Abstract
Fas-mediated apoptosis is a major physiologic mechanism by which activated T cells are eliminated after antigen-stimulated clonal expansion generates a specific cellular immune response. Because activated T cells are the major effectors of allograft rejection, we hypothesized that genetically modifying allogeneic bone marrow (BM) cells prior to transplantation could provide some protection from host T-cell attack, thus enhancing donor cell engraftment in bone marrow transplantation (BMT). We undertook studies to determine the outcome of lentiviral vector-mediated transduction of Fas ligand (FasL) into lineage antigen-negative (lin−) mouse BM cells (lin− BMs), in an allogeneic BMT model. FasL-modified lin− BMs killed Fas-expressing T cells in vitro. Mice that received transplants of allogeneic FasL+ lin−BMs had enhanced short-term engraftment, after nonmyeloablative conditioning, as compared to controls. We observed no major hepatic toxicity or hematopoietic or immune impairment in recipient mice at these time points. These results suggest potential therapeutic approaches by manipulating lymphohematopoietic stem-progenitor cells to express FasL or other immune-modulating genes in the context of BMT.
Introduction
General radiopharmacologic immunosuppression is the primary method used to decrease the immune rejection response of the host against allogeneic donor hematopoietic and organ transplant grafts. Development of more specific, cellular therapies designed to induce antigen-specific tolerance would be widely applicable in many transplantation settings. Studies have begun to investigate potential avenues for novel cellular-based therapies by producing tolerance using immature dendritic cells (DCs) alone,1,2 as well as DCs genetically modified to express a number of immunoregulatory genes, including interleukin 10 (IL-10),3 transforming growth factor β (TGF-β),4 CTLA4-Ig,4 and Fas ligand (FasL).5,6 These approaches confer tolerance in certain defined experimental settings. The Fas pathway offers an intriguing opportunity to manipulate the antidonor immune response in the bone marrow transplantation (BMT) setting, because allograft rejection is mediated primarily by activated host antidonor T cells and because activated T cells generally express high levels of the Fas receptor and are susceptible to Fas-mediated apoptosis, at least during the later stages of activation (“activation-induced cell death”).7-9
Conflicting results have been obtained on the effects of expressing FasL in experimental solid organ allografts. For pancreatic islet cell allografts, the initial report found increased graft acceptance,10 but later studies indicated that FasL+ pancreatic allografts became infiltrated with neutrophils and suffered enhanced rejection.11 Tolerance to FasL+ allografts was shown in thyroid12 and lung13 models. FasL expression was also shown to inhibit allogeneic recognition of tumor cells.14 The circumstances leading to these conflicting results are complex, and there are multiple differences among these model systems, including the strains of mice and the immunosuppressive regimens. In addition, the local environment may influence the nature of the response to FasL expression. For example, TGF-β inhibited the proinflammatory effects of FasL in a tumor rejection model.15
In this initial investigation of this approach, we sought to determine whether allogeneic hematopoietic grafts might be protected from acute rejection, early after nonmyeloablative transplantation, by genetically expressing FasL in donor lineage antigen-negative bone marrow (BM) cell preparations (lin− BMs) enriched in lymphohematopoietic stem-progenitor cells. Allogeneic BMT is an important treatment option for many cases of hematologic malignancies and blood diseases, as well as selected nonhematologic cancers and inherited disorders, but is limited by complications, including graft-versus-host disease (GVHD). Rigorous isolation of transplanted lymphohematopoietic stem-progenitor cells removes mature T cells and thereby prevents or reduces GVHD; but in the absence of donor T cells, host-versus-graft (HVG) rejection becomes a major problem. The effects of FasL expression have not been previously reported in a BMT model; “armed” FasL+ donor lin− BMs might engraft, and then they and their multilineage FasL+ progeny could kill T cells that attack them. This would tend to down-regulate the antidonor immune response and protect the donor graft. FasL+ lymphohematopoietic stem-progenitor cells for BMT might be more effective than FasL+ DCs have been in organ transplant models, because transduced lymphohematopoietic stem-progenitor cells could generate large numbers of donor FasL+ progeny cells. On this basis, we undertook studies to determine whether lentiviral transduction of donor lin− BMs with FasL protected the donor graft from acute rejection, in an allogeneic mouse nonmyeloablative BMT model. Results indicate that FasL+ lin− BMs killed activated antidonor T cells and enhanced short-term donor cell engraftment, without producing major acute hepatotoxicity or generalized acute myelosuppression or immunosuppression.
Materials and methods
Viral vector construction and production
The FasL gene expression vector was created by fusion of the mouse FasL coding region to the 5′ end of the enhanced green fluorescent protein (GFP) gene, and subsequent insertion into a lentivirus vector under the control of the cytomegalovirus β (CMV-β)–actin fusion promoter. The control vector expresses only GFP. The lentivirus packaging system16 and the self-inactivating lentiviral parent vector pRLL.hPGK.GFP SIn-1817 have been described previously. The PGK promoter was removed from the parent vector, and additional restriction sites were added (5′XhoI-EcoRV-BamHI-AgeI-NheI-KpnI-MluI-SpeI-HpaI-EcoRI-XBamHI) by insertion of a T4 kinase-treated oligonucleotide pair: (1) CGAGATATCGGATCCACCGGTGCTAGCGGTACCACGCGTACTAGTGTTAACGAATTC and (2) GATCGAATTCGTTAACACTAGTACGCGTGGTACCGCTAGCACCGGTGGATCCGATATC. Next, the 1.75-kb CMV-β–actin fusion promoter (CAG) derived from the pCAGGS vector from Dr J. Miyazaki (Osaka University Medical School, Suita, Japan)18 was inserted into the modified parent vector preceding the GFP reporter sequence, and the GFP was removed. A plasmid containing the mouse full-length FasL cDNA coding sequence, as an 880-bp insert in the p43 plasmid,19 was obtained from Dr T. August (Johns Hopkins Medical Institutions [JHMI]). The FasL cDNA was digested from the p43 plasmid usingBglII and SalI, then cloned into those sites of the pEGFP-C2 plasmid (Clontech, Palo Alto, CA). The resulting FasL-EGFP fusion sequence was digested from the plasmid with NheI andMluI, then using these sites, inserted into the lentiviral parent vector under the control of the CMV-β–actin fusion promoter. The insert sequence was verified by DNA sequencing.
For generation of producer lines, 293T cells cultured in Dulbecco modified Eagle medium (DMEM) containing 10% fetal calf serum (FCS; Life Technologies, Carlsbad, CA) were transfected using Effectene (Qiagen, Valencia, CA). Viral supernatants were collected for 3 days and filtered (0.45-μM Millipore filter, Fisher Scientific, Pittsburgh, PA). Supernatants were titered on 293T cells by adding 100 μL supernatant to 2 × 105 cells/well in 1 mL DMEM containing 10% FCS in a 6-well dish (Costar, Bedford, MA), incubating for 2 days, then measuring the percent GFP+ cells by fluorescence-activated cell sorting (FACS) analysis of 488-nm excited fluorescence in the FL1 channel using a FACScan flow cytometer and Cellquest software (Becton Dickinson, San Jose, CA). Supernatant from 293T cells was either used fresh for transduction or stored at −80°C until use. Prior to use, supernatants were concentrated using Centricon filters (100-kDa cutoff; Millipore, Bedford, MA). Sufficient supernatant to achieve a multiplicity of infection (MOI) of 3 to 5 was concentrated to a volume of 50 to 100 μL, then added to lin− BMs in the presence of 8 μg/mL polybrene (Sigma, St Louis, MO).
BM harvest, DC cultures, lin− BM enrichment, and transduction
All animal studies were conducted under approved animal protocols at JHMI. Mice were obtained from the National Cancer Institute at 6 to 8 weeks of age, except for the 2C transgenics,20 which were bred onto a C57BL/6 background and kindly provided to us by Dr J. Schneck (JHMI). For BM harvest, femurs and tibias were removed from mice that were killed, flushed with ice-cold isotonic phosphate-buffered saline (PBS; pH 7.4, 0.05 M phosphate), and the resulting BM cells were washed and counted. DCs were generated from BM cells as previously described.6After transduction, DCs were evaluated on day 8 by FACS analysis for expression of both the transgene (indicated by GFP fluorescence in the FACScan FL1 channel) and DC markers (including phycoerythrin [PE]–labeled major histocompatibility complex [MHC] class II, CD80, CD86, and DEC-205, measured in the FL2 channel). PE-labeled antibodies were obtained from Becton Dickinson-Pharmingen (San Diego, CA), except DEC-205 was from Serotec (Raleigh, NC).
The Lin− BMs were enriched from mouse BM by immunomagnetic depletion of cells expressing mature hematopoietic “lineage” antigens, following the manufacturer's procedure (Stem Cell Technologies, Vancouver, BC, Canada), then plated at 106cells/mL in RPMI 1640 with 5% serum (Life Technologies) containing recombinant flt-3 ligand (FL; 50 ng/mL; R & D Systems, Minneapolis, MN), kit ligand (KL; 100 ng/mL; Peprotech, Rocky Hill, NJ), and thrombopoietin (10 ng/mL; Peprotech). Lin− BMs were transduced 3 times, by addition of concentrated viral supernatant (MOI 3-5) as described above, on days 1, 2, and 3 of culture. On day 4 or 5, aliquots of the transduced cells were analyzed by FACS for GFP expression, plated for colony-forming cells (CFCs), or transplanted intravenously (via the dorsal tail vein) into recipient mice. In addition, transduced cells were analyzed for cytotoxic function by coincubation with PKH26-labeled (PKH; Sigma) Jurkat target cells (labeled according to the manufacturer's instructions), known to be sensitive to Fas-mediated killing. Transduced lin− BMs were sorted for GFP expression, on a FACSVantage flow cytometer (Becton Dickinson) for the dose-titration studies. Then, using multicolor flow cytometry, labeled Jurkat target cells (PKH+) were distinguished from (unlabeled) effector lin− BMs by PKH fluorescence (FACScan FL2 channel), and live versus dead PKH+ Jurkat target cells were quantified using 7-aminoactinomycin (7-AAD; Becton Dickinson-Pharmingen) incorporation (measured in FACScan FL3 channel), following the manufacturer's procedure.
Mixed lymphocyte reactions and T-cell proliferation assays
Spleen responder cells were incubated with irradiated (3000 cGy) DCs or spleen cell stimulators, depending on the experiment. 105 DC stimulators were incubated with 106responders. Then, 2 × 106 spleen cell stimulators were added to 2 × 106 responders. Cultures were incubated in 96-well U-bottom plates (Costar) for 4 days, then 1 μCi/mL (0.037 MBq) 3H-thymidine (Amersham, Piscataway, NJ) was added for 16 hours, at which time the plates were harvested and counted.
Allogeneic BMT and engraftment analysis
For nonmyeloablative allogeneic BMT in a multiple minor histocompatibility complex mismatch setting, B6.SJL (CD45.1+) donor lin− BMs were infused into 400-cGy irradiated recipient C3H.SW (CD45.2+) mice. These mice are MHC matched (H2b), but differ at multiple minor histocompatibility loci, many of which are still undefined.21 Mice were killed at 3 to 24 weeks after transplantation, then single-cell suspensions of organs were prepared for FACS analysis (BM and spleen), CFC assays (BM), and MLR assays including responsiveness to third-party stimulators (spleen). In these FACS analyses, BM and spleen were evaluated for the numbers of donor cells (CD45.1+) and transduced donor cells (GFP+/CD45.1+). PE-CD45.1 monoclonal antibody was obtained from Pharmingen.
CFC assays
Analysis of CFCs was conducted on BM cells prior to transplantation by plating 3 × 103 transduced lin− BMs (in triplicate) in 1 mL Marrow-Gro methylcellulose medium (generously provided by Quality Biologicals, Gaithersburg, MD) supplemented with recombinant KL (50 ng/mL), IL-3 (10 ng/mL), granulocyte-monocyte colony-stimulating factor (GM-CSF; 10 ng/mL), and erythropoietin (Epo; 5 U/mL). Unless otherwise specified, growth factors were obtained from Peprotech. After 7 days incubation, CFC-Mix, CFC–granulocyte-macrophage (CFC-GM), and erythroid burst-forming unit (BFU-E) colonies were counted. When the mice receiving transplants were killed, 3 × 105 whole BM cells were assayed for CFCs as above.
For the studies with soluble FasL (sFasL), BM cells were plated in QBSF-58 (Quality Biologicals) containing KL, GM-CSF, and Epo, with a range of concentrations of sFasL (Alexis Pharmaceuticals, San Diego, CA) for 48 hours prior to plating in CFC assays.
Listeria monocytogenes challenge
BALB/c mice, known to be susceptible toListeria from preliminary studies, were lethally irradiated (850 cGy) and received transplants of syngeneic BALB/c lin− BMs that had been transduced with either the control GFP or the FasL-GFP lentiviral vector. Three weeks later, the mice that underwent transplantation were tail bled to quantify GFP+ cells, then injected intraperitoneally with 106 colony-forming units (cfu) attenuated L monocytogenes bacteria.22Four days after challenge, mice were killed. Livers and spleens were removed, and portions were fixed in paraformaldehyde and analyzed histologically. The remainders of these organs were crushed to obtain single-cell suspensions that were stained with CD8 Cy-chrome and either CD3-PE or CD4-PE monoclonal antibodies (Becton Dickinson-Pharmingen), then analyzed by FACS.
Results
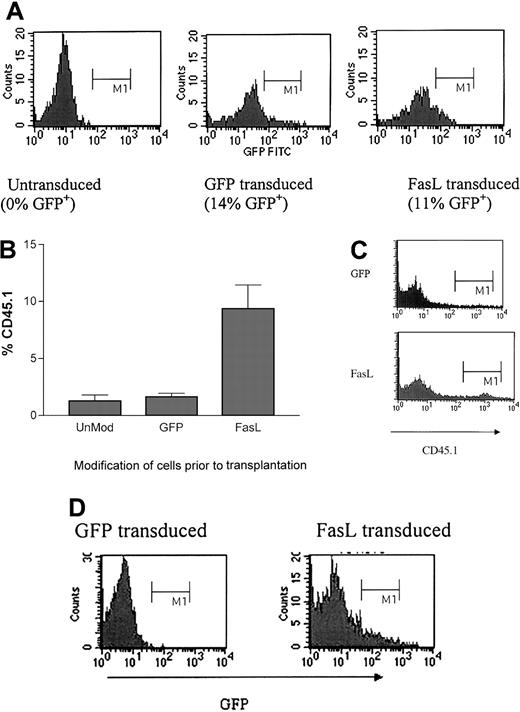
Transduced lin− BMs were analyzed for expression of the transgene by (1) determination of GFP fluorescence (which should be present in both control vector–transduced and experimental vector–transduced groups because the experimental lentivirus encodes FasL as a GFP-fusion) and (2) determination of function by analyzing killing of FasL-sensitive Jurkat T cells by transduced cells. Ten to 20% of the transduced lin− BMs used were GFP+, consistently in control and experimental groups throughout the experiments described herein; specific percentages are shown for representative experiments.
Transduced FasL+ lin− BMs killed activated T cells
For the functional assay, lin− BMs were incubated with PKH-labeled Jurkat cells, then the cocultured cells were analyzed by FACS for the presence of 7-AAD (indicating cell death) in PKH+ (Jurkat) cells (Figure1A). To determine whether there was a dose-response effect for the killing of the Jurkat cells by FasL+ cells, transduced lin− BMs were FACS sorted for GFP expression, then mixtures of FasL+ (ie, based on GFP fluorescence) with FasL− (ie, GFP−) cells were prepared and incubated for 24 hours with PKH-labeled Jurkat target cells. Mixtures containing 1% or 5% FasL+ cells mediated only a slight increase in killing compared to negative control cultures. The mixture containing 20% FasL+ lin− BMs was markedly more effective at killing Jurkat cells, and the mixture containing 80% FasL+lin− BMs was slightly more potent than the 20% mixture (Figure 1A shows 1 of 2 similar experiments).
FasL in the transduced cells was functional.
FasL+ lin− BMs killed Jurkat T cells in a dose-dependent fashion, and FasL+ DCs decreased T-cell proliferative responses. (A) B6.SJL lin− BMs were transduced 3 times to express either the control GFP vector or the vector expressing the GFP-FasL fusion. On day 5 of ex vivo transduction culture, the cells were FACS sorted into (FasL+)GFP+ or (FasL−)GFP− subsets. The (FasL+)GFP+ subset expressed more than 10-fold higher fluorescence than the (FasL−)GFP−subset; the lin− BMs with intermediate levels of fluorescence were discarded. Sorted (FasL+)GFP+cells were mixed in varying ratios with (FasL−)GFP− cells for a total of 105 lin− BMs, as indicated, in duplicate wells of a 96-well plate containing 105 PKH-labeled Jurkat cells. At 24 hours later, 7-AAD was added to the cultures, which were then analyzed by FACS for the percent PKH+ (Jurkat) cells that had incorporated 7-AAD. Shown are histograms of 7-AAD fluorescence in-gated PKH+ (Jurkat) cells. Mixtures containing 1%, 5%, 20%, or 80% (FasL+)GFP+ lin−BMs, indicated by the graph labels, resulted in 5%, 9%, 36%, and 42% dead Jurkat cells, respectively. (B) Function of modified DCs was tested in a standard T-cell proliferation assay. GFP control-transduced or FasL-transduced B6.SJL or BALB/c DCs were generated as described, then irradiated (3000 cGy) and incubated with responder spleen cells (B6, transgenic 2C [on a B6 background] or BALB/c, as indicated in the graph axis labels). Proliferation was determined by incorporation of 3H-thymidine. This plot shows the averages (± SD) of 3 separate experiments. (C) T-cell proliferative responses are shown from experiments in which allogeneic DCs were mixed with syngeneic DCs as stimulators, with either the allogeneic DCs (B6) or the syngeneic DCs (BALB/c) modified to express FasL. In this set of experiments, 106 BALB/c spleen cells were mixed with, from left to right: 105 control B6 DCs; 105FasL+ B6 DCs; 5 × 104 control B6 DCs plus 5 × 104 control BALB/c DCs; and 5 × 104control B6 DCs plus 5 × 104 FasL+ BALB/c DCs. After 3 days of incubation, 1 μCi (0.037MBq)3H-thymidine was added for 24 hours, then cells were harvested and proliferation was determined by 3H-thymidine incorporation. The results are shown for 2 separate experiments, with triplicates for each condition.
FasL in the transduced cells was functional.
FasL+ lin− BMs killed Jurkat T cells in a dose-dependent fashion, and FasL+ DCs decreased T-cell proliferative responses. (A) B6.SJL lin− BMs were transduced 3 times to express either the control GFP vector or the vector expressing the GFP-FasL fusion. On day 5 of ex vivo transduction culture, the cells were FACS sorted into (FasL+)GFP+ or (FasL−)GFP− subsets. The (FasL+)GFP+ subset expressed more than 10-fold higher fluorescence than the (FasL−)GFP−subset; the lin− BMs with intermediate levels of fluorescence were discarded. Sorted (FasL+)GFP+cells were mixed in varying ratios with (FasL−)GFP− cells for a total of 105 lin− BMs, as indicated, in duplicate wells of a 96-well plate containing 105 PKH-labeled Jurkat cells. At 24 hours later, 7-AAD was added to the cultures, which were then analyzed by FACS for the percent PKH+ (Jurkat) cells that had incorporated 7-AAD. Shown are histograms of 7-AAD fluorescence in-gated PKH+ (Jurkat) cells. Mixtures containing 1%, 5%, 20%, or 80% (FasL+)GFP+ lin−BMs, indicated by the graph labels, resulted in 5%, 9%, 36%, and 42% dead Jurkat cells, respectively. (B) Function of modified DCs was tested in a standard T-cell proliferation assay. GFP control-transduced or FasL-transduced B6.SJL or BALB/c DCs were generated as described, then irradiated (3000 cGy) and incubated with responder spleen cells (B6, transgenic 2C [on a B6 background] or BALB/c, as indicated in the graph axis labels). Proliferation was determined by incorporation of 3H-thymidine. This plot shows the averages (± SD) of 3 separate experiments. (C) T-cell proliferative responses are shown from experiments in which allogeneic DCs were mixed with syngeneic DCs as stimulators, with either the allogeneic DCs (B6) or the syngeneic DCs (BALB/c) modified to express FasL. In this set of experiments, 106 BALB/c spleen cells were mixed with, from left to right: 105 control B6 DCs; 105FasL+ B6 DCs; 5 × 104 control B6 DCs plus 5 × 104 control BALB/c DCs; and 5 × 104control B6 DCs plus 5 × 104 FasL+ BALB/c DCs. After 3 days of incubation, 1 μCi (0.037MBq)3H-thymidine was added for 24 hours, then cells were harvested and proliferation was determined by 3H-thymidine incorporation. The results are shown for 2 separate experiments, with triplicates for each condition.
FasL+ DCs inhibited allogeneic T-cell proliferation
Untransduced DCs or DCs transduced with the FasL (-GFP fusion) or the control GFP vector were irradiated and incubated with responder spleen cells. In the allogeneic mixture (B6 DCs and BALB/c splenocyte responders), the control GFP+ DCs stimulated a robust proliferative response, whereas the FasL+ DCs failed to stimulate a response above background (Figure 1B). 2C mice are transgenic for a CD8+ T-cell receptor that recognizes H2 Ld (displayed on BALB/c cells).18 Proliferation of 2C cells in response to BALB/c stimulators was essentially eliminated with the FasL+DCs.
To begin to address the question of the specificity of the effects of FasL+ DCs, we tested whether proliferation of responder T cells would be “nonspecifically” inhibited by FasL+cells syngeneic to the responders. The results in Figure 1C indicate that the proliferative response of BALB/c T-responder cells was potently inhibited by FasL+ allogeneic B6 DCs. A mixed population of B6 DC and FasL+ syngeneic BALB/c DC stimulators resulted in some inhibition of the response of BALB/c T cells to untransduced B6 DCs, but the inhibition was less. Thus, although some nonspecific inhibition was observed with syngeneic FasL+ cells, allogeneic FasL+ DCs mediated nearly complete inhibition of the proliferative immune response.
Constitutive FasL expression by lin− BMs did not impair generation of CFCs
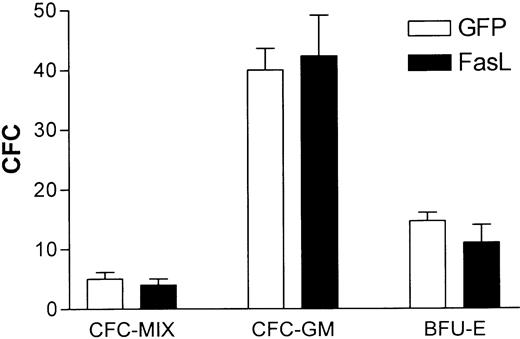
Lin− BMs were enriched from mouse BM as described, transduced with either the GFP or FasL vector (resulting in 10%-20% GFP+ cells before BMT, determined by FACS analysis prior to plating), and plated in CFC assays. Colonies were counted 7 days later. No significant difference was observed in numbers or types of CFCs from the 2 groups (Figure 2A).
FasL expression in lin− BMs did not inhibit generation of CFCs or syngeneic in vivo engraftment, assessed early after BMT.
(A) B6.SJL lin− BMs were transduced with either the FasL(-GFP fusion) or control GFP vector as described in “Materials and methods,” then 3 × 103 cells were plated in CFC assay medium as described in “Materials and methods.” The results are the averages (and SEM) of 4 separate experiments. (B) CFCs were assayed from untransduced B6.SJL lin− BMs that were exposed to sFasL in vitro, at the concentrations indicated, for 24 hours prior to plating in methylcellulose. Colonies were counted 7 days later; shown are the averages of 2 separate experiments. (C) BALB/c lin− BMs were transduced with either the GFP control or FasL vector, then 105 cells were transplanted into 850-cGy irradiated syngeneic mice (5 mice/group). Shown is the histogram of GFP expression in the starting population of transduced lin− BMs. (D) At 3 weeks after transplantation of the cells shown in panel C, mice were tail bled to determine the percentage of circulating transduced cells. Whole blood was collected by tail bleeds and red cells removed by hypotonic lysis, then analyzed by FACS for GFP expression. The graph shows the percent GFP+ blood cells after transplantation (each point represents one mouse).
FasL expression in lin− BMs did not inhibit generation of CFCs or syngeneic in vivo engraftment, assessed early after BMT.
(A) B6.SJL lin− BMs were transduced with either the FasL(-GFP fusion) or control GFP vector as described in “Materials and methods,” then 3 × 103 cells were plated in CFC assay medium as described in “Materials and methods.” The results are the averages (and SEM) of 4 separate experiments. (B) CFCs were assayed from untransduced B6.SJL lin− BMs that were exposed to sFasL in vitro, at the concentrations indicated, for 24 hours prior to plating in methylcellulose. Colonies were counted 7 days later; shown are the averages of 2 separate experiments. (C) BALB/c lin− BMs were transduced with either the GFP control or FasL vector, then 105 cells were transplanted into 850-cGy irradiated syngeneic mice (5 mice/group). Shown is the histogram of GFP expression in the starting population of transduced lin− BMs. (D) At 3 weeks after transplantation of the cells shown in panel C, mice were tail bled to determine the percentage of circulating transduced cells. Whole blood was collected by tail bleeds and red cells removed by hypotonic lysis, then analyzed by FACS for GFP expression. The graph shows the percent GFP+ blood cells after transplantation (each point represents one mouse).
sFasL pretreatment of untransduced lin− BMs did not affect CFCs
Because only 10% to 20% of the transduced BM cells in the experiments above expressed FasL, we undertook experiments to determine the effect of exposing 100% of the BM cells to agonistic sFasL. A range of concentrations of sFasL was added to cultures of untransduced lin− BMs for 48 hours prior to plating in CFC assays as shown. Pretreatment of lin− BMs with sFasL did not inhibit CFC numbers or alter the distribution of CFC types (Figure2B).
Constitutive FasL expression by lin− BMs did not impair syngeneic engraftment, assessed early after BMT
To investigate the effect of FasL expression on the capacity of transduced FasL+ lin− BMs to engraft, syngeneic transplantations were performed. BALB/c lin− BMs were transduced with either the GFP control or FasL vector, then 105 cells were transplanted into 850-cGy irradiated syngeneic mice (BALB/c). Figure 2C shows GFP fluorescence of the lin− BMs prior to transplantation. The entire population of cells (transduced and untransduced) was injected for transplantation.
FasL+ lin− BMs generated enhanced allogeneic engraftment early after BMT
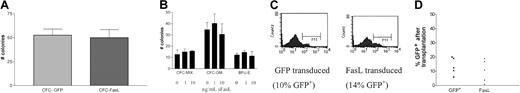
A multiple minor mismatch (B6.SJL→C3H.SW) was selected as an MHC-matched nonmyeloablative BMT model. Lin− BMs from B6.SJL mice (CD45.1+) were transduced with either the control (GFP) or experimental FasL (-GFP) vector. Figure3A shows GFP fluorescence of the B6.SJL lin− BMs prior to transplantation into sublethally irradiated recipient C3H.SW mice.
Mice that received transplants of FasL+ allogeneic lin− BMs had enhanced engraftment early after BMT.
(A) B6.SJL (CD45.1+) lin− BMs were transduced with either control GFP or FasL(-GFP) vector. Cells were analyzed by FACS to determine the level of GFP fluorescence of the transduced cells prior to transplantation. (B) 105transduced lin− BM cells were transplanted intravenously into 400-cGy irradiated C3H.SW (CD45.2+) recipients. After mice were killed at 3 weeks after BMT, mouse organs were analyzed by FACS for correlated expression of CD45.1 and GFP. The graph shows the compilation of data for all mice shown in Table 1, with the average percentage of CD45.1+ cells in BM of each group. (C) The figure shows a representative plot for CD45.1 for a mouse that received a transplant of GFP-modified cells (top) and a mouse that received a transplant of FasL-modified cells (bottom). (D) These histograms show the GFP fluorescence of gated CD45.1+(donor) cells (from panel B) of representative mice that received transplants of lin− BMs transduced with either control GFP or FasL and analyzed at 3 weeks after BMT.
Mice that received transplants of FasL+ allogeneic lin− BMs had enhanced engraftment early after BMT.
(A) B6.SJL (CD45.1+) lin− BMs were transduced with either control GFP or FasL(-GFP) vector. Cells were analyzed by FACS to determine the level of GFP fluorescence of the transduced cells prior to transplantation. (B) 105transduced lin− BM cells were transplanted intravenously into 400-cGy irradiated C3H.SW (CD45.2+) recipients. After mice were killed at 3 weeks after BMT, mouse organs were analyzed by FACS for correlated expression of CD45.1 and GFP. The graph shows the compilation of data for all mice shown in Table 1, with the average percentage of CD45.1+ cells in BM of each group. (C) The figure shows a representative plot for CD45.1 for a mouse that received a transplant of GFP-modified cells (top) and a mouse that received a transplant of FasL-modified cells (bottom). (D) These histograms show the GFP fluorescence of gated CD45.1+(donor) cells (from panel B) of representative mice that received transplants of lin− BMs transduced with either control GFP or FasL and analyzed at 3 weeks after BMT.
Approximately 3 weeks later, mice were killed and analyzed for donor cell hematopoietic engraftment. Donor cells and transduced cells were analyzed by correlated CD45.1 and GFP fluorescence. Table1 shows the percentages of transplanted mouse BM cells derived from donor (CD45.1+) cells or transduced donor (CD45.1+/GFP+) cells. Mice that received transplants of FasL+ lin− BMs had significantly higher levels of donor chimerism than those that received the control lin− BMs, in BMs (P = .01; Table 1 and Figure 3B).
Percentage of donor chimerism in each mouse receiving unmodified, GFP-modified, and FasL-modified grafts
| % CD45.1 . | % CD45.1 in Fas that are GFP . | ||
|---|---|---|---|
| UnMod . | GFP . | FasL . | % FasL (GFP+) . |
| 1.10 | 1.60 | 8.0 | 6.0 |
| 0.10 | 1.90 | 22.0 | 31.0 |
| 3.00 | 0.70 | 3.2 | 13.0 |
| 1.80 | 1.90 | 9.8 | 4.0 |
| 0.40 | 3.00 | 5.1 | 20.0 |
| — | 3.00 | 6.7 | 0.1 |
| — | 1.00 | 1.8 | 4.0 |
| — | 1.00 | 2.3 | 25.0 |
| — | 1.00 | 19.4 | 5.0 |
| — | — | 17.0 | 2.0 |
| — | — | 33.0 | 2.0 |
| — | — | 5.0 | 20.0 |
| — | — | 8.0 | 12.0 |
| — | — | 4.0 | 1.0 |
| — | — | 4.0 | 1.0 |
| — | — | 7.0 | 10.0 |
| — | — | 3.0 | 7.0 |
| % CD45.1 . | % CD45.1 in Fas that are GFP . | ||
|---|---|---|---|
| UnMod . | GFP . | FasL . | % FasL (GFP+) . |
| 1.10 | 1.60 | 8.0 | 6.0 |
| 0.10 | 1.90 | 22.0 | 31.0 |
| 3.00 | 0.70 | 3.2 | 13.0 |
| 1.80 | 1.90 | 9.8 | 4.0 |
| 0.40 | 3.00 | 5.1 | 20.0 |
| — | 3.00 | 6.7 | 0.1 |
| — | 1.00 | 1.8 | 4.0 |
| — | 1.00 | 2.3 | 25.0 |
| — | 1.00 | 19.4 | 5.0 |
| — | — | 17.0 | 2.0 |
| — | — | 33.0 | 2.0 |
| — | — | 5.0 | 20.0 |
| — | — | 8.0 | 12.0 |
| — | — | 4.0 | 1.0 |
| — | — | 4.0 | 1.0 |
| — | — | 7.0 | 10.0 |
| — | — | 3.0 | 7.0 |
This table shows the data for each mouse analyzed. The first column shows the percent donor cells for mice receiving unmodified lin− BMs; the second column, the percent donor cells for mice receiving GFP-modified BMs; the third, percent donor cells from the FasL-modified BMs; and the fourth, the percent donor cells in the FasL transplants that were also GFP+.
— indicates no data.
FasL+ lin− BMs generated enhanced allogeneic engraftment early after BMT. BM from mice was analyzed by FACS 3 weeks after BMT to determine the presence of both donor cells (CD45.1+) and donor cells that were transduced (GFP+ and CD45.1+). The graph (Figure 3B) summarizes the averages and SEM of all the mice shown in Table 1. In Table 1, each value is representative of a single mouse, with results combined from 4 separate experiments. The first 3 columns are the total percent CD45.1+ cells in mice receiving unmodified, GFP-modified, and FasL-modified transplants as indicated; the last row is the percentage of CD45.1+ cells that are GFP+ also, in the FasL transplants (CD45.1+/GFP+).
The BM cells from mice that underwent allotransplantation killed at 3 weeks after BMT (Table 1) were assessed for CFCs. No significant differences were observed in numbers or types of CFCs from the FasL-transduced versus control GFP-transduced groups of mice (Figure4).
Mice that received transplants of FasL+lin− BMs did not have diminished numbers of BM CFCs early after BMT.
After the mice described in Figure 3 were killed, whole BM (3 × 105 cells) was assayed for CFCs (triplicates). Seven days after plating, colonies were counted (averages ± SEM are shown).
Mice that received transplants of FasL+lin− BMs did not have diminished numbers of BM CFCs early after BMT.
After the mice described in Figure 3 were killed, whole BM (3 × 105 cells) was assayed for CFCs (triplicates). Seven days after plating, colonies were counted (averages ± SEM are shown).
Additional mice underwent transplantations as above in separate experiments; the transduction efficiency before BMT averaged 17% (Table 2). In 2 experiments (combined results), BM cells from mice were analyzed by FACS at 20 to 24 weeks after BMT for correlated expression of CD45.1 (representing donor) and GFP (representing transduced) cells. Each value shows the result for an individual mouse. The first column is the percent of donor cells in BM from mice that received GFP-modified transplants. The second column is the percent of donor cells in BM from mice that received FasL-modified transplants. The third column correlates with the second and shows the percent of donor cells that also are GFP+. None of 7 mice that received transplants of control GFP transduced lin− BMs, but 4 of 7 mice that received transplants of FasL-transduced lin− BMs, had more than 2% donor cells in BM. None of the mice in either group had detectable GFP+ BM cells.
Retention of graft at 4 to 6 months after transplantation
| GFP . | FasL . | % FasL (GFP+) . |
|---|---|---|
| < 0.5 | 9 | < 1 |
| < 0.5 | 3 | < 1 |
| 0.52 | 11 | < 1 |
| < 0.5 | 1 | < 1 |
| < 0.5 | 1 | < 1 |
| < 0.5 | 11 | < 1 |
| < 0.5 | < 1 | < 1 |
| GFP . | FasL . | % FasL (GFP+) . |
|---|---|---|
| < 0.5 | 9 | < 1 |
| < 0.5 | 3 | < 1 |
| 0.52 | 11 | < 1 |
| < 0.5 | 1 | < 1 |
| < 0.5 | 1 | < 1 |
| < 0.5 | 11 | < 1 |
| < 0.5 | < 1 | < 1 |
This table shows the percent donor chimerism of mice that received transplants of modified cells and analyzed 4 to 6 months later. Four of 7 FasL mice that underwent transplantation retained the graft at detectable levels, whereas 0 of 7 GFP mice that received transplants retained the graft. No GFP+ cells were detected at this later time point in the FasL mice that received transplants.
Mice that received transplants of FasL+lin− BMs did not have significant hepatic toxicity or immune impairment
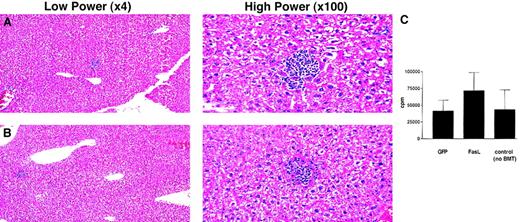
A significant concern with expressing FasL in lin−BMs is the potential for in vivo toxicity due to FasL. The mice that received transplants of syngeneic or allogeneic FasL+lin− BMs were not different from control groups in overall health, or on gross pathology at autopsy. Because hepatic cells express high levels of Fas and because hepatotoxicity was reported after administration of one, but not another, anti-Fas antibody,23 we evaluated whether transplant with FasL+ lin− BMs produced histologic hepatotoxicity. Histologic analysis of hematoxylin and eosin-stained slides by a pathologist (F.K.R.) revealed no detectable injury to hepatic cells of mice that had received a transplant of FasL+ versus control GFP+ lin− BMs (Figure 5A,B). Mild hepatic inflammation was noted in both groups but there was no difference in the levels of hepatic inflammation between the 2 groups. In the groups followed for 4 to 6 months, inflammation persisted to varying degrees in the mice that underwent transplantation; 1 of 4 FasL mice that were analyzed had detectably worse inflammation.
Mice that received transplants of FasL+lin− BMs did not have hepatocellular injury or enhanced hepatic inflammation and retained immune responsiveness to a third-party alloantigen.
(A,B) Livers from mice shown in Figure 3were fixed in formaldehyde, cut into paraffin blocks, then stained with hematoxylin and eosin for evaluation of inflammation. Panel A is a representative section from a control mouse that underwent GFP transplantation, and panel B is from the liver of a representative mouse that underwent FasL+ transplantation. (C) Splenocytes from mice that underwent transplantation were incubated as responders with irradiated (3000 cGy) allogeneic third-party stimulator (BALB/c) spleen cells. Four days after adding stimulators,3H-thymidine was added overnight. Cells were then harvested, and incorporation of 3H-thymidine was determined. This panel shows the proliferative responses (group averages ± SEM) from 8 mice that received transplants of FasL-transduced lin− BMs, 7 mice that received transplants of control GFP-transduced lin− BMs, 4 mice that received transplants of untransduced lin− BMs, and 3 control C3H.SW mice that did not undergo transplantation, taken from 3 separate experiments.
Mice that received transplants of FasL+lin− BMs did not have hepatocellular injury or enhanced hepatic inflammation and retained immune responsiveness to a third-party alloantigen.
(A,B) Livers from mice shown in Figure 3were fixed in formaldehyde, cut into paraffin blocks, then stained with hematoxylin and eosin for evaluation of inflammation. Panel A is a representative section from a control mouse that underwent GFP transplantation, and panel B is from the liver of a representative mouse that underwent FasL+ transplantation. (C) Splenocytes from mice that underwent transplantation were incubated as responders with irradiated (3000 cGy) allogeneic third-party stimulator (BALB/c) spleen cells. Four days after adding stimulators,3H-thymidine was added overnight. Cells were then harvested, and incorporation of 3H-thymidine was determined. This panel shows the proliferative responses (group averages ± SEM) from 8 mice that received transplants of FasL-transduced lin− BMs, 7 mice that received transplants of control GFP-transduced lin− BMs, 4 mice that received transplants of untransduced lin− BMs, and 3 control C3H.SW mice that did not undergo transplantation, taken from 3 separate experiments.
To assess the immune responsiveness of the mice that underwent allotransplantation, splenocytes were taken at the time of killing and used as responders in an MLR to a third-party antigen stimulator (BALB/c, H2d). No significant difference was observed between the 2 groups in the level of proliferation. (Figure 5C).
To further evaluate hepatotoxicity and to test the immune responsiveness of the mice that underwent transplantation, we challenged mice that underwent transplantation with a sublethal dose ofL monocytogenes as a model infectious agent. Listeriawas selected because it is known to produce hepatic inflammation, and thus any inflammatory or hepatic in vivo toxicity of FasL+ lin− BMs or their progeny might be highlighted by this challenge. In addition, because T cells have been reported to die in the liver based on Fas-FasL interactions,24 significant Fas-mediated toxicity should prevent accumulation of T cells recruited in response to this challenge. BALB/c lin− BMs were transduced with FasL or GFP as above, and transplanted into lethally irradiated syngeneic BALB/c recipients. All mice were then injected intraperitoneally with a sublethal dose of Listeria, observed for 4 days, and killed. All mice in both groups exhibited decreased activity, starting 1 day after Listeria injection. On pathologic examination, all mice had mild inflammation in the liver, with no gross differences between the 2 groups (Figure 6A). All mice had high numbers of T cells in the livers in response to this challenge (Figure 6B).
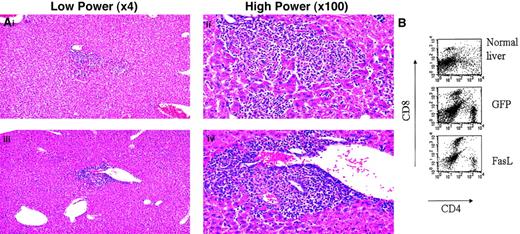
Mice that received transplants of syngeneic FasL+ lin− BMs responded to an antigenic infectious challenge.
(A) Representative liver histologies of mice 4 days afterL monocytogenes injection. Some hepatic inflammation was seen in all mice in both control (top) and experimental (bottom) groups. (B) Livers were analyzed for T-cell infiltration by making single-cell suspensions from a portion of the liver and staining for T-cell markers. Shown are representative FACS plots of correlated CD4 and CD8 staining of the liver cells from a normal uninfected mouse liver, a liver from a mouse that received a transplant of control GFP+ lin− BMs, and a liver from a mouse that received a transplant of FasL+ lin−BMs.
Mice that received transplants of syngeneic FasL+ lin− BMs responded to an antigenic infectious challenge.
(A) Representative liver histologies of mice 4 days afterL monocytogenes injection. Some hepatic inflammation was seen in all mice in both control (top) and experimental (bottom) groups. (B) Livers were analyzed for T-cell infiltration by making single-cell suspensions from a portion of the liver and staining for T-cell markers. Shown are representative FACS plots of correlated CD4 and CD8 staining of the liver cells from a normal uninfected mouse liver, a liver from a mouse that received a transplant of control GFP+ lin− BMs, and a liver from a mouse that received a transplant of FasL+ lin−BMs.
Discussion
Results of this study suggest that HVG rejection was inhibited at time points early after transplantation when FasL was genetically expressed in a fraction of the transplanted donor BM cells. Increased levels of donor cells were detected in the host early after nonmyeloablative BMT, and no significant toxicity to the recipient mice was detected. These results may serve as a paradigm for developing systems in which immune modulatory genes may be inserted into donor lin− BMs prior to BMT to engineer the recipient's immune response after BMT. Insertion of immunomodulatory genes into BM prior to transplantation has the potential to be applied to diseases for which BMT is already used, to enhance the transplantation effects, or to develop new therapies in which transplantation could be used for the purpose of introducing such genes. Thus, the capacity to modify immune responses through BMT would provide a significant potential for improvement of therapies for a number of diseases.
In the present experiments, transduced FasL+lin− BMs killed Fas-sensitive T cells in vitro. Expression of FasL in mouse lin− BMs did not appear to be toxic to BM function because (1) there was no difference in the CFC numbers or types from FasL+ lin− BMs compared to controls, and (2) FasL+ donor lin− BMs engrafted to as high (in syngeneic transplants) or higher (in allogeneic transplants) levels than did control lin− BMs. Although syngeneic engraftment was assessed at only the early time point of 3 weeks after BMT, this finding is consistent with other studies in which human CD34+ cells were shown to not be susceptible to Fas-mediated apoptosis, possibly due to high level expression of the caspase pathway inhibitor, FLICE inhibitory protein (FLIP).25
FasL+ lin− BMs directly killed Fas-sensitive Jurkat target cells, and FasL+ DCs inhibited allogeneic T-cell proliferation. Transplanted FasL+ lin−BMs have the potential to differentiate in vivo into DCs, which could potentiate the tolerizing effect mediated by the FasL+lin− BMs, per se. Our observation that FasL+DCs decreased an immune response is consistent with findings in multiple other in vitro and model organ transplant systems. Mice that received transplants of FasL+ donor lin− BMs had significantly higher levels of donor hematopoietic cell chimerism than did those that received transplants of control donor lin− BMs. This was likely the result of inhibition of HVG attack by the FasL+ lin− BMs and progeny, because in the syngeneic transplantations conducted for theListeria challenge, no significant differences in levels of total or GFP+ cells were observed between the FasL+ and control groups. Presumably, FasL expression would confer no selective advantage in a syngeneic transplantation. In the nonmyeloablative allogeneic BMT recipient hematopoietic chimeras, not all of the donor cells were transduced or expressed high levels of FasL, as assessed by GFP fluorescence from the fusion protein. Thus, HVG rejection was significantly down-regulated, even though only a fraction of the donor cells were FasL+. It is possible that FasL+ cells generated donor tolerance, and once tolerance to donor cells was achieved, the FasL+ cells no longer had a selective advantage over the untransduced donor cells. Over time, the percent FasL+ cells appeared to decrease, consistent with this hypothesis. Because the percent donor cells decreased somewhat over time, ongoing studies will address relative contribution of the graft in longer term experiments using this approach, as compared to (or combined with) other nonmyeloablative approaches. The goal of the present studies was to determine effects on acute allograft rejection. In addition, these studies do not address the potential for long-term nonspecific toxicity, for example, late GVHD or late nonspecific immunosuppression. However, it appears that the FasL expression provides bystander protection and that not all the cells need to express FasL. In these experiments, clonal expansion of FasL+ cells did not occur. Thus, it may be possible to translate the effects of FasL+ cells to clinical use in the future using only a low percent FasL+ cells that express FasL for a relatively short time. However, more extensive analysis of long-term transplants will be necessary to determine the full extent of the beneficial and potential toxic effects.
These results are consistent with other model systems in which organs modified to express FasL have been protected from rejection, as discussed above. One still unresolved issue in the use of FasL is the results from studies in which FasL generated enhanced rejection of organs and inflammatory responses. The conditioning regimen may affect the level of engraftment or rejection and the degree of nonspecific immunosuppression. For example, BMT preparative radiation may nonspecifically sensitize cells that may up-regulate Fas, potentially leading to nonspecific killing by FasL+ cells. It is likely that the microenvironment surrounding the FasL+ cells may contribute to differences in published results, because FasL has been shown to have different effects depending on the cytokines present in the host. A greater understanding of these phenomena would increase the utility of FasL in vivo.
One significant potential for a limitation in this approach of using FasL+ BM cells is that constitutive hematopoietic cellular expression of FasL might be toxic to the host. For example, many subsets of immune cells express Fas and so might be nonspecifically killed by FasL+ BM cells and their progeny.26Long-term effects of constitutively expressed FasL by donor cells could lead to chronic GVHD or potentially generate cells that would be inappropriately resistant to killing. Inducible vector systems would be one potential method to address these limitations. In addition, Fas is not the sole determinant of sensitivity to FasL-mediated apoptosis.27-30 As examples, DCs may express Fas but are protected by high levels of FLIP, and T cells are only highly sensitive to FasL on activation.31 In addition, we have recently found that CD34+ cells are resistant to Fas-mediated cytotoxicity and express low levels of Fas and high levels of FLIP.25 However, because the detailed long-term effects of in vivo administered transduced FasL+ BM cells are not fully known, this significant concern must be investigated. In these studies, the mice that received transplants of FasL+lin− BMs appeared as healthy as the controls. No significant difference was observed in BM cellularity or CFCs of mice that received transplants of FasL+ versus control lin− BMs. Because FasL has been shown to produce acute hepatotoxicity in some systems, we analyzed livers histologically from mice that underwent transplantation. Although there was minimal inflammation in both experimental and control mice (mild inflammation might be expected after a BMT), no difference was noted between the groups.
An evaluation of immune function of mice that underwent transplantation was conducted in 2 separate ways. First, spleen cells from the mice that underwent transplantation were used as responders in an MLR at the time of killing, as a general determinant of intact immune responsiveness to an alloantigen. Mice that were received transplants of FasL+ lin− BMs had no decrease in the ability to respond to allogeneic third-party stimulation. Second, mice were evaluated for their ability to respond to an infectious agent (L monocytogenes) at a dose that was determined empirically to produce severe but sublethal toxicity (A. Jain, R. Schulick, D. Pardoll, unpublished observations, February 2001). Mice that were significantly immunocompromised would be unable to mount an immune response to the agent and succumb. At 1 to 4 days after injection of the Listeria, all mice were alive but lethargic. At this time, they were killed, and livers were analyzed by FACS for T-cell infiltration and by histology. Because each liver had a significant T-cell infiltrate, the ability to mobilize T cells in response to an infectious antigen appeared to be intact in all mice.
These studies provide a novel approach to down-regulate graft rejection in BMT. Because FasL has the potential to kill multiple cell types and to produce organ toxicity, comprehensive analysis of potential toxicity in long-term engrafted recipients of FasL+ BM cells needs to be conducted prior to clinical application, and results at longer time points are needed, for example, for assessment of potential effects on long-term engrafting lymphohematopoietic stem cells or chronic hepatotoxicity. In addition, the percentages of cells that express the FasL may need to be titrated to achieve effective killing of T cells with the minimum toxicity. Our results in vitro showed that only marginal killing of T cells was achieved if 1% to 5% of the effector cells expressed FasL. Therefore, values below this would not likely result in an effective decrease in graft rejection. Additional studies are currently under way, both to assess the long-term stability of a nonmyeloablative transplant and for the expression of FasL. The present studies tested the acute effects mediated by FasL expression in lin− BMs In potential future application in BMT, permanent immunosuppression may not be necessary to achieve stable hematopoietic engraftment and tolerance. If so, one might minimize exposure of the recipient to FasL by coengineering the FasL+ BM cells with a suicide gene so that the FasL+ cells could be deleted as soon as engraftment and tolerance were observed.
Thanks to Ajay Jain and Richard Schulick for providingListeria monocytogenes and to Ephraim Fuchs and Leo Luznik for bone marrow transplantation expertise.
Prepublished online as Blood First Edition Paper, June 21, 2002; DOI 10.1182/blood-2002-01-0118.
Supported in part by grant 6663 from The Leukemia & Lymphoma Society and a grant from the National Foundation for Cancer Research.
The Johns Hopkins University holds patents on CD34 monoclonal antibodies and related inventions. C.I.C. is entitled to a share of the sales royalty received by the University under licensing agreements between the University, Becton Dickinson Corporation, and Baxter Health Care Corporation. This arrangement is being managed by the University in accordance with its conflict of interest policies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Katharine A, Whartenby, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Bunting-Blaustein Cancer Research Bldg, Room 2M44, 1650 Orleans St, Baltimore, MD 21231; e-mail:whartka@jhmi.edu.

![Fig. 1. FasL in the transduced cells was functional. / FasL+ lin− BMs killed Jurkat T cells in a dose-dependent fashion, and FasL+ DCs decreased T-cell proliferative responses. (A) B6.SJL lin− BMs were transduced 3 times to express either the control GFP vector or the vector expressing the GFP-FasL fusion. On day 5 of ex vivo transduction culture, the cells were FACS sorted into (FasL+)GFP+ or (FasL−)GFP− subsets. The (FasL+)GFP+ subset expressed more than 10-fold higher fluorescence than the (FasL−)GFP−subset; the lin− BMs with intermediate levels of fluorescence were discarded. Sorted (FasL+)GFP+cells were mixed in varying ratios with (FasL−)GFP− cells for a total of 105 lin− BMs, as indicated, in duplicate wells of a 96-well plate containing 105 PKH-labeled Jurkat cells. At 24 hours later, 7-AAD was added to the cultures, which were then analyzed by FACS for the percent PKH+ (Jurkat) cells that had incorporated 7-AAD. Shown are histograms of 7-AAD fluorescence in-gated PKH+ (Jurkat) cells. Mixtures containing 1%, 5%, 20%, or 80% (FasL+)GFP+ lin−BMs, indicated by the graph labels, resulted in 5%, 9%, 36%, and 42% dead Jurkat cells, respectively. (B) Function of modified DCs was tested in a standard T-cell proliferation assay. GFP control-transduced or FasL-transduced B6.SJL or BALB/c DCs were generated as described, then irradiated (3000 cGy) and incubated with responder spleen cells (B6, transgenic 2C [on a B6 background] or BALB/c, as indicated in the graph axis labels). Proliferation was determined by incorporation of 3H-thymidine. This plot shows the averages (± SD) of 3 separate experiments. (C) T-cell proliferative responses are shown from experiments in which allogeneic DCs were mixed with syngeneic DCs as stimulators, with either the allogeneic DCs (B6) or the syngeneic DCs (BALB/c) modified to express FasL. In this set of experiments, 106 BALB/c spleen cells were mixed with, from left to right: 105 control B6 DCs; 105FasL+ B6 DCs; 5 × 104 control B6 DCs plus 5 × 104 control BALB/c DCs; and 5 × 104control B6 DCs plus 5 × 104 FasL+ BALB/c DCs. After 3 days of incubation, 1 μCi (0.037MBq)3H-thymidine was added for 24 hours, then cells were harvested and proliferation was determined by 3H-thymidine incorporation. The results are shown for 2 separate experiments, with triplicates for each condition.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/9/10.1182_blood-2002-01-0118/4/m_h82123345001.jpeg?Expires=1767749016&Signature=OZ-r2WsoLzkb8jeL0eX2CkDbb2UVnQ0HLV2MP5pYVe5q0H745DtxKkfbK63ldBdFMnQ0h~8O7zbAyRAnQlqjr9Jcgssw5FO7aFIRcyZTmU5-i64IwDS9MYZ1TQfM~zJBmkWdp~5HougLO9E7PwsOVe32~bveEmWLXXEQvgsGslZuFvo1f-BxT0zVduqqtiVrjDXdjbL9ltmf78QKypADFtc6vcMmM8KCDb9zXuRH~~KJVffY1pWxFi3kiI5QkbVqMUdpDsU7QvLW1MDxQrqWWRU~yXqzZyZTaqVjGm0Kzn3sJxoMskLoEs9WOuGw6OjfzNrJrmsaTVwSjoHSxdZqYg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal