Abstract
It has been established that low-density lipoprotein receptor-related protein (LRP) is involved in the cellular uptake and degradation of coagulation factor VIII (FVIII) in vitro. To address the physiologic role of LRP in regulating plasma FVIII in vivo, we used cre/loxP–mediated conditional LRP- deficient mice (MX1cre+LRPflox/flox). Upon inactivation of the LRP gene, MX1cre+LRPflox/flox mice had significantly higher plasma FVIII as compared with control LRPflox/floxmice (3.4 and 2.0 U/mL, respectively; P < .001). Elevated plasma FVIII levels in MX1cre+LRPflox/flox mice coincided with increased plasma von Willebrand factor (VWF) (2.0 and 1.6 U/mL for MX1cre+LRPflox/flox and control LRPflox/flox mice, respectively; P < .05). Elevation of plasma FVIII and VWF persisted for at least 6 weeks after inactivation of the LRP gene. Upon comparing plasma FVIII and VWF in individual mice, we observed an increase of the FVIII/VWF ratio in MX1cre+LRPflox/flox mice as compared with control LRPflox/flox mice. Administration of either a vasopressin analog or an endotoxin resulted in increased plasma VWF, but not FVIII. In clearance experiments, MX1cre+LRPflox/flox mice displayed a 1.5-fold prolongation of FVIII mean residence time. Adenovirus-mediated overexpression of the 39-kDa receptor–associated protein (RAP) in normal mice resulted in a 3.5-fold increase of plasma FVIII. These data confirm that the regulation of plasma FVIII in vivo involves a RAP-sensitive mechanism. Surprisingly, plasma FVIII in MX1cre+LRPflox/flox mice increased 2-fold after RAP gene transfer. We propose that RAP-sensitive determinants other than hepatic LRP contribute to the regulation of plasma FVIII in vivo.
Introduction
Coagulation factor VIII (FVIII) is a 300-kDa glycoprotein that acts as a cofactor for activated factor IX (FIX) in the middle phase of the coagulation cascade.1,2 The fact that deficiency or dysfunction of FVIII is associated with the bleeding disorder hemophilia A demonstrates that this cofactor is indispensable for appropriate hemostasis. FVIII is synthesized in various tissues, including liver, spleen, and kidney.3,4 In plasma, FVIII circulates in complex with its carrier protein von Willebrand factor (VWF), which is produced and secreted by vascular endothelial cells.5,6 VWF serves a predominant role in stabilizing FVIII and prevents its premature clearance.7 Upon triggering of the coagulation cascade, FVIII is activated upon multiple proteolytic cleavages by thrombin or factor Xa, which results in the dissociation from VWF.6,8 After the participation of activated FVIII in the factor X–activating complex, it is rapidly inactivated by spontaneous subunit dissociation or via enzymatic degradation.9 10
Of the many parameters that regulate the plasma level of FVIII in vivo, its removal from plasma plays a central role. Investigation of the molecular basis thereof has recently started.11-13 It has been demonstrated that FVIII clearance is facilitated by cell surface heparan sulfate proteoglycans.13 These are thought to act by preconcentrating ligands on the cell surface that subsequently transfer their ligands to endocytic receptors.14 Recently, it has been demonstrated that FVIII comprises multiple binding sites that mediate the interaction with the low-density lipoprotein receptor–related protein (LRP).11,12 LRP is a large cell surface receptor that is ubiquitously expressed in a variety of tissues and is present on a wide range of different cell types, including hepatocytes, monocytes, and smooth muscle cells.15 LRP is a member of the low-density lipoprotein (LDL) receptor family of endocytic receptors and recognizes a wide range of structurally and functionally distinct ligands.14,16 Among these ligands, the 39-kDa receptor–associated protein (RAP) serves a unique role as an LRP chaperone, which blocks all ligand binding to the receptor.17 18
It has been established that LRP contributes to the cellular uptake and subsequent lysosomal delivery of FVIII in vitro.11,12,19In vivo, FVIII half-life is markedly prolonged in mice in the presence of a bolus administration of purified RAP.12 Similar effects of RAP on FVIII half-life are observed in a mouse model of VWF deficiency.20 Moreover, administration of RAP triggers a sustained rise in endogenous plasma FVIII in these mice.21In addition to LRP, however, RAP is known to block ligand binding to many other endocytic cell surface receptors, including other members of the LDL receptor family.22-25 With the use of the antagonist RAP, only indirect evidence is collected regarding the physiologic role of LRP in the removal of FVIII from plasma. Therefore, it remains inconclusive whether LRP indeed plays a role in regulating plasma FVIII in vivo.
In this study, we took advantage of a unique mouse model of LRP deficiency as a tool to investigate whether LRP contributes to the regulation of plasma FVIII in vivo. Previously, this LRP-deficient mouse model proved to be successful for studying the role of LRP in regulating plasma lipoprotein levels in vivo.26 27In addition, other RAP-sensitive mechanisms involved in regulating plasma FVIII in vivo were addressed with the use of adenovirus-mediated gene transfer of RAP in LRP-deficient mice.
Materials and methods
Transgenic animals
Mice (LRPflox/flox) carrying loxP sites within the LRP gene were created by homologous recombination in embryonic stem cells as described previously.28 Mice transgenic for the MX1cre expression construct were generated by pronuclear injection of hybrid mice (SJL × C57BL/6J) as described.26 Mice homozygous for the “floxed” LRP allele, either with or without the MX1cre transgene (MX1cre+LRPflox/flox or LRPflox/flox, respectively) were used. For experiments, female mice, 8 to 10 weeks of age were used (20 to 25 g). Nontransgenic littermates (ie, MX1cre− animals) served as controls (LRPflox/flox). Mice were determined for the presence of “floxed” LRP allele or MX1cre status by polymerase chain reaction (PCR) analysis as described.26Induction with polyinosinic:polycytidylic ribonucleic acid (pI:pC) (Sigma-Aldrich, St Louis, MO) was done by intraperitoneal injection of 250 μg of a 1 mg/mL solution of pI:pC in water. Injections were repeated 3 times at 2-day intervals. Mice were housed under standard conditions in conventional cages and given free access to food (ie, standard rodent chow diet [Hope Farms, Woerden, the Netherlands]) and water. All animal experiments were approved by the institutional committees on animal welfare of TNO Prevention and Health.
Quantification of mouse plasma factors VIII, V, and IX
Blood was obtained by tail bleeding. Samples were collected in polypropylene eppendorf tubes containing 1/10 vol of 3.2% (wt/vol) trisodium citrate. Plasma was prepared by centrifugation of blood at 2000g for 10 minutes at 4°C, immediately snap-frozen in liquid nitrogen, and stored at −80°C prior to analysis. Mouse plasma FVIII activity was measured by means of the Coatest FVIII Chromogenic assay as described by the supplier (Chromogenix, Mölndal, Sweden). Mouse plasma FIX activity was determined by means of the FIX Chromogenic assay as described by the supplier (Dade Behring, Düdingen, Switzerland). Mouse plasma factor V (FV) activity was assessed by measuring the conversion of prothrombin into thrombin, with the use of purified human-derived components. Briefly, plasma was diluted in 100 mM NaCl, 0.1% (wt/vol) bovine serum albumin (BSA), and 50 mM Tris (tris(hydroxymethyl)aminomethane) (pH 7.4) and was added to a mixture of 26 pM factor Xa, 20 μM phospholipids, and 10 mM CaCl2 for 5 minutes at 37°C. Subsequently, the reaction was started by adding 250 nM prothrombin in the same buffer. After 5 minutes, prothrombin conversion was stopped with 100 mM EDTA (ethylenediaminetetraacetic acid), and the amount of thrombin was measured with the use of the chromogenic substrate S2238 (Chromogenix). FVIII, FV, and FIX activity levels were expressed in murine plasma–equivalent units per milliliter, with the use of MF-1 (FVIII) (Sanbio, Uden, The Netherlands) or C57BL/6J (FV and FIX) normal pooled mouse plasma as a reference.
Quantification of mouse plasma VWF
Plasma VWF antigen levels were measured by means of an enzyme-linked immunosorbent assay, with the use of a rabbit antihuman VWF polyclonal antibody (DAKO, Glostrup, Denmark) for both capture and detection of VWF. We found that this antibody cross-reacts with mouse VWF. Plasma was prepared as described, diluted in 100 μL buffer containing 1% (wt/vol) BSA and 0.1% (vol/vol) Tween 20 in phosphate-buffered saline (PBS) (pH 7.4), and incubated with the immobilized rabbit antihuman VWF polyclonal antibody (3.3 pmol per well) for 2 hours at 37°C. After washing with 0.1% (vol/vol) Tween 20 in PBS (pH 7.4), 0.3 pmol peroxidase-labeled anti-VWF polyclonal antibody (DAKO) was incubated in 100 μL of the same buffer for 1 hour at 37°C. VWF antigen levels were expressed in murine plasma–equivalent units per milliliter, with the use of MF-1 normal pooled mouse plasma as a reference.
Administration of DDAVP and endotoxin
DDAVP (1-deamino-8-D-arginine vasopressin) was obtained from Ferring (Malmö, Sweden), and endotoxin (Escherichia coli, serotype 0111:B4) was purchased from Sigma-Aldrich. Female C57BL/6J mice received 12 μg DDAVP per kg body weight diluted in 200 μL physiologic saline by intraperitoneal injection. Female MX1cre+LRPflox/flox mice received 2 mg endotoxin per kg body weight diluted in 200 μL physiologic saline by intraperitoneal injection. Before injection and at 0.5, 1, 2, 4, and 6 hours after injection, blood samples of 50 μL were taken via tail bleeding. Plasma was prepared and analyzed for FVIII and VWF as described.
Plasma clearance of human FVIII
FVIII clearance studies were performed with the use of human immunoaffinity–purified FVIII concentrate (Aafact; Sanquin Plasma Products, Amsterdam, The Netherlands). This comprises homogeneous heterodimeric FVIII in a human albumin–containing formulation.29 After reconstitution, 200 μL (20 IU FVIII) was injected into the tail vein of weight-matched female MX1cre+LRPflox/flox and LRPflox/flox mice. At 1, 30, 60, 120, 180, 300, and 420 minutes after injection, blood samples of 50 μL were drawn via tail bleeding in EDTA-coated capillary tubes (Sarstedt, Nümbrecht, Germany). Plasma was prepared by centrifugation of blood at 2000g for 10 minutes at 4°C, immediately snap-frozen in liquid nitrogen, and stored at −80°C prior to analysis. Human FVIII antigen was measured with an immunosorbent assay, with the use of the antihuman FVIII monoclonal antibodies CLB-CAg A and CLB-CAg 117 as described previously.29 FVIII antigen levels were expressed in international units per milliliter, with calibrated human plasma used as a reference. The amount of FVIII recovered in the plasma 1 minute after injection was 90% to 100%. Values are expressed as the percentage of FVIII remaining in the circulation, with the amount of FVIII present at 1 minute after injection considered as 100%. Pharmacokinetic parameters were calculated by means of a model-independent (ie, noncompartmental) approach.30 A double-exponential fit was used to calculate the standard parameters area under the curve (AUC), fractional catabolic rate (FCR), and mean residence time (MRT).
Detection of LRP in mouse livers
Mouse liver membranes were prepared as described previously.27 Briefly, mouse liver membrane extracts (50 μg per lane) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis on 4% to 15% polyacrylamide gels under nonreduced conditions. Nitrocellulose membranes were blocked for 30 minutes at room temperature in 0.5% (vol/vol) Tween 20, 2% (wt/vol) BSA, 5% (wt/vol) milk powder, and PBS (pH 7.4), followed by a 60-minute incubation of a peroxidase-labeled rabbit polyclonal antibody directed against the 85-kDa subunit of LRP in the same buffer.27 Bound immunoglobulin G (IgG) was detected by means of the enhanced chemiluminescence system (Amersham Pharmacia Biotech, Uppsala, Sweden).
Recombinant adenovirus transduction
Recombinant adenovirus containing the rat RAP cDNA (Ad-RAP) or β-galactosidase cDNA (Ad-β-Gal) driven by the cytomegalovirus promotor were generated, grown, and purified as described previously.31 For in vivo adenovirus transduction, 6 weeks after the last pI:pC injection, 2 × 109 plaque-forming units (PFUs) in 200 μL physiologic saline was injected into the tail vein. Blood samples (50 μL) were withdrawn via tail bleeding before and 3 days after adenovirus injection. Expression of RAP in mouse plasma was quantified by enzyme-linked immunosorbent assay, with the use of polyclonal antibodies against RAP for capture and detection.27
Statistical analysis
Data are represented as geometric means and 68% confidence intervals (CI), which represent one standard deviation from the geometric mean if a log-normal distribution is assumed. Data are analyzed by means of the Mann-Whitney U test.P < .05 was regarded as statistically significant.
Results
Induction of LRP deficiency results in accumulation of plasma FVIII and VWF
Transgenic mice that both were homozygous for a loxP-flanked LRP gene and expressed cre recombinase under the control of the interferon-inducible MX1 promotor (ie, MX1cre+LRPflox/flox) were used to inactivate the LRP gene by administration of pI:pC. PI:pC-induced littermates that were MX1cre− (ie, LRPflox/flox) served as controls. We previously demonstrated that the cre/loxP recombination system effectively achieves inducible disruption of LRP in adult mice, thereby allowing in vivo studies on the role of LRP for at least 6 weeks after pI:pC injection.26 27 To investigate the role of LRP in regulating plasma FVIII in vivo, both plasma FVIII and its carrier protein VWF were measured in MX1cre+LRPflox/flox and control LRPflox/flox mice.
Noninduced MX1cre+LRPflox/flox animals had plasma FVIII levels (1.9 U/mL; CI, 1.5-2.2 U/mL) that were not statistically different (P = .2) from those of noninduced LRPflox/flox mice (1.6 U/mL; CI, 1.4-2.0 U/mL). Noninduced MX1cre+LRPflox/flox animals had plasma VWF (1.3 U/mL; CI, 1.1-1.5 U/mL) levels similar (P = .7) to those observed in noninduced LRPflox/flox mice (1.4 U/mL; CI, 1.1-1.6 U/mL). In addition, pI:pC-induced control LRPflox/flox mice had plasma FVIII and VWF levels similar to those of their noninduced littermates (Table1). These observations indicate that neither pI:pC nor MX1cre status alone affects plasma FVIII or VWF.
Plasma FVIII and VWF levels in MX1cre+LRPflox/flox and control LRPflox/flox mice following pI:pC induction
| Genotype . | Day 10 after pI:pC induction . | Week 6 after pI:pC induction . | ||
|---|---|---|---|---|
| FVIII levels, U/mL . | VWF levels, U/mL . | FVIII levels, U/mL . | VWF levels, U/mL . | |
| Noninduced | 1.9 (1.5-2.4) | 1.2 (1.0-1.6) | 1.5 (0.9-2.3) | 0.8 (0.5-1.1) |
| LRPflox/flox | 2.0 (1.5-2.7) | 1.6 (1.2-2.0) | 1.5 (1.1-1.9) | 0.8 (0.6-1.1) |
| MX1cre+LRPflox/flox | 3.4 (2.4-4.9)* | 2.0 (1.4-2.8)† | 2.1 (1.6-2.9)† | 1.1 (0.8-1.5)† |
| Genotype . | Day 10 after pI:pC induction . | Week 6 after pI:pC induction . | ||
|---|---|---|---|---|
| FVIII levels, U/mL . | VWF levels, U/mL . | FVIII levels, U/mL . | VWF levels, U/mL . | |
| Noninduced | 1.9 (1.5-2.4) | 1.2 (1.0-1.6) | 1.5 (0.9-2.3) | 0.8 (0.5-1.1) |
| LRPflox/flox | 2.0 (1.5-2.7) | 1.6 (1.2-2.0) | 1.5 (1.1-1.9) | 0.8 (0.6-1.1) |
| MX1cre+LRPflox/flox | 3.4 (2.4-4.9)* | 2.0 (1.4-2.8)† | 2.1 (1.6-2.9)† | 1.1 (0.8-1.5)† |
MX1cre+LRPflox/flox mice (n = 29 and 21 for 10 days and 6 weeks after pI:pC induction, respectively) or control LRPflox/flox mice (n = 18 and 9, for 10 days and 6 weeks after pI:pC induction, respectively) were injected 3 times intraperitoneally with 250 μg pI:pC at 2-day intervals. A parallel group of MX1cre+LRPflox/flox littermates were not induced with pI:pC (n = 5 and 6 for 10 days and 6 weeks after pI:pC induction, respectively). At 10 days and 6 weeks after the final pI:pC injection, blood samples were withdrawn and analyzed for plasma factor VIII and VWF, as described in “Materials and methods.” Data represent geometric mean values and 68% confidence intervals.
P < .001, significantly different from control LRPflox/flox mice with the use of the Mann-WhitneyU test.
P < .05, significantly different from control LRPflox/flox mice with the use of the Mann-WhitneyU test.
As demonstrated in Figure 1, at 10 days following pI:pC induction, plasma FVIII showed an approximately 2-fold increase in MX1cre+LRPflox/flox mice as compared with control LRPflox/flox (3.4 and 2.0 U/mL, respectively;P < .001). Induction with pI:pC also resulted in elevated plasma VWF in MX1cre+LRPflox/flox mice as compared with control LRPflox/flox mice (2.0 and 1.6 U/mL, respectively;P < .05) (Figure 1). As shown in Table 1, both plasma FVIII and VWF remained elevated in MX1cre+LRPflox/flox mice for at least 6 weeks after induction. In contrast, inactivation of the LRP gene did not affect plasma levels of the FVIII-related cofactor FV (0.6 U/mL [CI, 0.5-0.7 U/mL], 0.5 U/mL [CI, 0.4-0.6 U/mL], and 0.7 U/mL [CI, 0.6-0.8 U/mL], for noninduced, control LRPflox/flox, and MX1cre+LRPflox/flox, respectively).1 Similarly, LRP deficiency did not affect the plasma level of the FIX zymogen, which does not interact with LRP (0.5 U/mL [CI, 0.4-0.6 U/mL], 0.5 U/mL [CI, 0.4-0.6 U/mL], and 0.5 U/mL [CI, 0.4-0.6 U/mL], for noninduced, control LRPflox/flox, and MX1cre+LRPflox/flox, respectively).32 Collectively, these data demonstrate that inactivation of the LRP gene results in an elevation of plasma FVIII and, to a lesser extent, of plasma VWF in vivo.
Plasma FVIII and VWF levels in MX1cre+LRPflox/flox and control LRPflox/flox mice.
MX1cre+LRPflox/flox mice ( ) (n = 29) and control LRPflox/flox mice (○) (n = 18) were injected 3 times intraperitoneally with 250 μg pI:pC at 2-day intervals. At 10 days after the final pI:pC injection, blood was drawn and plasma was analyzed for FVIII and VWF, as described in “Materials and methods.” (C) Replotting of individual FVIII levels (A) against the corresponding VWF levels (B).
) (n = 29) and control LRPflox/flox mice (○) (n = 18) were injected 3 times intraperitoneally with 250 μg pI:pC at 2-day intervals. At 10 days after the final pI:pC injection, blood was drawn and plasma was analyzed for FVIII and VWF, as described in “Materials and methods.” (C) Replotting of individual FVIII levels (A) against the corresponding VWF levels (B).
Plasma FVIII and VWF levels in MX1cre+LRPflox/flox and control LRPflox/flox mice.
MX1cre+LRPflox/flox mice ( ) (n = 29) and control LRPflox/flox mice (○) (n = 18) were injected 3 times intraperitoneally with 250 μg pI:pC at 2-day intervals. At 10 days after the final pI:pC injection, blood was drawn and plasma was analyzed for FVIII and VWF, as described in “Materials and methods.” (C) Replotting of individual FVIII levels (A) against the corresponding VWF levels (B).
) (n = 29) and control LRPflox/flox mice (○) (n = 18) were injected 3 times intraperitoneally with 250 μg pI:pC at 2-day intervals. At 10 days after the final pI:pC injection, blood was drawn and plasma was analyzed for FVIII and VWF, as described in “Materials and methods.” (C) Replotting of individual FVIII levels (A) against the corresponding VWF levels (B).
Relation between plasma VWF and plasma FVIII in mice
It has been well established that VWF is a major regulator of FVIII in plasma, as VWF deficiency is associated with low FVIII levels in many species, including humans, pigs, dogs, and mice.5,33 34 In agreement with this notion, MX1cre+LRPflox/flox mice displayed an increase of both plasma FVIII and plasma VWF (Figure 1). A closer look at these data, however, revealed that inactivation of the LRP gene is associated with an increased FVIII/VWF ratio (P < .001) (Figure1C). This demonstrates that plasma FVIII does not merely covary with plasma VWF in these mice.
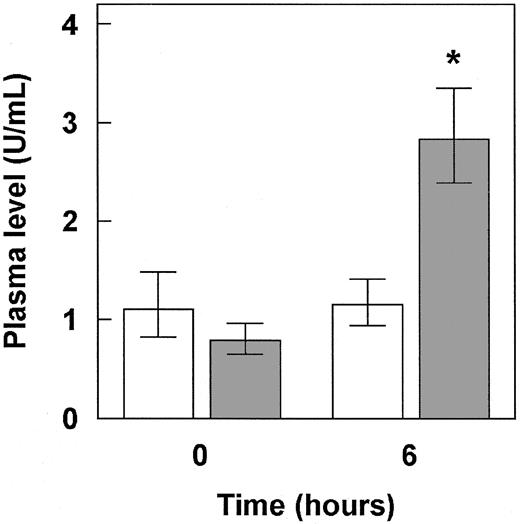
To further investigate the relation between plasma FVIII and VWF, we triggered an increase in plasma VWF employing DDAVP or endotoxin. Administration of DDAVP had only limited effect on plasma VWF in normal C57BL/6J mice. Plasma VWF increased from 1.4 U/mL (CI, 1.2-1.7 U/mL) before the experiment to 1.8 U/mL (CI, 1.5-2.1 U/mL) 30 minutes after DDAVP administration. This increase was not associated with a change in plasma FVIII (1.3 U/mL [CI, 1.2-1.4 U/mL] and 1.2 U/mL [CI, 1.2-1.3 U/mL] for 0 and 30 minutes after DDAVP administration, respectively). As for noninduced MX1cre+LRPflox/flox mice, DDAVP did not affect the VWF level at all (data not shown). To obtain a more pronounced increase of plasma VWF, we administered endotoxin into noninduced MX1cre+LRPflox/flox mice to elicit a VWF response. As shown in Figure 2, plasma VWF increased by about 3-fold after the administration of endotoxin, whereas plasma FVIII did not respond to endotoxin. These observations support previous data demonstrating that VWF, but not FVIII, is an acute-phase protein in mice.35 We therefore conclude that, in mice, a rise in plasma VWF is not necessarily associated with a secondary rise in plasma FVIII.
Effect of intraperitoneal injection of endotoxin on plasma FVIII and VWF levels in noninduced MX1cre+LRPflox/flox mice.
Endotoxin (2 mg/kg body weight) was administered to noninduced MX1cre+LRPflox/flox mice (n = 4). Before and 6 hours after endotoxin administration, blood was drawn and plasma was analyzed for FVIII (■) and VWF (░). Values represent geometric means and 68% confidence intervals. *P < .05, significantly different from plasma VWF levels before endotoxin administration, with the use of the Mann-Whitney Utest.
Effect of intraperitoneal injection of endotoxin on plasma FVIII and VWF levels in noninduced MX1cre+LRPflox/flox mice.
Endotoxin (2 mg/kg body weight) was administered to noninduced MX1cre+LRPflox/flox mice (n = 4). Before and 6 hours after endotoxin administration, blood was drawn and plasma was analyzed for FVIII (■) and VWF (░). Values represent geometric means and 68% confidence intervals. *P < .05, significantly different from plasma VWF levels before endotoxin administration, with the use of the Mann-Whitney Utest.
Plasma FVIII clearance in LRP-deficient mice
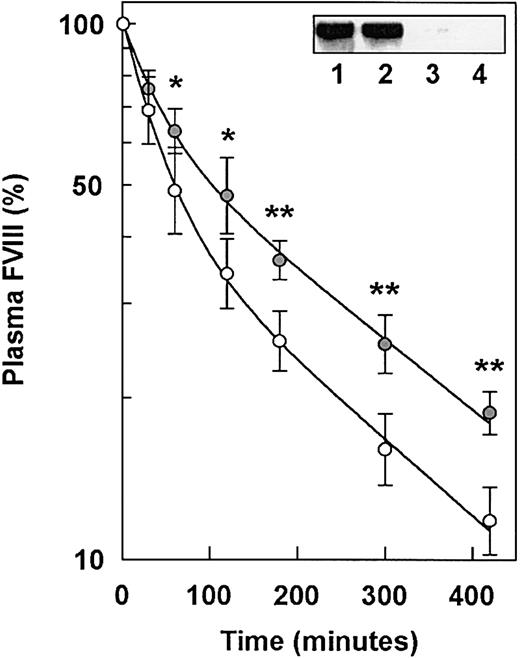
Several in vitro studies using LRP-deficient cells revealed that LRP contributes to the cellular degradation of FVIII.11,12,19 In vivo, a bolus administration of RAP is known to prolong FVIII half-life, suggesting that a RAP-sensitive mechanism contributes to the clearance of FVIII from plasma.12,20 21 To investigate whether increased plasma FVIII in MX1cre+LRPflox/flox mice is the result of a slower plasma FVIII elimination rate, we intravenously injected purified human FVIII into MX1cre+LRPflox/floxand control LRPflox/flox mice. As demonstrated in Figure 3, FVIII clearance was slower in MX1cre+LRPflox/flox mice than in control LRPflox/flox mice. The mean residence time was calculated to be 155 minutes in control LRPflox/flox mice and 230 minutes in MX1cre+LRPflox/flox mice, with 68% confidence intervals of 133 to 180 and 209 to 254 minutes, respectively (P = .002). As FVIII clearance in MX1cre+LRPflox/flox mice was still fairly efficient, we considered the possibility that this was due to residual hepatic LRP. Therefore, mouse liver membrane extracts were subjected to immunoblotting analysis, with the use of an antibody directed against the 85-kDa subunit of LRP. Whereas LRP was readily detectable in the livers of control LRPflox/flox mice, it was lacking in MX1cre+LRPflox/flox mice 10 days after pI:pC induction (Figure 3 inset). Collectively, these data indicate that the mean residence time of injected human FVIII is prolonged in hepatic LRP-deficient mice. This suggests that the increased plasma FVIII in MX1cre+LRPflox/flox mice is, at least in part, due to a slower clearance of FVIII from the circulation.
Plasma removal of human FVIII in MX1cre+LRPflox/flox and control LRPflox/flox mice.
MX1cre+LRPflox/flox mice ( ) (n = 6) and control LRPflox/flox mice (○) (n = 6) were injected 3 times intraperitoneally with pI:pC at 2-day intervals. At 10 days after the final injection, endogenous mouse plasma FVIII levels were 1.7 U/mL (range, 1.5-1.9 U/mL) and 3.3 U/mL (range, 2.8-3.8 U/mL) for control LRPflox/flox and MX1cre+LRPflox/flox mice, respectively. Endogenous plasma VWF levels were 1.1 U/mL (range, 1.0-1.3 U/mL) and 1.3 U/mL (range, 1.2-1.4 U/mL), for control LRPflox/floxand MX1cre+LRPflox/flox mice, respectively. Then, purified human FVIII (20 IU) was administered intravenously. Plasma removal was monitored at 1, 30, 60, 120, 180, 300, and 420 minutes after injections, and FVIII levels were determined. Data represent geometric mean values and 68% confidence intervals, and are expressed as a percentage of the amount of FVIII present in thet = 1 minute plasma sample. The curves were analyzed by means of double-exponential fits. *P < .02 and **P < .001, significantly different from control LRPflox/flox mice, with the use of the Mann-WhitneyU test. Inset shows detection of LRP in the liver membrane extracts of control LRPflox/flox mice (n = 2) (lanes 1-2), and MX1cre+LRPflox/flox mice (n = 2) (lanes 3-4). Immunoblotting experiments were performed as described in “Materials and methods.”
) (n = 6) and control LRPflox/flox mice (○) (n = 6) were injected 3 times intraperitoneally with pI:pC at 2-day intervals. At 10 days after the final injection, endogenous mouse plasma FVIII levels were 1.7 U/mL (range, 1.5-1.9 U/mL) and 3.3 U/mL (range, 2.8-3.8 U/mL) for control LRPflox/flox and MX1cre+LRPflox/flox mice, respectively. Endogenous plasma VWF levels were 1.1 U/mL (range, 1.0-1.3 U/mL) and 1.3 U/mL (range, 1.2-1.4 U/mL), for control LRPflox/floxand MX1cre+LRPflox/flox mice, respectively. Then, purified human FVIII (20 IU) was administered intravenously. Plasma removal was monitored at 1, 30, 60, 120, 180, 300, and 420 minutes after injections, and FVIII levels were determined. Data represent geometric mean values and 68% confidence intervals, and are expressed as a percentage of the amount of FVIII present in thet = 1 minute plasma sample. The curves were analyzed by means of double-exponential fits. *P < .02 and **P < .001, significantly different from control LRPflox/flox mice, with the use of the Mann-WhitneyU test. Inset shows detection of LRP in the liver membrane extracts of control LRPflox/flox mice (n = 2) (lanes 1-2), and MX1cre+LRPflox/flox mice (n = 2) (lanes 3-4). Immunoblotting experiments were performed as described in “Materials and methods.”
Plasma removal of human FVIII in MX1cre+LRPflox/flox and control LRPflox/flox mice.
MX1cre+LRPflox/flox mice ( ) (n = 6) and control LRPflox/flox mice (○) (n = 6) were injected 3 times intraperitoneally with pI:pC at 2-day intervals. At 10 days after the final injection, endogenous mouse plasma FVIII levels were 1.7 U/mL (range, 1.5-1.9 U/mL) and 3.3 U/mL (range, 2.8-3.8 U/mL) for control LRPflox/flox and MX1cre+LRPflox/flox mice, respectively. Endogenous plasma VWF levels were 1.1 U/mL (range, 1.0-1.3 U/mL) and 1.3 U/mL (range, 1.2-1.4 U/mL), for control LRPflox/floxand MX1cre+LRPflox/flox mice, respectively. Then, purified human FVIII (20 IU) was administered intravenously. Plasma removal was monitored at 1, 30, 60, 120, 180, 300, and 420 minutes after injections, and FVIII levels were determined. Data represent geometric mean values and 68% confidence intervals, and are expressed as a percentage of the amount of FVIII present in thet = 1 minute plasma sample. The curves were analyzed by means of double-exponential fits. *P < .02 and **P < .001, significantly different from control LRPflox/flox mice, with the use of the Mann-WhitneyU test. Inset shows detection of LRP in the liver membrane extracts of control LRPflox/flox mice (n = 2) (lanes 1-2), and MX1cre+LRPflox/flox mice (n = 2) (lanes 3-4). Immunoblotting experiments were performed as described in “Materials and methods.”
) (n = 6) and control LRPflox/flox mice (○) (n = 6) were injected 3 times intraperitoneally with pI:pC at 2-day intervals. At 10 days after the final injection, endogenous mouse plasma FVIII levels were 1.7 U/mL (range, 1.5-1.9 U/mL) and 3.3 U/mL (range, 2.8-3.8 U/mL) for control LRPflox/flox and MX1cre+LRPflox/flox mice, respectively. Endogenous plasma VWF levels were 1.1 U/mL (range, 1.0-1.3 U/mL) and 1.3 U/mL (range, 1.2-1.4 U/mL), for control LRPflox/floxand MX1cre+LRPflox/flox mice, respectively. Then, purified human FVIII (20 IU) was administered intravenously. Plasma removal was monitored at 1, 30, 60, 120, 180, 300, and 420 minutes after injections, and FVIII levels were determined. Data represent geometric mean values and 68% confidence intervals, and are expressed as a percentage of the amount of FVIII present in thet = 1 minute plasma sample. The curves were analyzed by means of double-exponential fits. *P < .02 and **P < .001, significantly different from control LRPflox/flox mice, with the use of the Mann-WhitneyU test. Inset shows detection of LRP in the liver membrane extracts of control LRPflox/flox mice (n = 2) (lanes 1-2), and MX1cre+LRPflox/flox mice (n = 2) (lanes 3-4). Immunoblotting experiments were performed as described in “Materials and methods.”
Effect of adenovirus-mediated gene transfer of RAP on plasma FVIII and VWF in LRP-deficient mice
Previous studies have demonstrated that a bolus injection of the LRP antagonist RAP prolongs FVIII half-life in normal mice.12,20,21 In addition, RAP induces a rise in endogenous plasma FVIII in VWF-deficient mice, thus underscoring that FVIII levels are dependent on a RAP-sensitive mechanism.21Since RAP is known to antagonize ligand binding to a variety of other cell surface receptors involved in endocytosis, including the entire LDL receptor family,22-25 other LRP-independent RAP-sensitive mechanisms that contribute to FVIII catabolism were addressed. To this end, we employed adenovirus-mediated gene transfer to overexpress the RAP protein in both MX1cre+LRPflox/flox and control LRPflox/flox mice. We injected a dose of 2 × 109 PFUs of adenovirus containing the rat RAP cDNA under the control of a cytomegalovirus promotor (Ad-RAP). As a control, mice received an adenovirus encoding the β-galactosidase gene (Ad-β-Gal).
To evaluate the functionality of Ad-RAP, we first verified whether this dose effectively induced RAP expression. In LDL-receptor–deficient mice, Ad-RAP induced hypercholesterolemia (higher than 30 mM) and hypertriglycideridemia (higher than 15 mM), which is in agreement with our previous studies.27 31 Furthermore, RAP expression was measured in the plasma C57BL/6J mice after transfection of either Ad-β-Gal (n = 5) or Ad-RAP (n = 7). Whereas no RAP was observed in mice that received Ad-β-Gal (less than 0.03 μg/mL), 2.7 ± 0.2 μg/mL (mean ± standard deviation [SD]) RAP was detected in Ad-RAP–treated animals.
Administration of control Ad-β-Gal into normal C57BL/6J and MX1cre+LRPflox/flox mice evoked a rise in plasma VWF, whereas plasma FVIII was not affected (Table2). As for Ad-RAP, its administration to C57BL/6J mice increased plasma VWF to an extent similar to that observed for Ad-β-Gal. In contrast to control Ad-β-Gal, however, Ad-RAP also resulted in a 3.5-fold increase of plasma FVIII (Table 2). These results indicate that overexpression of RAP in normal C57BL/6J mice results in a specific increase of plasma FVIII, confirming that a RAP-sensitive mechanism indeed contributes to the regulation of plasma FVIII in vivo. Following the administration of Ad-RAP to MX1cre+LRPflox/flox mice, plasma VWF increased as also observed in the C57BL/6J controls (Table2). Surprisingly, however, plasma FVIII increased in addition to VWF, reaching values between 3.5 and 4.4 U/mL. Thus, the elevated FVIII levels that are associated with induced LRP deficiency are further increased by adenovirus-mediated gene transfer of RAP (Table 2). One explanation for this finding might be that RAP affects FVIII levels by directly interacting with FVIII. This possibility was addressed by surface plasmon resonance analysis, employing purified proteins.11 However, we were not able to detect any association between RAP and heterodimeric FVIII, its isolated heavy chain, or its light chain. Therefore, it seems unlikely that RAP affects FVIII catabolism by interacting with FVIII. Collectively, these results suggest that, besides hepatic LRP, additional RAP-sensitive mechanisms contribute to the regulation of plasma FVIII in vivo.
Plasma FVIII and VWF levels after adenovirus-mediated overexpression of RAP
| Genotype and plasma protein . | Adenovirus . | Levels before adenovirus injection, U/mL . | Levels at day 3 after adenovirus injection, U/mL . |
|---|---|---|---|
| C57BL/6J | |||
| FVIII | Ad-β-Gal | 0.9 (0.8-1.0) | 1.3 (0.8-2.1) |
| Ad-RAP | 0.9 (0.9-1.0) | 3.2 (2.4-4.2)* | |
| VWF | Ad-β-Gal | 0.7 (0.5-0.9) | 2.0 (1.2-3.3) |
| Ad-RAP | 0.7 (0.6-0.7) | 2.4 (2.3-2.5) | |
| MX1cre+LRPflox/flox | |||
| FVIII | Ad-β-Gal | 2.4 (1.9-2.9) | 1.9 (1.0-3.4) |
| Ad-RAP | 1.9 (1.2-3.1) | 3.9 (3.5-4.4)* | |
| VWF | Ad-β-Gal | 1.4 (1.1-1.8) | 2.8 (2.5-3.2) |
| Ad-RAP | 1.3 (0.8-2.1) | 4.5 (3.5-5.6)* |
| Genotype and plasma protein . | Adenovirus . | Levels before adenovirus injection, U/mL . | Levels at day 3 after adenovirus injection, U/mL . |
|---|---|---|---|
| C57BL/6J | |||
| FVIII | Ad-β-Gal | 0.9 (0.8-1.0) | 1.3 (0.8-2.1) |
| Ad-RAP | 0.9 (0.9-1.0) | 3.2 (2.4-4.2)* | |
| VWF | Ad-β-Gal | 0.7 (0.5-0.9) | 2.0 (1.2-3.3) |
| Ad-RAP | 0.7 (0.6-0.7) | 2.4 (2.3-2.5) | |
| MX1cre+LRPflox/flox | |||
| FVIII | Ad-β-Gal | 2.4 (1.9-2.9) | 1.9 (1.0-3.4) |
| Ad-RAP | 1.9 (1.2-3.1) | 3.9 (3.5-4.4)* | |
| VWF | Ad-β-Gal | 1.4 (1.1-1.8) | 2.8 (2.5-3.2) |
| Ad-RAP | 1.3 (0.8-2.1) | 4.5 (3.5-5.6)* |
Normal C57BL/6J and MX1cre+LRPflox/floxmice were injected 3 times intraperitoneally with 250 μg pI:pC at 2-day intervals. At 6 weeks after the last pI:pC injection, mice were injected intravenously with 2 × 109 PFUs of Ad-RAP or Ad-β-Gal. Before and 3 days after adenovirus injection, blood was drawn and plasma was analyzed for FVIII and VWF, as described in “Materials and methods.” Values represent geometric mean values and 68% confidence intervals of 4 animals per group.
P < .05, effect of Ad-RAP significantly different from control Ad-β-Gal by means of the Mann-WhitneyU test.
Discussion
FVIII requires complex assembly with its carrier protein VWF to be protected from premature clearance from the circulation.7In the presence of VWF, FVIII half-life varies between 12 and 14 hours in humans7,36 and about 1 hour in mice.37 In VWF deficiency, however, FVIII half-life is only to 2 to 3 hours in humans38 and less than 10 minutes in mice.20While FVIII clearance from the circulation seems to be driven by a particularly efficient process, the mechanism by which this occurs has remained poorly understood. In 1999, we and others reported that FVIII binds to the multifunctional endocytic receptor LRP and that this receptor mediates the cellular uptake and subsequent degradation of FVIII in vitro.11 12 If LRP were to play a major role in the clearance of FVIII in vivo, one would expect LRP deficiency to be associated with elevated FVIII levels in the circulation. In the present study, we have addressed this question employing the cre/loxP homologous recombination system to inactivate the LRP gene in mice. This unique model of LRP deficiency allowed us to investigate the potential role of LRP in regulating plasma FVIII in vivo.
We found that both constituents of the FVIII-VWF complex are elevated in LRP-deficient mice (Figure 1; Table 1). Although the increase of plasma VWF was statistically significant, it was minor in comparison with FVIII. This is reflected by an upward shift in stoichiometry within the FVIII-VWF complex in LRP-deficient mice (Figure1C). Why VWF is slightly increased in LRP-deficient mice remains unclear. Unlike FVIII, VWF has been reported not to be a ligand of LRP in vitro.11,12 Therefore, we have no reason to suppose that LRP is involved in the clearance of VWF. On the other hand, it might be possible that the MX1-induced expression of cre recombinase causes some VWF increase as part of an inflammatory response. In humans, elevation in plasma concentration of VWF usually results in corresponding changes of FVIII. In mice, however, VWF increase has been reported without concomitant rise in FVIII.35 Similarly, we did not observe any rise in FVIII after increasing the plasma VWF level using DDAVP or endotoxin (Figure2), or after adenovirus-mediated Ad-β-Gal gene transfer (Table 2). These data led us conclude that plasma FVIII can vary independently of plasma VWF. This conclusion is further supported by the observation that interleukin 11 (IL-11), while elevating both VWF and FVIII levels in normal mice, effectively increases plasma FVIII in VWF-deficient mice.39
The molecular mechanisms that underlie elevated plasma FVIII in LRP-deficient mice can be due to increased FVIII biosynthesis, impaired FVIII clearance, or a combination thereof. As LRP is known to play a role in endocytosis of its ligands, we studied the elimination of FVIII from the circulation in both LRP-deficient and control mice. Indeed, hepatic LRP contributes to FVIII clearance and, in particular, to the initial 120 minutes of FVIII decay (Figure 3). However, the question may arise whether or not this effect is sufficient to fully explain FVIII elevation in LRP deficiency. In this context, we cannot exclude a role for LRP in the biosynthesis of FVIII. Our current understanding of the mechanism of FVIII biosynthesis in mice is limited by the lack of a positive correlation between FVIII mRNA and FVIII protein levels.39,40 Because FVIII mRNA and LRP mRNA have been identified in the same cell type,3,4,16,41 we cannot exclude the possibility that LRP plays an as-yet-unidentified role along the intracellular secretory pathway of FVIII. This would be consistent with the recent observation that the LDL receptor within the endoplasmic reticulum mediates the presecretory degradation of its ligand apolipoprotein B.42 43
As plasma FVIII is elevated in LRP-deficient mice, one would expect that, in normal mice, plasma FVIII should accumulate following the administration of the LRP-antagonist RAP. As RAP is rapidly cleared from the circulation,44 bolus injections are impractical for maintaining RAP in the circulation for an extended period of time. Therefore, we employed adenoviral gene transfer to evoke a sustained level of RAP in the circulation. Indeed, we found that RAP overexpression resulted in a marked increase of plasma FVIII in normal mice (Table 2). It is important to note that, while the control Ad-β-Gal virus caused a pronounced increase in VWF, this did not occur for FVIII. Apparently, nonspecific effects that are secondary to adenoviral genes cannot explain the increase of FVIII. It therefore seems reasonable to conclude that the regulation of plasma FVIII involves a RAP-sensitive mechanism. In line with this observation, it has been reported that FVIII half-life is prolonged in normal mice after a bolus injection of RAP.12,20,21 However, the effect of RAP may also involve other RAP-sensitive mechanisms. In this respect, it is striking that overexpression of RAP using adenovirus-mediated gene transfer in LRP-deficient mice further increased plasma FVIII (Table 2). It has been well established (Figure3) that cre/loxP–mediated gene inactivation is complete in the liver.26-28,45 Considering that FVIII clearance occurs predominantly in the liver,12 it remains difficult to explain why the FVIII level of mice that totally lack hepatic LRP still increases upon RAP overexpression. One explanation may be that the liver expresses RAP-sensitive FVIII receptors that differ from LRP in that they either are weakly expressed or display low affinity for RAP, and therefore have remained undetected by ligand blotting.27 This would be analogous to the transthyretin receptor and LDL receptor, which both occur in the liver and are RAP sensitive.22,46 Alternatively, if FVIII clearance is partially extrahepatic, it may also involve other RAP-sensitive receptors such as megalin (LRP2/glycoprotein 330 [gp330]), intrinsic factor–vitamin B12 receptor (IF-B12-R), very low density lipoprotein (VLDL) receptor, or apolipoprotein E (apoE)–receptor 2 (LRP8).23-25,47 One receptor-independent pathway of FVIII clearance may involve heparan sulfate proteoglycans.13Although this mechanism is apparently insensitive to RAP,13 we cannot fully exclude the possibility that RAP overexpression affects FVIII levels by weakly interfering in FVIII association with heparan sulfate proteoglycans. Finally, it may seem conceivable that other ligands that accumulate upon RAP overexpression might sequester FVIII in LRP-deficient mice. Further studies will be needed to distinguish among these possibilities.
It has been generally accepted that elevated FVIII in plasma is a risk factor for venous thrombosis.48 In this report, we show that disruption of the LRP gene results in the accumulation of FVIII in plasma (Figure 1). Moreover, the mechanism of plasma FVIII regulation may not be limited to LRP, as overexpression of RAP further increases plasma FVIII in LRP-deficient mice (Table 2). These observations open the possibility that LRP or another RAP-sensitive receptor is associated with an increased risk for developing thrombosis. The mechanisms by which FVIII levels are elevated in plasma are poorly understood. So far, attempts to associate elevated plasma FVIII with mutations in the FVIII gene have remained unsuccessful. To date, numerous variations that are associated with familial hypercholesterolemia have been found in the gene encoding the LDL receptor.49 The molecular consequences of these genetic variations include loss of LDL receptor function, resulting in the accumulation of its ligand in plasma.49-51 Whether or not such genetic variations that are associated with increased plasma FVIII or thrombosis exist within the LRP gene or other LDL receptor family member genes is an intriguing question that deserves further study.
We thank Dr J. A. van Mourik for critically reading the manuscript and Dr B. Teusink for advice on pharmacokinetics.
Prepublished online as Blood First Edition Paper, January 9, 2003; DOI 10.1182/blood-2002-07-2081.
Supported by grants from the Royal Netherlands Academy of Art and Sciences (B.J.M.v.V.) and the Landsteiner Foundation for Blood Transfusion Research (K.M. and B.J.M.v.V.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Koen Mertens, Department of Plasma Proteins, Sanquin Research at CLB, Plesmanlaan 125, 1066 CX Amsterdam, the Netherlands; e-mail: k.mertens@sanquin.nl.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal