2-null mice is, if anything, increased.
TSP2-null MKs display a fragmented peripheral zone.
Representative TEM images of (A) wild-type and (B) TSP2-null bone marrow (KO). A MK with extensive fragmentation of the peripheral zone (PZ) is seen in panel B. Bar represents 0.5 μm.
TSP2-null MKs display a fragmented peripheral zone.
Representative TEM images of (A) wild-type and (B) TSP2-null bone marrow (KO). A MK with extensive fragmentation of the peripheral zone (PZ) is seen in panel B. Bar represents 0.5 μm.
Compromised MK-vascular sinus interactions in TSP2-null marrow.
Representative TEM images of (A) wild-type and (B) TSP2-null (KO) marrow demonstrate the association between MKs and the sinusoidal endothelium. A TSP2-null MK displaying a lack of close continuous association with the subendothelium is shown in panel B. Sinusoidal lumen (L) and endothelium (arrows) are marked. Bar represents 0.5 μm.
Compromised MK-vascular sinus interactions in TSP2-null marrow.
Representative TEM images of (A) wild-type and (B) TSP2-null (KO) marrow demonstrate the association between MKs and the sinusoidal endothelium. A TSP2-null MK displaying a lack of close continuous association with the subendothelium is shown in panel B. Sinusoidal lumen (L) and endothelium (arrows) are marked. Bar represents 0.5 μm.
Discussion
Our investigation into the basis for the bleeding diathesis in the TSP2-null mouse has yielded the surprising finding that TSP2-null platelets have an intrinsic defect in aggregation, despite the fact that normal platelets lack TSP2. Aggregation was compromised in response to a mild agonist, ADP, but was normal when platelets were treated with thrombin or collagen. As a possible reflection of this defect, we observed that platelets adhered to the vessel wall but did not aggregate normally when the carotid artery endothelium was denuded in the TSP2-null mouse. In searching for the basis for the intrinsic platelet defect, we discovered that normal MKs synthesize small amounts of TSP2 in vitro, but take up a far larger proportion in an integrin-dependent manner when the recombinant protein is added to the culture medium. We speculate that a similar process occurs in vivo since a subset of MKs stain strongly with an antibody to TSP2, and BMSCs actively synthesize and secrete the protein into the bone marrow microenvironment. In addition, TSP2-null MKs display fragmentation of peripheral zones and loss of close associations with vascular sinuses, which we presume reflect the lack of TSP2.
The uptake and subsequent loss of TSP2 from MKs
The endocytic uptake of a number of proteins from plasma by MKs has been documented31-33 and in the case of fibrinogen involves an integrin.34 The storage of proteins, such as factor V and fibrinogen, in granules ensures that these proteins will be available during platelet activation. However, there is no precedent for the uptake of proteins by MKs that are subsequently lost in the formation of platelets. Since MKs express low levels of TSP2 it is unclear why these cells should take up additional TSP2 from the extracellular milieu, rather than increase endogenous synthesis. Perhaps the synthesis and uptake of TSP2 is regulated by a variation in receptor levels during maturation of MKs. Such regulation has been demonstrated for glycoprotein VI (GPVI) and α2β1 during expansion of MKs.35-37 Partial support for the hypothesis that the uptake of TSP2 is dependent on the temporal availability of surface receptors may be found in our results that demonstrate the ability of plasma to influence this process. Specifically, when MKs were cultured in media containing plasma, the distribution pattern of TSP2 was predominantly peripheral. The pattern perhaps better reflects the situation in vivo, and is consistent with the suggestion that TSP2 may localize to the peripheral zone.
Previously we have shown that TSP2-null mice have increased numbers of BMSCs,38 and recently we have determined that BMSCs are a major source of TSP2 in the bone marrow.28 The influence of BMSCs on MKs has been demonstrated by coculture studies in which these cells were shown to induce MK growth and proplatelet formation by secreting TPO and stroma derived factor-1.39-41 However, the paracrine effects of BMSCs on the maturation of MKs and proplatelet formation are rendered ineffective when BMSCs and MKs are in direct contact. We have initiated in vitro studies to investigate whether the effect of TSP2-null BMSCs and their conditioned media on wild-type MKs differs from that of their control counterparts.
The mechanism by which TSP2 is lost from MKs as they form platelets remains unknown. Our in vitro studies suggest that MKs that have taken up TSP2 proceed to lose it when induced to differentiate. A possible explanation for how this loss could occur in vivo may be the targeted localization of TSP2 to the peripheral zone of the MKs, since this zone is excluded from platelet formation.42 43 TSP2 may function in the peripheral zone to assist the MKs in migrating to and interacting with the sinusoidal subendothelium during proplatelet formation.
Significance of the abnormalities in TSP2-null platelets
The inability of TSP2-null platelets to respond fully to ADP is consistent with the observation that these cells can adhere and spread on an exposed subendothelium but fail to form large aggregates. The latter process depends on the release of ADP from the granules of activated platelets, which appears deficient in TSP2-null platelets. This finding is also supported by the suboptimal release of ATP from ADP-activated TSP2-null platelets. We have therefore initiated studies to investigate the response of TSP2-null platelets to ADP in greater detail. We are interested in determining whether all ADP-induced signaling pathways are intact and whether the levels of the 2 known ADP receptors are normal. ADP induces platelet aggregation by binding purinergic receptors such as P2Y1 and P2Y12.44 45P2Y1 is responsible for mobilization of ionized calcium from internal stores and for initiation of aggregation. The recently discovered ADP receptor, P2Y12, couples to Gito reduce adenylyl cyclase activity and is essential for the full aggregation response to ADP. Thus, determination of the extent to which these processes are altered in TSP2-null platelets should permit the identification of the basis for possible deficiencies in ADP-induced aggregation.
Other defects in platelet formation from MKs
The process of platelet release from MKs by fragmentation at the ends of proplatelet processes has been observed in vitro in an elegant study.46 However, the interpretation of this study was limited because platelet release occurred in the absence of other cell types, such as endothelial cells, that are normally present in the marrow. Mori et al performed coculture experiments with endothelial cells and MKs in 3-dimensional collagen gels and were able to observe proplatelet formation by morphologic criteria.47 Platelet release has been shown to occur in the lung and spleen in a similar fashion.27 48 It is clear from such studies that MKs release proplatelets into the lumen of sinusoids. Despite the wealth of evidence demonstrating MK–vascular sinus interactions and proplatelet release, nothing is known about the molecules that regulate these events.
It is clear, however, that abnormalities that originate in MKs can lead to the formation of platelets with compromised function. For example, deletion of the transcription factor, GATA-1, in mice led to structural abnormalities in MKs and a prolonged bleeding time.49 In addition, deletion of the transcription factor NF-E2 resulted in profound defects in MKs and severe thrombocytopenia.50,51 Although such studies provide a strong link between MK maturation and platelet function, at the molecular level little is understood about the actual processes involved. Since MKs expanded in vitro, where the levels of TSP2 are minimal, can undergo proplatelet release (Figure 6), we believe that this process is not dependent on TSP2. This conclusion is also supported by our observation of proplatelets in the vascular lumens of TSP2-null animals (T.R.K., unpublished data, June 2001). On the other hand, our in vitro studies suggest that TSP2 uptake may inhibit or delay platelet formation. Perhaps this may account for the 20% increase in circulating platelets in TSP2-null mice.8How then do TSP2-null platelets acquire an intrinsic defect? Although we cannot provide an answer to this question, we speculate that the defect originates either in MKs during their transmural migration through vascular sinuses and/or in proplatelets during their release from MKs.
Contribution of a matrix abnormality to the bleeding defect
Since the aggregation defect in TSP2-null platelets is subtle, it may not account solely for the prolonged bleeding. Thus, the contribution of a subendothelial matrix abnormality should be considered. Exposed subendothelial collagen fibers have been shown to be the major inducer of platelet aggregation.52,53 In vitro studies have also shown that changes in the nature of collagen can alter its aggregative activity.54,55 Supporting evidence in vivo may exist in anecdotal reports that have linked bleeding diatheses with abnormalities in collagen fibrillogenesis in patients with the Ehlers-Danlos syndrome.56,57 In fact, analyses of the thrombogenicity of TSP2-null fibroblast-derived extracellular matrix indicate that it is compromised (F. Almus, T.R.K., P.B., and Z. Ruggeri, manuscript in preparation). It would be interesting to determine whether this deficiency extends to the subendothelial matrix. An analogous situation may exist in PECAM-1–null mice, in which prolonged bleeding times could not be corrected by bone marrow transplantation. This finding prompted the investigators to conclude that the defect was in endothelial cells.13
How might the lack of TSP2 influence the thrombogenicity of cell-derived matrices in vitro and subendothelial matrices in vivo? In a number of studies we have reported that TSP2-null mice produce an abnormal matrix associated with defects in collagen fibrillogenesis.58 Furthermore, we have found that the solubility of dermal collagen in TSP2-null mice is 2-fold greater than in control animals (T.R.K., unpublished observation, May 2001). Thus, it seems possible that subendothelial collagen fibrils, which are altered in structure and in cross-linking, are present in TSP2-null mice and can influence the formation of platelet aggregates.
The present study has revealed the existence of a complex mechanism that implicates TSP2 in platelet formation and function. TSP2, which is most likely produced by BMSCs in the bone marrow, is taken up by MKs and directed to the peripheral zone of these cells where it is required for their interaction with the matrix underlying the sinusoidal endothelium. A lack of TSP2 results in abnormalities in the ultrastructural appearance of the MKs and reduces the intimacy of their association with the vascular sinus. Consequently, TSP2-null mice form platelets that are intrinsically abnormal, as reflected in their reduced response to ADP and the marked reduction in their ability to form aggregates on an injured subendothelium. These findings provide a partial explanation for the bleeding diathesis in TSP2-null mice, and a novel resolution of the paradox of a genetically induced abnormality in a cell type that does not express the deleted gene product. Furthermore, our results highlight the functional importance of a matricellular protein, such as TSP2, in the bone marrow microenvironment, and emphasize the need to analyze cell-matrix interactions in this system in greater detail.
We thank Colleen Irvin for assistance with carotid artery denudations and Emily Stainbrook, Zhantao Yang, Henrik Andersen, and Dan Greenberg for assistance with platelet studies. We also thank Greg Priestley for assistance with FACS analysis and Brian Lanutti, Jonathan Drachman, and Norma Fox for assistance with the isolation of megakaryocytes. Confocal microscopy was performed with the assistance of the Keck Imaging Center, University of Washington. EM was performed with the assistance of the University of Washington EM Center and lumi-aggregometry with the assistance of the University of Washington Hospital Hematology Laboratory. We are indebted to Alan Nurdin, Paquita Nurdin, and Christel Poujol for assistance with the interpretation of EM images. Finally, we are indebted to Zaverio Ruggeri for helpful discussions and a critical reading of the manuscript.
Supported by the National Institute of Health grants HL 18645 and AR 45418.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Themis R. Kyriakides, Department of Biochemistry, Box 357350, University of Washington, Seattle, WA 98195; e-mail: themi@u.washington.edu.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal