Abstract
De novo erythroleukemia (EL) is a rare disease. Reported median survival are poor and vary from 4 to 14 months. The value of hematopoietic stem cell transplantation (HSCT) for EL is unknown. This EBMT registry study reports on the largest series of patients with EL treated with HSCT in first complete remission—103 autologous and 104 HLA identical sibling allogeneic HSCT. Outcome and identification of prognostic factors for each type of transplantation were evaluated. For autologous HSCT, outcome at 5 years showed a leukemia-free survival (LFS) of 26% ± 5%, a relapse incidence (RI) of 70% ± 6%, and a transplant-related mortality (TRM) of 13% ± 4%. By multivariate analysis, the only prognostic factor was age. For allogeneic HSCT, outcome at 5 years showed an LFS of 57% ± 5%, an RI of 21% ± 5%, and a TRM of 27% ± 5%. By multivariate analysis, prognostic factors were graft-versus-host disease and age. This study represents the largest series of de novo EL treated with HSCT and shows that allogeneic HSCT is by far the most effective treatment.
Introduction
Erythroleukemia (EL) is an uncommon form of acute myeloblastic leukemia that was recognized in 1912 by Copelli1 as a hematologic disorder named erythematosus. In 1917, Di Guglielmo2 reported additional observations of a neoplastic disorder with proliferation of the erythroid precursors and involvement of the granulocytic series. In 1940, Moeschlin3 definitively established the term erythroleukemia, and later, Dameshek proposed the term Di Guglielmo's syndrome4 describing 3 sequential phases of morphologic alteration within the bone marrow: an erythremic phase, an erythromyeloblastic phase, and a myeloblastic phase indistinguishable from acute myeloblastic leukemia.5
In 1976, the French-American-British (FAB) cooperative group classified EL as acute myeloid leukemia (AML) M6.6 In fact, criteria for diagnosis of AML M6 were confused with myelodysplastic syndrome, megaloblastic anemia, congenital dyserythropoietic anemia, and secondary acute leukemia. Finally, the 1985 revised FAB classification defined EL as leukemia comprising an erythroid component of at least 50% and a myeloid component of at least 30% of the nonerythroid cells.7
EL represents only 1% to 3% of all leukemias and 4.5% of all AMLs. The age range of patients with EL is 2 to 85 years, with a median in the fifth and sixth decades.8 EL is de novo for approximately half of patients, or secondary to myelodysplastic syndrome or chemotherapy and is frequently associated with multiple chromosomal abnormalities.9 It has been reported that EL is less sensitive to chemotherapy than other AML subtypes, that median survival varies from 4 to 14 months,8,10-15 and that it has a tendency to transform into other AML subtypes.9
Whereas the benefit of high-dose therapy with autologous or allogeneic hematopoietic stem cell transplantation (HSCT) for AML is now well established by several randomized studies comparing chemotherapy to autologous or allogeneic HSCT,16-20 the real value of high-dose therapy with HSCT for EL is unknown. In this study we used the prospectively collected data of the EBMT acute leukemia registry to analyze the fate of patients with de novo EL who underwent transplantation while in first complete remission (CR1). This study reports the largest series of patients with such a rare disease treated with autologous or allogeneic HSCT.
Patients, materials, and methods
Patients
The study concerned patients receiving transplants from January 1986 to December 2000 in 117 European centers, and consisted of patients older than 16 years with de novo EL who underwent transplantation in CR1. Results were reported to the Acute Leukemia (AL) registry of the EBMT. Secondary acute leukemias were usually reported to the EBMT Chronic Leukemia Registry, and familial ELs were not included.
EBMT teams are supposed to submit consecutive cases, though for logistic reasons this was not the case for all teams throughout the observation period from 1979 to 2000. Since 1996, EBMT is annually subjected to a possible audit program. Participating institutions are listed in Appendix 1.
The AL registry contained information on 312 adult patients with de novo EL who were registered from January 1986, with the AML M6 FAB subtype according to the 1985 revised FAB classification. Among these, 207 patients underwent HSCT while in CR1—103 autologous HSCT and 104 HLA identical sibling allogeneic HSCT.
To ensure the reliability of the diagnosis of EL, a subset of 92 (43.6%) initial diagnostic marrow aspirates was reviewed by a panel of experts to confirm the diagnosis of EL according to the 1985 revised FAB classification. Confirmation in this subset was obtained in 95.6% of the cases.
Cytogenetics data were available in the registry for 112 patients. Information on karyotype analysis was given as not performed, failed, normal, or abnormal.
Statistical analysis
The aim of this study was to evaluate the outcome of patients with de novo EL in CR1 undergoing autologous HSCT or HLA identical sibling allogeneic HSCT and to identify prognostic factors for autologous and allogeneic HSCT. It is not a comparative study between autologous and allogeneic HSCT for AML but a study demonstrating actual outcome of a rare disease (EL) treated with high-dose therapy and HSCT.
All analyses were performed using the SPSS computer program (SPSS, Chicago, IL). Values reported for quantitative variables were median and range.
For all analyses, continuous variables were categorized as follows: each variable was first divided into 5 categories at approximately the 20th, 40th, 60th, and 80th percentiles. If the relative event rates (ratio of the observed number of events to the expected number of events in a category assuming no variation across categories) in 2 or more adjacent categories (and the mean times-to-event) were not substantially different, these categories were grouped. If a linear trend was observed in the relative event rates, the variable was used as a continuous factor. Otherwise, the median was used as a cut-off point.
Leukemia-free survival (LFS) was defined as survival without evidence of relapse; the event under study was death or relapse. Relapse was defined as hematologic recurrence at any site. To evaluate the probability of relapse incidence (RI), patients dying from direct toxicity of the procedure or from any other cause not related to leukemia were censored. Transplant-related mortality (TRM) was defined as death occurring in continuous CR after HSCT.
Patients were censored at the time of relapse or at last follow-up. Probabilities of LFS, RI, and TRM were estimated by the product-limit method.21 By univariate analysis, the following variables were analyzed: patient age and sex, leukocyte count at diagnosis, year of transplantation, time from diagnosis to CR1, time from CR1 to HSCT, total body irradiation (TBI), busulfan or other chemotherapy in the conditioning regimen, and source of stem cells. Acute graft-versus-host disease (GVHD) for allogeneic HSCT was included in the model as a time-dependent covariate. The significance of differences between curves was estimated by the log-rank test (Mantel-Cox). All variables associated with outcomes withP < .1 in univariate analysis were included in a multivariate analysis. Then a backward stepwise procedure was used to select the covariates (P ≤ .05) included in the Cox proportional hazards model.22
Results
Distribution of patients receiving autologous HSC transplants
One hundred three patients underwent high-dose therapy followed by autologous HSCT (Table 1). The median age was 40 years (range, 17-69 years), the male/female ratio was 57:46, and the median leukocyte count at diagnosis was 3.8 × 109/L (range, 0.9 × 109/L-32 × 109/L).
Distribution of adult patients with de novo EL treated by autologous or identical sibling allogeneic HSCT in first CR
| . | Autologous HSCT (103 patients) . | Identical sibling allogeneic HSCT (104 patients) . |
|---|---|---|
| Median age, y (range) | 40 (17-69) | 36 (16-63) |
| Male/female ratio | 57:46 | 54:50 |
| Median leukocyte count at diagnosis, × 109/L (range) | 3.8 (0.9-32) | 3 (0.8-200) |
| Median time from diagnosis to CR1, d (range) | 44 (24-199) | 47 (21-245) |
| Median time from CR1 to HSCT, d (range) | 120 (37-324) | 99 (10-351) |
| Conditioning regimen, % | ||
| With TBI | 26 | 56 |
| With busulfan | 38 | 31 |
| Other chemotherapy alone | 26 | 9 |
| Missing data | 10 | 5 |
| Source of stem cells, % | ||
| Bone marrow | 51 | 74 |
| Peripheral blood | 43 | 23 |
| Both | 6 | 3 |
| GVHD prophylaxis, % | ||
| Cyclosporin + methotrexate | — | 62 |
| T-cell depletion | — | 22 |
| Other | — | 17 |
| . | Autologous HSCT (103 patients) . | Identical sibling allogeneic HSCT (104 patients) . |
|---|---|---|
| Median age, y (range) | 40 (17-69) | 36 (16-63) |
| Male/female ratio | 57:46 | 54:50 |
| Median leukocyte count at diagnosis, × 109/L (range) | 3.8 (0.9-32) | 3 (0.8-200) |
| Median time from diagnosis to CR1, d (range) | 44 (24-199) | 47 (21-245) |
| Median time from CR1 to HSCT, d (range) | 120 (37-324) | 99 (10-351) |
| Conditioning regimen, % | ||
| With TBI | 26 | 56 |
| With busulfan | 38 | 31 |
| Other chemotherapy alone | 26 | 9 |
| Missing data | 10 | 5 |
| Source of stem cells, % | ||
| Bone marrow | 51 | 74 |
| Peripheral blood | 43 | 23 |
| Both | 6 | 3 |
| GVHD prophylaxis, % | ||
| Cyclosporin + methotrexate | — | 62 |
| T-cell depletion | — | 22 |
| Other | — | 17 |
Median time from diagnosis to CR1 was 44 days (range, 24-199 days), and from CR1 to autologous HSCT 120 days (range, 37-324 days). TBI was part of the conditioning regimen for 26% of patients, and busulfan for 38% of patients.
The source of stem cells was bone marrow in 51% of patients, peripheral blood in 43% of patients, and both in 6% of patients. Bone marrow was used from 1986 and peripheral blood from 1990.
Distribution of patients receiving HLA identical sibling allogeneic HSC transplants
One hundred four patients received high-dose therapy followed by allogeneic transplantation from an HLA identical sibling donor (Table1). The median age was 36 years (range, 16-63 years), the male/female ratio was 54:50, and the median leukocyte count at diagnosis was 3 × 109/L (range, 0.8 × 109/L-200 × 109/L).
Median time from diagnosis to CR1 was 47 days (range, 21-245 days), and from CR1 to HSCT 99 days (range, 10-351 days). Conditioning regimen was TBI for 56% of patients and busulfan for 31% of patients.
The source of stem cells was bone marrow in 74% of patients, peripheral blood in 23% of patients, and both in 3% of patients. Bone marrow was used from 1986 and peripheral blood from 1995. For GVHD prophylaxis, a combination of cyclosporin A and methotrexate was used in 62% of patients, T-cell depletion in 22%, and other types of prophylaxis in 17% of patients.
Cytogenetics analysis
Information on cytogenetics was available in 112 patients. In 27 patients karyotype analysis technically failed or was not performed.
In the remaining 85 evaluable patients, 52 had no chromosomal abnormalities and 33 had chromosomal abnormalities (described in only 27). For the 79 (52 + 27) informative patients, cytogenetics were reclassified according to Southwest Oncology Group (SWOG) as favorable, intermediate, unfavorable, and unknown17; 43 patients underwent allogeneic HSCT and 36 underwent autologous HSCT. Results are shown in Table 2.
HSCT for adult de novo EL in first CR
| Cytogenetic risk groups (SWOG criteria) . | Identical-sibling allogeneic HSCT n = 43 . | Autologous HSCT n = 36 . |
|---|---|---|
| Favorable | n = 0 | n = 0 |
| n = 0 | ||
| Intermediate | n = 30 | n = 28 |
| n = 58 | Normal, n = 28 | Normal, n = 24 |
| Trisomy 8, n = 2 | Trisomy 8, n = 4 | |
| Unfavorable | n = 8 | n = 4 |
| n = 12 | abn 3q and 9q | abn 20q and t(5;7),del(17q) |
| abn 11q | −7 in complex karyotype | |
| del(7) | abn 3q, −7, and ins(6p) | |
| abn 11q | del(5q),−7, abn 17p in complex karyotype | |
| abn 3q | ||
| −7 in complex karyotype | ||
| abn 3q | ||
| abn 20q | ||
| Unknown | n = 5 | n = 4 |
| n = 9 | i(18q) | Tetraploidy |
| Pseudo/hypodiploidy | Hypodiploidy | |
| del(6) | Hypodiploidy | |
| −20,+mar | Pseudo near-triploidy and tetraploidy | |
| Hypodiploidy |
| Cytogenetic risk groups (SWOG criteria) . | Identical-sibling allogeneic HSCT n = 43 . | Autologous HSCT n = 36 . |
|---|---|---|
| Favorable | n = 0 | n = 0 |
| n = 0 | ||
| Intermediate | n = 30 | n = 28 |
| n = 58 | Normal, n = 28 | Normal, n = 24 |
| Trisomy 8, n = 2 | Trisomy 8, n = 4 | |
| Unfavorable | n = 8 | n = 4 |
| n = 12 | abn 3q and 9q | abn 20q and t(5;7),del(17q) |
| abn 11q | −7 in complex karyotype | |
| del(7) | abn 3q, −7, and ins(6p) | |
| abn 11q | del(5q),−7, abn 17p in complex karyotype | |
| abn 3q | ||
| −7 in complex karyotype | ||
| abn 3q | ||
| abn 20q | ||
| Unknown | n = 5 | n = 4 |
| n = 9 | i(18q) | Tetraploidy |
| Pseudo/hypodiploidy | Hypodiploidy | |
| del(6) | Hypodiploidy | |
| −20,+mar | Pseudo near-triploidy and tetraploidy | |
| Hypodiploidy |
Results of karyotype at diagnosis classified according to the SWOG cytogenetics risk groups in 79 informative patients—52 with normal karyotype and 27 with abnormal karyotype.
Outcome following autologous HSCT
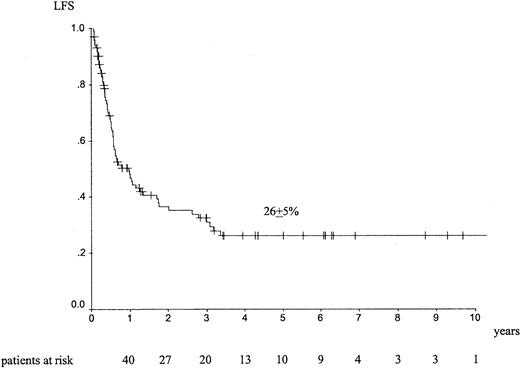
Median follow-up was 26 months (range, 1-157 months). The outcome at 5 years showed an LFS of 26% ± 5% (Figure1), an RI of 70% ± 6% (Figure2), a TRM of 13% ± 4%, and an overall survival (OS) of 34% ± 6%. Forty-four patients died of EL relapse, and 11 patients died of toxicity (5 infections, 1 graft failure, 3 hemorrhage, and 1 interstitial pneumonitis); information was missing for 2 patients.
LFS at 5 years in adult patients with de novo EL treated with autologous HSCT in CR1.
LFS at 5 years in adult patients with de novo EL treated with autologous HSCT in CR1.
RI at 5 years in adult patients with de novo EL treated with autologous HSCT in CR1.
RI at 5 years in adult patients with de novo EL treated with autologous HSCT in CR1.
Outcome following allogeneic identical sibling HSCT
Median follow-up was 46 months (range, 2-169 months). The outcome at 5 years showed an LFS of 57% ± 5% (Figure3), an RI of 21% ± 5% (Figure4), a TRM of 27% ± 5%, and an OS of 57% ± 5%. Seventeen patients died of EL relapse, and 26 died of toxicity (8 infections, 8 GVHD, 3 interstitial pneumonitis, 1 hemorrhage); information was missing for 6 patients.
LFS at 5 years in adult patients with de novo EL treated with identical sibling allogeneic HSCT in CR1.
LFS at 5 years in adult patients with de novo EL treated with identical sibling allogeneic HSCT in CR1.
RI at 5 years in adult patients with de novo EL treated with allogeneic identical sibling HSCT in CR1.
RI at 5 years in adult patients with de novo EL treated with allogeneic identical sibling HSCT in CR1.
Acute GVHD of grade 2 or more developed in 36% of patients. Of 87 patients alive and well at 100 days after transplantation, chronic GVHD developed in 41% (limited in 24% and extensive in 17%) of patients.
Prognostic factors
For autologous HSCT, univariate analyses indicated that ages below the median of 40 years was associated with a better LFS (34% ± 8% vs 19% ± 6%; P = .04), a trend for a lower RI (61% ± 8% vs 79% ± 7%; P = .08), and better survival (44% ± 8% vs 24% ± 7%; P = .045). Peripheral blood transplantation was associated with a trend for a lower TRM (5% ± 3% vs 20% ± 6%; P = .09). There was no statistical difference on LFS (23% ± 9% vs 27% ± 6%;P = .91) and on RI (76% ± 9% vs 66% ± 8%;P = .35) between peripheral blood and bone marrow. A conditioning regimen with TBI was associated with a better LFS than regimens containing busulfan or consisting of other chemotherapies (40% ± 10% vs 32% ± 8% vs 24% ± 9%;P = .03), respectively. With TBI, there was a trend for lower RI (63% ± 11% vs 67% ± 9% vs 79% ± 9%;P = .06). Using multivariate analysis, young age below the median value was associated with a better LFS (relative risk [RR] = 1.67 [1.02-2.73]; P = .044) and better OS (RR = 1.74 [1.01-3.04]; P = .05).
For allogeneic HSCT, univariate analyses indicated that female patients had a trend for a better LFS (67% ± 7% vs 49% ± 7%;P = .09). Acute GVHD of grade 2 or more was associated with a lower LFS (45% ± 8% vs 64% ± 6%;P = .053), a higher TRM (38% ± 8% vs 21% ± 6%;P = .07), and a lower OS (44% ± 8% vs 63% ± 7%;P = .047). Seventy-six patients aged 45 years and younger had a statistically significant better outcome than 26 patients older than 45 years. LFS was 64% ± 6% and 42% ± 10% (P = .02), TRM was 23% ± 5% and 39% ± 11% (P = .06), and OS was 63% ± 6% and 39% ± 11% (P = .014), respectively. No difference was found on RI—17% ± 5% and 31% ± 11% (P = .15), respectively. By multivariate analysis, acute GVHD of grade 2 or more was associated with higher TRM (RR = 2.65 [1.17-6.02];P = .02). Age older than 45 years was associated with lower LFS (RR = 2.1 [1.12-3.94]; P = .02), higher TRM (RR = 2.58 [1.16-5.74]; P = .02), and lower OS (RR = 2.14 [1.15-4.01]; P = .017).
Discussion
This EBMT study on 207 adult patients with de novo EL who underwent transplantation in CR1 indicates that HLA identical sibling allogeneic HSCT can cure more than 50% of patients. Indeed, the LFS at 5 years after allogeneic transplantation was 57% ± 5%; median survival was not reached. In contrast, it also indicates that outcome following autologous HSCT was poor—the LFS rate was 26% ± 5% at 5 years, and median survival was 12 months. The low RI observed after allogeneic HSCT (22% ± 5%), in contrast to the high RI after autologous HSCT (70% ± 6%), suggests the existence of a graft-versus-leukemia effect following allogeneic transplantation. Results with autologous transplantation were, in fact, not different from those of conventional chemotherapy alone, which showed median survival from 4 to 14 months.8 10-15
EL is a rare disease. This study is the only one giving actual information on HSCT in a large series of patients with EL. The low incidence of EL is indicated in the acute leukemia EBMT registry, in which only 559 (2.9%) patients with EL are registered for 19 513 patients with de novo AML (M. L., unpublished data, December 2000). Therefore, outside the registry, it is difficult to obtain sufficient data to study EL. The identification of patients with EL in prospective studies of HSCT for de novo AML, when FAB subtype description is mentioned, confirms that EL represents a small proportion of patients—1.6% to 3%—of all patients included in the studies.16,19,23,24 In details it corresponds in the MRC study with 12 EL patients out of 381 patients (6 EL patients underwent autologous HSCT and 6 chemotherapy alone),16 and it corresponds in the EORTC study with 6 EL patients out of 362 patients (2 underwent chemotherapy, 2 autologous HSCT, and 2 allogeneic HSCT).19 In the Catalan study 5 EL patients out of 159 patients were randomized between autologous and allogeneic HSCT,23 and in the Goelam study 9 EL patients out of 517 patients were included.24
Data indicating the specific outcome of EL following allogeneic or autologous HSCT are scarce. In 1994, a Japanese group25reported as a single case a 2-year-old girl who had a survival up to 2 years at the time of publication after allogeneic bone marrow transplantation (BMT). More recently, the outcome of 27 patients with EL following allogeneic or autologous BMT was reported by the Royal Marsden group.26 Nineteen patients had de novo EL: 5 could not receive BMT, 1 received autologous BMT, 9 sibling allogeneic BMT, 2 mismatch allogeneic BMT, and 2 matched unrelated BMT. For these 19 patients with de novo EL, the overall survival was 66% at 2 years; it was 53% in adults.
The results for the 104 allografted patients of our series matched those of most prospective trials of allogeneic HSCT for de novo AML in CR1 using an HLA identical family donor: LFS was 48% at 5 years in the Goelam study,24 43% at 4 years in the EORTC study,19 43% at 4 years in the US intergroup study,27 and 66% at 3 years in the BGMT study18; the Dutch study indicated an overall survival of 66% at 3 years.28
Results of autologous HSCT in the present study were low. LFS at 5 years was 26%. This low LFS was attributed mainly to a high RI of 70% at 5 years. These results compared unfavorably with the data in the literature for de novo AML. Prospective comparative studies, including those mentioned above, have shown LFS of 54% at 5 years in the MRC AML 10 trial,16 44% at 4 years in the Goelam study,24 48% at 5 years in the EORTC study,19 51% at 3 years in the BGMT study18and 67% at 4 years in the Catalan study.23 Results of the present series were worse than those of the US intergroup study27 and the Dutch study,28 which reported the lowest LFS rates of all prospective comparative studies: 35% at 5 years and 37% at 3 years, respectively. However, the LFS of 26% for autografted EL patients in the present study concerns a mixture of all cytogenetics groups. The number of patients with informative karyotype in each group was too small, and we could not exclude that patients with favorable karyotype had better outcomes.
Results with autologous HSCT in this series look similar to those observed with autologous HSCT for myelodysplastic syndrome (MDS). A retrospective EBMT study29 analyzed 55 patients with MDS or secondary AML; these patients underwent autologous BMT in first CR; LFS was 28% and RI was 69% at 2 years. This observation supports the hypothesis that primary MDS and EL are closely related disorders.15 Indeed, studies showed that EL is a trilineage disease8,9,15,30 similar to MDS. In fact, allogeneic HSCT is currently the only curative treatment for MDS, with reported LFS from 35% to 56%.31-38 In younger patients (younger than 40) with low-risk MDS, long-term LFS was as high as 75%.39
Prognostic factors found in this study were not different from those observed in HSCT for de novo AML (ie, age and GVHD). For autologous HSCT, a trend for a lower TRM was observed with peripheral blood; the absence of expected improvement of LFS was probably related to the small size of the population studied. However, in a previous EBMT study of 1393 patients who received autografts for AML in CR and comparing autologous blood cell to bone marrow transplantation, there was no difference on LFS and RI between blood cell transplantation and unpurged BMT.40 For allogeneic HSCT, acute GVHD and age were the main prognostic factors identified in the present study; these factors are commonly found in allogeneic HSCT for de novo AML.
Unfortunately, informative cytogenetics with karyotype description was available for only 79 of 207 (38%) of the patients. Therefore, no prognostic evaluation of chromosomal abnormalities was possible, and outcome analysis according to the SWOG cytogenetics prognostic groups was not performed. Results were known for 79 patients; 52 patients showed no chromosomal abnormality, and 27 patients showed clonal acquired abnormalities. For these 27 patients the abnormalities were heterogeneous. This is consistent with previous studies in which no specific pattern was found. In our series, involvement of chromosomes 5 or 7 was observed in 6 patients; these abnormalities were also found in other series of patients with EL9,15,26,30,41 and were associated with poor prognosis.15 Trisomy 8 was observed in 6 patients and reported in previous series of patients with EL.9,15,30 These abnormalities on chromosomes 5, 7, and 8 are also described in MDS.42
We conclude that patients with EL who achieve CR1 should proceed to HLA matched allogeneic HSCT if a family donor is available. Allogeneic HSCT remains the treatment of choice to cure this rare and severe disease. In the absence of a family donor, autologous HSCT may cure a subset of patients, albeit at a lower proportion. There is little information on the value of unrelated allogeneic transplantation in EL.
We thank the following EBMT centers for registering patients with erythroleukemia analyzed in the study:
Abecasis, Manuel, Inst. Portugues Oncologia, Lisboa, Portugal; Alessandrino, E. Paolo, Policlinico San Matteo, Pavia, Italy; Amadori, Sergio, St Eugenio Hospital, Rome, Italy; Attal, Michel, Hopital de Purpan, Toulouse, France; Bacigalupo, Andrea, Ospedale San Martino, Genova, Italy; Barbui, Tiziano, Ospedale Bergamo, Bergamo, Italy; Beelen, Dietrich, University of Saarland, Homburg, Germany; Benedetti, Fabio, Policlinico Borgo Roma Ematologia, Verona, Italy; Bergmann, L., Universität Ulm, Germany; Blaise, Didier, Institut Paoli Calmettes, Marseille, France; Boasson, Marc, CHRU, Angers, France; Boogaerts, Marc A., University Hospital Gasthuisberg, Leuven, Belgium; Bosi, Alberto, Ospedale di Careggi, Firenze, Italy; Bron, Dominique, Institut Jules Bordet, Brussels, Belgium; Cahn, Jean-Yves, Hopital Jean Minjoz, Besancon Cedex, France; Chapuis, B., Hopital Cantonal Universitaire, Geneva, Switzerland; Clark, R. E., Royal Liverpool University Hospital, Liverpool, United Kingdom; Cordonnier, Catherine, Hôpital Henri Mondor, Creteil, France; Cornelissen, J. J., Daniel den Hoed Cancer Centre (AZR), Rotterdam, The Netherlands; Coser, Paolo, Hospital San Maurizio, Bolzano, Italy; Davies, John M., Western General Hospital, Edinburgh, Scotland, United Kingdom; De Souza, Carmino, Univ. Est. de Campinas, Campinas -SP, Brazil; Degos, Laurent, Hôpital St Louis, Paris, France; Evensen, Stein A., Rikshospitalet, Oslo, Norway; Falda, Michele, Azienda Ospedaliera S. Giovanni, Turin, Italy; Fanin, Renato, Udine University Hospital, Udine, Italy; Fassas, Athanasios, The George Papanicolaou General Hospital, Exokhi, Greece; Fasth, Anders, Queen Silvia Children's Hospital, Goeteborg, Sweden; Fauser, A. A., Klinik für Knochenmarktransplantation, Idar-Oberstein, Germany; Fernández-Ranada, José M., Hospital de la Princesa, Madrid, Spain; Ferrant, Augustin, Cliniques Universitaires St Luc, Brussels, Belgium; Fischer, Alain, Hôpital Necker, Paris, France; Franklin, Ian, Glasgow Royal Infirmary, Glasgow, Scotland, United Kingdom; Garcı́a-Conde, Javier, Hospital Clı́nico Universitario, Valencia, Spain; Giustolisi, Rosario, Ferrarotto Hospital, Catania, Italy; Gluckman, Eliane, Hopital St Louis, Paris, France; Goldman, John M., Hammersmith Hospital, London, United Kingdom; Goldstone, Antony H., University College London Hospital, London, United Kingdom; Gorin, Norbert C., Hopital Saint Antoine, Paris, France; Gratecos, Nicole, Hôpital de l'ARCHET I, Nice, France; Gratwohl, Alois, Kantonsspital, Basel, Switzerland; Greco, Michele Mario, Hospital Casa Sollievo Della Sofferenza, San Giovanni Rotondo, Italy; Greinix, Hildegard T., AKH Vienna, Vienna, Austria
Guilhot, F., Hopital La Miletrie, Poitiers, France; Hansz, J. K., Marcinkowski University, Poznan, Poland; Harhalakis, Nicholas, Evangelismos Hospital, Athens, Greece; Harousseau, Milpied, Hotel Dieu, Nantes, France; Hellmann, Andrzej, Medical University of Gdansk, Poland; Herrmann, R. P., Royal Perth Hospital, Western Australia, Australia; Hertenstein, Bernd, Medical School of Hannover, Germany; Holowiecki, J., Silesian Medical Academy, Katowice, Poland; Iacopino, Pasquale, Azienda Ospedaliera Bianchi-Melacrino-Morelli, Reggio Calabria, Italy; Iriondo, A., Hospital Universitario ‘Marqués de Valdecilla’, Santander, Spain; Izzi, Teodosio, Spedali Civili, Brescia, Italy; Jacobsen, Niels, Rigshospitalet, Copenhagen, Denmark; Jouet, J. P., Hopital Claude Huriez, Lille Cedex, France; Kanz, L., Medizinische Klinik, Tübingen, Germany; Karianakis, G., Diagnostic & Therapeutic Center of Athens, Greece; Koc, Haluk, Ibni Sina Hospital, Ankara, Turkey; Labar, Boris, University Hospital Centre, Rebro, Zagreb, Croatia; Lambertenghi Deliliers, G., Ospedale Maggiore di Milano, Milan, Italy; Lasa Isasti, Ramon, Hospital Aranzazu, San Sebastian, Spain; Leblond, Veronique, Hôpital la Pitie-Salpetriere, Paris, France; Lenhoff, Stig, University Hospital, Lund, Sweden; Leone, Giuseppe, Universita Cattolica S. Cuore, Rome, Italy; Littlewood, Tim, The Oxford Radcliffe Hospital, Oxford, United Kingdom; Ljungman, Per, Huddinge University Hospital, Sweden; Lucarelli, G., Pesaro Hospital, Pesaro, Italy; Mandelli, Franco, Universita “La Sapienza,” Rome, Italy; Marcus, Robert, Addenbrookes Hospital, Cambridge, United Kingdom; Martelli, Massimo F., Policlinico Monteluce, Perugia, Italy; McCann, Shaun, St James Hospital Trinity College, Dublin, Ireland; Michallet, Mauricette, Hopital E. Herriot, Lyons, France; Montserrat, Emilio, Hospital Clinic, Barcelona, Spain; Morris, T. C. M., Belfast City Hospital, Belfast, Northern Ireland, United Kingdom; Mufti, G. J., King's College School of Medicine, London, United Kingdom; Niederwieser, Dietger, University of Leipzig, Germany; Noens, L. A., University Hospital Ghent, Belgium; Novitzky, Nicolas, UCT Medical School, Cape Town, South Africa; Ossenkoppele, G. J., Free University Hospital Amsterdam, The Netherlands; Pico, José Luis, Institut Gustave Roussy, Villejuif Cedex, France; Pogliani, Enrico Maria, Ospedale S. Gerardo, Monza, Italy; Powles, Ray, Royal Marsden Hospital, Sutton, Surrey, United Kingdom; Prentice, H. G., Royal Free Hospital, London, United Kingdom; Proctor, S. J., Royal Victoria Infirmary, Newcastle upon Tyne, United Kingdom; Reiffers, J., Hopital du Haut Leveque, Pessac, France; Remes, Kari, Turku University Central Hospital, Turku, Finland; Rio, Bernard, Hotel Dieu, Paris, France; Rizzoli, Vittorio, University of Parma, Italy; Rodeghiero, Francesco, S. Bortolo Hospital, Vicenza, Italy; Rodrı̀guez Fernández, Juan M., Hospital ‘Virgen del Rocio’, Sevilla, Spain; Rossi, Jean Francois, University Hospital, Montpellier, France; Rotoli, Bruno, Federico II Medical School, Napoli, Italy; Russell, N. H., Nottingham City Hospital, Nottingham, United Kingdom; Ruutu, Tapani, Helsinki University Central Hospital, Helsinki, Finland; Sanz, Miguel A., Hospital Universitario La Fe, Valencia, Spain; Schaefer, U. W., University Hospital, Essen, Germany; Schanz, Urs, University Hospital, Zurich, Switzerland; Schattenberg, A., Nijmegen University Medical Center, The Netherlands; Schey, S. A. M, Guy's Hospital, London, United Kingdom; Schmitz, Norbert, Christian-Albrechts-University, Kiel, Germany; Schots, R., University Hospital VUB, Brussels, Belgium; Schroyens, W., Universiteit Antwerpen (UZA), Antwerp, Edegem, Belgium; Scimè, Rosanna, Ospedale V. Cervello, Palermo, Italy; Selleslag, D., A. Z. Sint-Jan, Bruges, Belgium; Sierra, Jorge, Hospital Santa Creu i Sant Pau, Barcelona, Spain; Simonsson, Bengt, University Hospital, Uppsala, Sweden; Slavin, Shimon, Hadassah University Hospital, Jerusalem, Israel; Tura, Sante, Hospital San Orsola, Bologna, Italy; Van den Berg, H., Academic Medical Centre van Amsterdam, The Netherlands; van Marwijk Kooy, M., Isala Klinicken Zwolle, The Netherlands; Verdonck, Leo F., University Medical Centre, Utrecht, The Netherlands; Vitek, Antonin, Institute of Hematology and Blood Transfusion, Prague, Czech Republic; Volpe, Ettore, Ematologia ‘Giovanni di Guglielmo’, Avellino, Italy; Wahlin, Anders, Umea University Hospital, Sweden; Wandt, Hannes, Klinikum Nürnberg, Nuremberg, Germany; Zander, Axel R., University Hospital Eppendorf, Hamburg, Germany.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Loı̈c Fouillard, Service des maladies du sang, Hôpital Saint-Antoine, 184 rue du faubourg Saint-Antoine, 75571 Paris Cedex 12, France; e-mail: fouillar@ext.jussieu.fr.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal