Abstract
Shortly before the all-trans retinoic acid (ATRA) era, the GIMEMA cooperative group initiated a randomized study comparing idarubicin (IDA) alone with IDA plus arabinosylcytosine (Ara-C) as induction treatment in patients with newly diagnosed hypergranular acute promyelocytic leukemia (APL). Of the 257 patients evaluable for induction treatment, 131 were randomized to receive IDA alone (arm A) and 126 to receive IDA + Ara-C (arm B). Treatment in arm A consisted of 10 mg/m2 IDA daily for 6 consecutive days, whereas in arm B it consisted of 12 mg/m2 IDA daily for 4 days combined with 200 mg/m2 Ara-C daily in continuous infusion for 7 days. Once in complete remission (CR), patients received 3 consolidation courses of standard chemotherapy, and those still in CR at the end of the consolidation were randomized to receive or not receive 1 mg/kg 6-mercaptopurine daily and intramuscular injections of 0.25 mg/kg methotrexate weekly for 2 years. Overall, 100 (76.3%) patients in arm A and 84 (66.6%) patients in arm B achieved CR (P = NS). Event-free survival (EFS) rates were 35% and 23% for patients in arm A and arm B, respectively (P = .0352). Multivariate analysis revealed that EFS was favorably influenced by induction treatment with IDA alone (P = .0352) and unfavorably influenced by white blood cell (WBC) counts greater than 3000/μL (P = .0001) and increasing age (P = .0251). These results indicate that anthracycline monochemotherapy with IDA favorably influences the EFS of patients with newly diagnosed hypergranular APL.
Introduction
The use of all-trans retinoic acid (ATRA) in the treatment of acute promyelocytic leukemia (APL) has greatly modified the prognosis of this peculiar subtype of acute myelogenous leukemia (AML).1 However, ATRA, when given alone, does not cure APL, and all patients eventually have relapses.2 Therefore, a modern approach to the treatment of APL requires that ATRA be combined with standard chemotherapeutic protocols to achieve a high cure rate, as demonstrated by several cooperative groups.3-6
Despite these good results, concern still exists regarding which is the best chemotherapeutic protocol for use in APL. Early reports, pioneered by Bernard et al,7 indicated a peculiarly high sensitivity of APL to the anthracycline drug daunorubicin (DNR) when used as monochemotherapy during the induction phase, with high complete remission (CR) rates.7 Recently, the high sensitivity of APL to high-dose DNR was retrospectively confirmed by the Southwest Oncology Group (SWOG).8 Among the main limitations to the use of high-dose DNR are its acute and chronic cardiotoxicity.9,10 To overcome or reduce this problem, the new anthracycline idarubicin (IDA) has been proposed as a therapeutic option to the use of DNR. In an early Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) pilot study, we achieved 82% CR in a small number of APL patients using IDA as a single-induction chemotherapeutic agent; no cardiac toxicity was observed among the treated patients.11 These results prompted us to initiate, in 1989, a randomized study comparing IDA with IDA+ arabinosylcytosine (Ara-C) as induction treatment in APL. Patients who achieved CR received 3 consolidation courses that, at random, were or were not followed by maintenance treatment. After 7.5 years from the accrual of the last patient in the protocol, we report here the results of this study, which was the basis for the development of our current AIDA protocol.12 13
Materials and methods
Eligibility
Eligibility criteria were a diagnosis of hypergranular APL (microgranular APL was officially excluded from the study) morphologically defined according to the FAB guidelines,14age ranging from 12 to 62 years, and informed consent.
Exclusion criteria were inability to meet eligibility criteria, presence of severe cardiac disease, left ventricular ejection fraction less than 50% as measured by bidimensional echocardiography, history of neoplastic disease including myelodysplasia, serum creatinine levels greater than 3.0 mg/dL, serum bilirubin levels greater than 3.0 mg/dL, serum glutamic oxaloacetic transaminase levels more than 5 times the normal levels, and previous antineoplastic treatment.
Study design
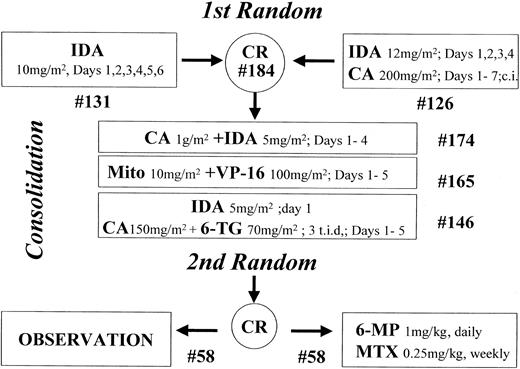
The study design is summarized in Figure1.
Study design.
The number of patients starting each step is indicated (#).
Induction treatment.
Patients were randomly assigned to receive 10 mg/m2 IDA intravenously daily from day 1 to day 6 (arm A) or 12 mg/m2IDA intravenously daily from day 1 to day 4 combined with 200 mg/m2 Ara-C daily as continuous infusion from day 1 to day 7 (arm B).
Consolidation therapy.
Patients who achieved CR received consolidation treatment with 3 chemotherapy courses, as follows: (1) 4 days of a 6-hour infusion of 1 g/m2 Ara-C followed, 3 hours after the end of each Ara-C infusion, by a brief intravenous infusion of 5 mg/m2 IDA (course 1); (2) brief daily intravenous infusion of 10 mg/m2 mitoxantrone on days 1, 2, 3, 4, and 5 followed, 12 hours after the start of each mitoxantrone infusion, by an intravenous infusion of 100 mg/m2 etoposide lasting 45 to 60 minutes (course 2); and (3) rapid intravenous infusion of 12 mg/m2IDA on day 1, associated with subcutaneous administration of 150 mg/m2 Ara-C every 8 hours (total daily dosage, 450 mg/m2) and oral administration of 70 mg/m26-thioguanine (6-TG) every 8 hours (total daily dosage, 210 mg/m2). Ara-C and 6-TG were also administered on days 2, 3, 4, and 5 at the same dosage as on day 1 (course 3).
Each consolidation course was administered at recovery from the previous one, when polymorphonuclear cells numbered 1500/μL or more and platelets numbered 100 000/μL or more.
Maintenance therapy.
Patients still in CR after consolidation therapy and with serum bilirubin levels less than 3.0 mg/dL, serum creatinine levels less than 3.0 mg/dL, and SGOT levels less than 5 times normal values were randomized to receive (arm A) or not (arm B) maintenance treatment consisting of 1 mg/kg 6-mercaptopurine (6 MP) by mouth each day and 0.25 mg/kg methotrexate (MTX) intramuscularly each week for a period of 2 years.
Supportive treatment
During the induction phase, all patients received oral antifungal and antimicrobial prophylaxis until polymorphonuclear cells numbered more than 1000/μL. All febrile episodes were treated with cephalosporin and aminoglycoside.
Supportive platelet transfusions were administered only in the presence of overt hemorrhage or if the platelet count was less than 30 000/μL with or without laboratory signs of severe coagulopathy (fibrinogen less than 150 mg/dL and fibrinogen degradation products (FDPs) greater than 40 μg/mL or X fragments of FDPs (XDPs) greater than 400 μg/mL). Platelet transfusion data are expressed as the median number of platelet units transfused during the considered period of treatment. In the case of single-donor apheresis, the transfusions provided the equivalent of 8 platelet units. When needed, it was common practice to transfuse 1 U platelets/10 kg body weight. As for prophylaxis and treatment of coagulopathy during induction, any decision was left to the discretion of the participating center.
Packed red blood cell units were transfused to maintain hemoglobin levels of 8 g/dL or higher.
Evaluation of response
Criteria for CR were the presence of normal BM cellularity with less than 5% of leukemic promyelocytes, a normal coagulation profile, polymorphonuclear cells numbering 1500/μL or more, and platelets numbering 100 000/μL or more. Resistant disease was defined as persistence of more than 5% leukemic promyelocytes 40 days after the start of treatment.
Toxicity
Acute and subacute toxicities were graded according to the World Health Organization (WHO) recommendations.
Follow-up
During the first 2 years of CR, physical examination was performed and clinical chemistry, blood cell count, and bone marrow aspirate were evaluated each month. After the first 2 years of follow-up, physical examination, clinical chemistry, blood cell count, and bone marrow aspirate were repeated every 3 months or when clinically required for another 3 years. Thereafter, patients were followed up annually with a physical examination and a blood cell count, but bone marrow was aspirated only if clinically required.
Randomization
Investigators were masked to the potential treatment assignment by central randomization performed according to permuted blocks. An approximate balance between the 2 arms of randomization was maintained within and across participating centers.
Statistical analysis
The study was designed to permit a 33% reduction in the rate of event, adding Ara-C in induction or maintenance in postconsolidation therapy. Therefore, 76 events in each induction or postconsolidation arm were needed to achieve a significance level of 0.05 with a power (1-β) of 0.80 after a follow-up period of 5 years from the inclusion of the last patient in the protocol.
Event-free survival (EFS) was defined as the time from first randomization to the event. An event in EFS was failure to achieve CR, relapse after a prior CR, or death from any cause. The endpoints of disease-free survival (DFS) and overall survival (OS) were also analyzed. During DFS, all relapses and deaths in CR were considered failures. For OS, death from any cause was considered failure. DFS was defined as the time from the achievement of CR to relapse or death in CR. OS was defined as the time from first randomization to death from any cause.
Analysis was based on follow-up data as of September 2000. All patients were evaluated according to the treatment assigned at randomization using intention-to-treat analysis. Characteristics of the patients and their responses to treatment were compared using χ2analysis or Fisher exact test when appropriate.
Survival distributions were estimated according to the Kaplan-Meier method.15 Differences in survival distributions were tested with the log-rank test,16 and all reportedP values were 2-sided. Adjusted Cox regression analysis was used to determine the influence of prognostic factors on the primary treatment effect.17
Results
Accrual
Between March 1989 and June 1993, 280 consecutive patients from 43 centers of GIMEMA were registered in the study. Of these, 13 patients were found to be ineligible because of misdiagnosis (n = 5), age older than 62 years (n = 1), previous neoplastic disease (n = 3), and severe organ failure (n = 4).
Of the 267 eligible patients, 139 (52%) were males, and 202 (76%) had hemorrhage at diagnosis. Median age was 36.9 years (range, 12.3-61.7 years), median WBC count was 2600/μL (range, 100/μL-161 600/μL), and median platelet count was 24 000/μL (range, 1000/μL-201 000/μL).
Induction therapy
All 267 patients eligible for the study were randomized; however, 2 died before the initiation of treatment, 2 refused the assigned treatment, 4 did not complete the induction phase because of toxicity, and 2 had protocol violations. Therefore, 257 patients were fully evaluable for induction—131 in arm A and 126 in arm B.
Complete remission.
Among the 131 patients who received IDA as induction treatment, 100 (76.3%) achieved CR, 11 (8.4%) were resistant, and 20 (15.3%) died during induction. Of the 126 patients who received IDA + Ara-C, 84 (66.6%) had CR, 15 were resistant (11.9%), and 27 died (21.4%). These differences between the 2 arms were not statistically significant. Moreover, including the 10 patients previously considered not evaluable for induction, this difference remains not statistically significant. In particular, the CR rates for arm A and arm B were 74.4% and 64.5%, respectively.
Toxic effects.
The causes of induction deaths in arm A were early (before day 7) and late (after day 7) hemorrhage in 5 and 2 patients, respectively; severe infection in 8 patients; and renal, hepatic, and cardiac failure in 3, 1, and 1 patients, respectively. The causes of induction death in arm B were early (before day 7) and late (after day 7) hemorrhage in 11 and 5 patients respectively; severe infection in 10 patients; and renal failure in 1 patient. Overall there were 7 hemorrhagic deaths in arm A and 16 in arm B (χ2 statistic, 4.26;P < .05) for a total of 23 hemorrhagic deaths during induction. Moreover, there were 18 deaths from infection and 6 from organ failure (4 renal, 1 hepatic, and 1 cardiac), equally distributed between the 2 randomization arms (Table1).
Deaths during induction
| Cause . | IDA . | IDA + Ara-C . | Total . |
|---|---|---|---|
| Early hemorrhage (day 7 or before) | 5 | 11 | 16 |
| Late hemorrhage (after day 7) | 2 | 5 | 7 |
| Infection | 8 | 10 | 18 |
| Renal failure | 3 | 1 | 4 |
| Hepatic failure | 1 | — | 1 |
| Cardiac failure | 1 | — | 1 |
| Cause . | IDA . | IDA + Ara-C . | Total . |
|---|---|---|---|
| Early hemorrhage (day 7 or before) | 5 | 11 | 16 |
| Late hemorrhage (after day 7) | 2 | 5 | 7 |
| Infection | 8 | 10 | 18 |
| Renal failure | 3 | 1 | 4 |
| Hepatic failure | 1 | — | 1 |
| Cardiac failure | 1 | — | 1 |
Other grade 3 and 4 toxicities were equally distributed among the 2 arms (Table 2). Median times to WBC count greater than 1000/μL and platelet level greater than 100 000/μL were 23 days (range, 12-54 days) and 19 days (range, 10-36 days) in arm A and 21 days (range, 11-49 days) and 18 days (range, 10-65 days) in arm B.
Toxicity during induction
| Type of toxicity (WHO grade 3-4) . | IDA . | IDA + Ara-C . | Total . |
|---|---|---|---|
| Mucositis | 22 | 21 | 43 |
| Infection | 24 | 16 | 40 |
| Hemorrhage | 15 | 20 | 35 |
| Vomiting | 8 | 14 | 22 |
| Hepatic | 10 | 9 | 19 |
| Diarrhea | 5 | 5 | 10 |
| Renal | 5 | 3 | 8 |
| Cardiac | 2 | 2 | 4 |
| Type of toxicity (WHO grade 3-4) . | IDA . | IDA + Ara-C . | Total . |
|---|---|---|---|
| Mucositis | 22 | 21 | 43 |
| Infection | 24 | 16 | 40 |
| Hemorrhage | 15 | 20 | 35 |
| Vomiting | 8 | 14 | 22 |
| Hepatic | 10 | 9 | 19 |
| Diarrhea | 5 | 5 | 10 |
| Renal | 5 | 3 | 8 |
| Cardiac | 2 | 2 | 4 |
Supportive care.
Mean ± standard error of platelet transfusions (considering 1 apheresis unit equivalent to 8 random units of platelets transfused) was 76 ± 14.3 and 68 ± 5.3 in patients randomized to arm A and arm B, respectively. The number (mean ± standard error) of packed red cells transfused was 10.4 ± 0.4 and 9.5 ± 0.4 in arm A and arm B, respectively.
Patients in arm A received intravenous antibiotic therapy for 20.8 ± 0.9 days and were hospitalized for a total of 35.5 ± 1.0 days, whereas patients randomized to arm B received intravenous antibiotic therapy for 20.2 ± 1.0 days and were hospitalized for a total of 34.7 ± 1.2 days.
Data on antihemorrhagic treatment were available in 246 of 257 (95.7%) evaluable patients for induction (122 in arm A and 124 in arm B). Of these 246 patients, 88 (35.8%) (40.2% in arm A and 31.5% in arm B) received only platelet transfusions, 100 (40.7%) (38.5% in arm A and 42.7% in arm B) received tranexamic acid at a dosage of 100 mg/kg body weight as a continuous infusion, 24 (9.8%) (8.2% in arm A and 11.3% in arm B) received prophylactic heparin (intravenously in 12 and subcutaneously in 12), 9 (3.7%) received antithrombin III (AT III) concentrates, and 4 (1.6%) received aprotinin prophylaxis. The remaining 21 (8.5%) patients received antihemorrhagic treatment consisting of a combination of 2 of the previous drugs.
Follow-up of consolidation therapy
Of the 184 patients who had CR, 10 (5.4%) did not initiate consolidation therapy because of toxicity (n = 7) or death in CR (n = 3) (Table 3). Therefore, the first consolidation cycle was administered to 174 patients. During this cycle 4 patients died, 2 had severe toxicity, 2 had protocol violations, and 1 was lost to follow-up. As a consequence, the second consolidation cycle was administered to 165 patients. Of these patients, 9 were withdrawn because of toxicity, 4 died while in CR, 2 underwent allogeneic bone marrow transplantation, 2 refused to continue the protocol, and 2 had protocol violations. The third consolidation cycle was, therefore, initiated in 146 patients. During and at recovery from the third cycle, there were 2 protocol violations, 14 patients underwent bone marrow transplantation, 2 patients had relapses before randomization to maintenance treatment, 7 refused to be randomized, 1 was not randomized because of toxicity, 2 were lost to follow-up, and 2 died while in CR. Therefore, 116 of 174 (66.6%) patients who initiated the consolidation phase were available for randomization to maintenance or observation group. Of the 16 patients who underwent allogeneic bone marrow transplantation, 7 were randomized to arm A and 9 to arm B.
Follow-up of consolidation therapy
| Treatment step . | No. patients . | Toxicity . | Reasons for going off study . | Total . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Death in CR . | Violation . | Refusal . | Lost to FU . | BMT . | Relapse . | ||||
| CR | 184 | 7 | 3 | — | — | — | — | — | 10 |
| 1st consolidation | 174 | 2 | 4 | 2 | — | 1 | — | — | 9 |
| 2nd consolidation | 165 | 9 | 4 | 2 | 2 | — | 2 | — | 19 |
| 3rd consolidation | 146 | 1 | 2 | 2 | 7 | 2 | 14 | 2 | 30 |
| Total | 19 | 13 | 6 | 9 | 3 | 16 | 2 | 68 | |
| Treatment step . | No. patients . | Toxicity . | Reasons for going off study . | Total . | |||||
|---|---|---|---|---|---|---|---|---|---|
| Death in CR . | Violation . | Refusal . | Lost to FU . | BMT . | Relapse . | ||||
| CR | 184 | 7 | 3 | — | — | — | — | — | 10 |
| 1st consolidation | 174 | 2 | 4 | 2 | — | 1 | — | — | 9 |
| 2nd consolidation | 165 | 9 | 4 | 2 | 2 | — | 2 | — | 19 |
| 3rd consolidation | 146 | 1 | 2 | 2 | 7 | 2 | 14 | 2 | 30 |
| Total | 19 | 13 | 6 | 9 | 3 | 16 | 2 | 68 | |
Toxicity during consolidation.
The toxic effects of consolidation cycles, grade 3 and 4 according to WHO grading system, are listed by induction randomization arm in Table4.
Toxicity during each consolidation course
| Type of toxicity (WHO grade 3-4) . | 1st course (n = 174) . | 2nd course (n = 165) . | 3rd course (n = 146) . | |||
|---|---|---|---|---|---|---|
| Arm A . | Arm B . | Arm A . | Arm B . | Arm A . | Arm B . | |
| Mucositis | — | 1 | 5 | 3 | — | 2 |
| Infection | 4 | 2 | 3 | 5 | 3 | 2 |
| Hemorrhage | — | — | 1 | — | 3 | — |
| Vomiting | 2 | 5 | — | 3 | — | 4 |
| Hepatic | 1 | 1 | 7 | 1 | 2 | 1 |
| Diarrhea | — | — | — | — | 1 | — |
| Renal | — | — | — | — | 1 | — |
| Cardiac | — | — | 1 | — | — | — |
| Total | 7 | 9 | 17 | 12 | 10 | 9 |
| Type of toxicity (WHO grade 3-4) . | 1st course (n = 174) . | 2nd course (n = 165) . | 3rd course (n = 146) . | |||
|---|---|---|---|---|---|---|
| Arm A . | Arm B . | Arm A . | Arm B . | Arm A . | Arm B . | |
| Mucositis | — | 1 | 5 | 3 | — | 2 |
| Infection | 4 | 2 | 3 | 5 | 3 | 2 |
| Hemorrhage | — | — | 1 | — | 3 | — |
| Vomiting | 2 | 5 | — | 3 | — | 4 |
| Hepatic | 1 | 1 | 7 | 1 | 2 | 1 |
| Diarrhea | — | — | — | — | 1 | — |
| Renal | — | — | — | — | 1 | — |
| Cardiac | — | — | 1 | — | — | — |
| Total | 7 | 9 | 17 | 12 | 10 | 9 |
Serious infections were observed in 19 patients, and their incidence was equally distributed among the 3 courses. Vomiting and hepatic toxicity were experienced by 14 and 13 patients, respectively. Mucositis developed in 11 patients and was mainly observed during the second course. Only 4 patients had severe hemorrhage secondary to thrombocytopenia. Median numbers of days to polymorphonuclear cell levels greater than 1000/μL and platelet levels greater than 100 000/μL after the first, second, and third consolidation courses were 18 (range, 3-32) and 16 (range, 6-40); 23 (range, 8-88) and 25 (range, 10-77); 21 (range, 11-49) and 21 (range, 7-80), respectively.
Supportive care during consolidation.
Platelet and packed red blood cell transfusion levels and days on antibiotic treatment during the 3 consolidation courses are summarized in Table 5. No statistically significant differences were observed in supportive treatment during the 3 consolidation cycles.
Supportive care during consolidation
| Type of supportive care . | 1st course . | 2nd course . | 3rd course . |
|---|---|---|---|
| No. platelet transfusions | 6.0 ± 1.7 | 8.8 ± 7.4 | 5.7 ± 8.3 |
| No. packed red cell transfusions | 2.9 ± 2.3 | 3.6 ± 3.0 | 3.0 ± 2.5 |
| Days of antibiotic therapy | 6.3 ± 7.6 | 2.8 ± 1.0 | 4.8 ± 6.6 |
| Days of hospitalization | 22.8 ± 16.1 | 8.2 ± 8.4 | 14.9 ± 13.8 |
| Type of supportive care . | 1st course . | 2nd course . | 3rd course . |
|---|---|---|---|
| No. platelet transfusions | 6.0 ± 1.7 | 8.8 ± 7.4 | 5.7 ± 8.3 |
| No. packed red cell transfusions | 2.9 ± 2.3 | 3.6 ± 3.0 | 3.0 ± 2.5 |
| Days of antibiotic therapy | 6.3 ± 7.6 | 2.8 ± 1.0 | 4.8 ± 6.6 |
| Days of hospitalization | 22.8 ± 16.1 | 8.2 ± 8.4 | 14.9 ± 13.8 |
Values are mean ± SD.
Maintenance therapy
After completion of the 3 consolidation cycles, 116 patients were randomized to maintenance therapy (n = 58) or observation (n = 58). Five patients in the maintenance arm did not complete the programmed therapy because of toxicity, 1 underwent allogeneic bone marrow transplantation, and 1 refused to continue maintenance. Therefore, of the 58 patients randomized to maintenance for 2 years, 51 (87.9%) completed the maintenance arm.
Event-free, overall, and disease-free survival
Effect of induction treatment.
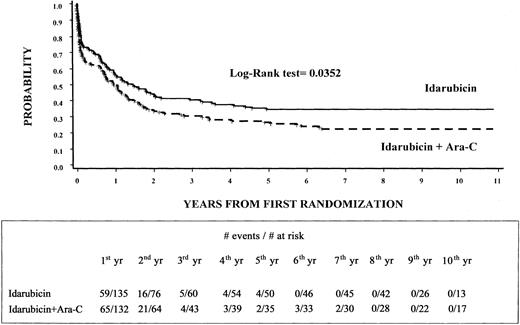
The estimated 8-year EFS rate was 35% (95% CI, 27%-43%) for the 135 patients randomly assigned to arm A and 23% (95% CI, 16%-30%) for the 132 patients assigned to arm B (P = .0352) (Figure2). Moreover, DFS was 46% (95% CI, 37%-56%) for patients in arm A and 32% (95% CI, 22%-43%) for those in arm B (P = NS). Finally, the OS rate was 45% (95% CI, 37%-54%) for patients randomized to arm A and 36% (95% CI, 27%-44%) for those randomized to arm B (P = NS).
Effect of maintenance treatment.
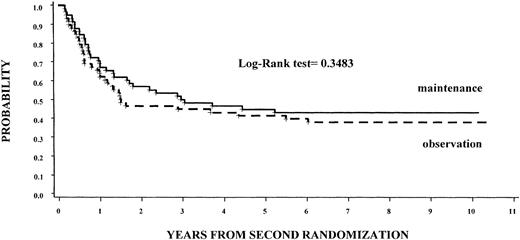
Of the 58 patients assigned to maintenance treatment 32 (55%) had relapses compared with 35 (60%) assigned to observation (P = NS). Seven years from the randomization time, the DFS rate was 43% (95% CI, 30%-56%) for those receiving maintenance therapy versus 38% (95% CI, 25%-50%) for those assigned to the observation group (P = NS), regardless of the induction regimen (Figure 3). The OS rates were 51% for those receiving maintenance therapy versus 61% for those assigned to the observation group (P = NS), regardless of the induction regimen.
Combined effects of induction and maintenance treatments.
Events occurred in 17 of 32 (53%) patients who received IDA followed by maintenance therapy and in 14 of 29 (48%) patients treated with IDA and no maintenance. They occurred in 16 of 26 (61%) patients treated with IDA + Ara-C followed by maintenance and in 22 of 29 (76%) who received combination induction treatment not followed by maintenance. As a consequence, the DFS rates for the above considered subgroups were 47%, 52%, 24%, and 38%, respectively (P = NS). Results of Cox model considering the combined effects of the 2 randomization arms indicated no effect for maintenance treatment (P = NS) and a trend in favor of induction treatment with IDA alone (P = .0570).
Prognostic factors for EFS.
The disease-related variables of WBC count, age, lactate dehydrogenase level, hemoglobin level, and platelet count significantly influenced EFS at univariate analysis. After adjustment for disease-related variables, multivariate analysis revealed that EFS was favorably influenced by induction treatment with IDA alone (P = .0101) and was unfavorably influenced by WBC count greater than 3000/μL (P = .0001) and increasing age (P = .0251) (Table 6).
Cox proportional hazards model on EFS
| Parameters . | RR . | 95% CI . | P . |
|---|---|---|---|
| Age | 1.014 | 1.002 -1.026 | .0251 |
| WBC count | |||
| Lower than 3000/μL | 1 | — | — |
| Higher than 3000/μL | 1.307 | 1.141 -1.496 | .0001 |
| 1st randomization | |||
| IDA | 1 | — | — |
| IDA + Ara-C | 1.495 | 1.101 -2.031 | .0101 |
| Parameters . | RR . | 95% CI . | P . |
|---|---|---|---|
| Age | 1.014 | 1.002 -1.026 | .0251 |
| WBC count | |||
| Lower than 3000/μL | 1 | — | — |
| Higher than 3000/μL | 1.307 | 1.141 -1.496 | .0001 |
| 1st randomization | |||
| IDA | 1 | — | — |
| IDA + Ara-C | 1.495 | 1.101 -2.031 | .0101 |
Discussion
The policy of using daunorubicin (DNR) or idarubicin (IDA) alone as induction therapy for APL has always been a characteristic of the Italian cooperative group GIMEMA.11,18 Therefore, to help determine whether cytarabine is a useful drug in the induction treatment of newly diagnosed hypergranular APL, the group designed this prospective, randomized trial before the so-called ATRA era. Multivariate analysis of this study, closed to patient accrual on June 1993, indicated that WBC count greater than 3000/μL, increasing age, and randomization to the IDA arm at diagnosis were the main prognostic factors influencing the EFS of these patients. However, we must take into account that the total dose of IDA was different in the 2 randomized arms—60 mg in arm A and 48 mg in arm B. This difference is important and may explain the trend to a better CR rate considering that a dose efficacy relationship of anthracycline has been shown in APL.8 As for CR rate, toxicity, and supportive care, no differences were observed between the 2 induction arms, even though a trend to a better CR rate was obtained in patients treated with IDA alone. Acute cardiotoxicity was limited to 4 (2%) patients, 2 in each randomization arm, and no chronic or late cardiotoxicity has been observed so far. These data indirectly confirm that IDA is less cardiotoxic than DNR, and, as suggested, it can be used up to a cumulative dose of 150 mg/m2 in patients without cardiac dysfunction.19 20 Moreover, patients randomized to maintenance therapy with 6-MP and MTX did not have better outcomes than those randomized to observation.
Although WBC count greater than 3000/μL and increasing age are well-known prognostic factors in APL, little is known about the influence of anthracycline monochemotherapy on EFS. A possible explanation for this behavior could be that higher doses of anthracycline, such as those used in monochemotherapy, play a favorable role in the control of the APL coagulopathy through rapid reduction of leukemic promyelocytes. In fact, the number of hemorrhagic deaths observed during induction in arm B compared to those observed in arm A were significantly higher (16 vs 7; P < .05). As a consequence, considering that the difference in EFS curves occur early, this higher rate of hemorrhagic deaths observed in arm B may partially explain the difference observed in EFS curves. The early study of Bernard et al in 1973 indicated the high sensitivity of APL to DNR.7 This result was later confirmed by the same group21 and by other investigators.22,23However, the use of this peculiar approach to APL was limited to few investigators because most were skeptical about the use of an induction protocol without cytarabine. The introduction of ATRA and the evidence of its dramatic effect on the outcome of APL rapidly convinced all the investigators that APL was unique among the AMLs and, therefore, required appropriate tailor-made treatments by combining ATRA and chemotherapy. Despite this recognition of the peculiarity of APL, there are still concerns about whether cytarabine should be used in the treatment of APL. A recent retrospective analysis of the SWOG8 has demonstrated that CR and survival rates of 141 APL patients were significantly influenced by higher dose DNR and that the use of high-dose cytarabine, during consolidation, had a significant detrimental effect on outcomes for these patients. Therefore, from this SWOG study, it seems that cytarabine has a limited role in APL treatment. As a consequence, based on the retrospective SWOG analysis on the early results of the GIMEMA studies with the AIDA protocol,12,13 and particular on the results obtained by the M.D. Anderson Group in the only study that did not include any Ara-C at any time,24 the Spanish cooperative group PETHEMA has recently published the results of a study indicating that a modification of the original AIDA protocol through the elimination of cytarabine, etoposide, and thioguanine from the consolidation program resulted in high antileukemic efficacy and reduced toxicity.25 The reasons why APL is highly sensitive to the anthracycline drugs are unclear; a possible important factor might be the absence on APL cells of the multidrug resistance glycoprotein P-170.26
In conclusion, the main result of this multicenter randomized study designed before the ATRA era is the demonstration that cytarabine is not needed for the induction treatment of hypergranular APL, but no conclusions can be drawn regarding the requirement of cytarabine during consolidation courses and its role overall. Therefore, this result may only contribute to determining which may be the best induction chemotherapy that can be combined with ATRA during the treatment of APL. Further studies are needed to investigate whether cytarabine has a role during the consolidation phase of APL.
We thank Sandra Simone, who managed the data entry, Dr Paola Fazi and Dr Marco Vignetti, who participated in the statistical analysis and coordinated the randomization procedures, and the nurses and physicians at the participating institutions.
The following Centers and investigators from the GIMEMA Group participated in this study:
Ancona: Ematologia, Nuovo Ospedale Le Torrette, (P. Leone, M. Montillo, S. Rupoli); Avellino: Ematologia, Ospedale Civile (E. Volpe, N. Cantore); Aviano: Ematologia, Centro di riferimento Oncologico (G. Cartei, I. Milan); Bari: Ematologia, Policlinico Universitario (V. Liso, G. Specchia); Bergamo: Divisione di Ematologia, Ospedale Riuniti (T. Barbui, M. Buelli); Bologna: Istituto di Ematologia ed Oncologia (M. Baccarani, G. Visani); Bolzano: Ematologia, Ospedale Centrale Regionale (P. Coser, P. Fabris); Cagliari: Ematologia, Ospedale Oncologico A. Businco (G. Broccia, W. Deplano); Catania: Ematologia, Ospedale Ferrarotto (R. Giustolisi, F. Di Raimondo); Catanzaro: Ospedale Regionale A. Pugliese (A. Peta); Cremona: Istituti Ospitalieri (P. Bodini, S. Morandi); Cuneo: Ospedale S. Croce (A. Gallamini); Firenze: Ematologia, Policlinico di Careggi (P. Rossi Ferrin, F. Leoni); Foggia: Ematologia, Ospedali Riuniti (M. Monaco, E. Capussela); Genova: Ematologia, Azienda Ospedaliera S. Martino (E. Damasco, R. Cerri) and Università degli Studi (M. Gobbi, M. Clavio); Latina: Divisione di Ematologia Ospedale S. Maria Goretti (A. De Blasio, A. Chierichini); Milano: Istituto di Medicina Interna dell'Università (G. Lambertenghi, C. Annaloro) and Ospedale Niguarda Ca' Granda (E. Morra, A.M. Nosari); Napoli: Ematologia, Università Federico II (B. Rotoli, C. Selleri), and Divisione di Ematologia, Ospedale A.Cardarelli (F. Ferrara); Nuoro: Ematologia, Ospedale S. Francesco (A. Gabbas, G. Latte); Orbassano: Az. Ospedaliera “S. Luigi Gonzaga” (G. Saglio, F. Vischia); Palermo: Ematologia, Università degli Studi (G. Mariani, M. Musso), Istituto di Clinica Medica (P. Citarella, S. Miceli) and Ematologia, Ospedale Cervello (S. Mirto); Pavia: Medicina Interna (E. Ascari, R. Invernizzi) and Ematologia (M. Lazzarino) IRCCS San Matteo; Perugia: Medicina Interna (A. Del Favero, A.M. Liberati) and Ematologia (M. Martelli, A. Tabilio) Policlinico Monteluce; Pescara: Ematologia, Ospedale Civile (G. Fioritoni, A. Recchia); Potenza: Ematologia, Ospedale S. Carlo (F. Ricciuti, M. Pizzuti); Reggio Calabria: Ematologia, Azienda Ospedaliera Bianchi-Melacrino-Morelli (F. Nobile, D. Vincelli); Rome: Dipartimento di Biotecnologie Cellulari ed Ematologia, Università La Sapienza (F. Mandelli, G. Avvisati, F. Lo-Coco, M.C. Petti, A. Testi, M.L. Vegna); Ematologia, Università Cattolica del Sacro Cuore (G. Leone, S. Sica); Ematologia, Università Tor Vergata (S. Amadori, S. Buccisano); San Giovanni Rotondo: Ematologia, Ospedale Casa Sollievo della Sofferenza (M. Carotenuto, S. Ladogana, L. Melillo); Torino: Ematologia, Azienda Ospedaliera S. Giovanni Battista (E. Gallo, B. Allione) and Ematologia, Università degli Studi (M. Boccadoro, D. Ferrero); Udine: Ematologia, Università degli Studi Policlinico (R. Fanin, D. Russo); Verona: Ematologia, Università degli Studi, Ospedale Policlinico Borgo Roma (G. Zizzolo, D. Veneri); Vicenza: Divisione di Ematologia, Ospedale S. Bortolo (F. Rodeghiero, E. Di Bona)
Prepublished online as Blood First Edition Paper, July 5, 2002; DOI 10.1182/blood-2002-02-0352.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Giuseppe Avvisati, Ematologia, UniversitàCampus Bio-Medico, 83 Via Emilio Longoni, 00155 Rome, Italy; e-mail:g.avvisati@unicampus.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal