Natural killer (NK) cells are the major effectors of acute rejection of incompatible bone marrow cell (BMC) grafts in lethally irradiated mice. The immunogenetics of BMC rejection are largely controlled by the coexpression (or not) of inhibitory and stimulatory Ly49 receptors whose ligands are class I major histocompatibility complex (MHC) molecules. The majority of the BMC rejection studies involved low numbers of BMCs that were resisted by host NK cells. In the present study, larger numbers of BMCs were given in which rejection was not detected and the role of different Ly49 NK subsets not presumably involved in the rejection of a particular BMC haplotype was examined. Surprisingly, the data show that the removal of NK cell subsets expressing Ly49 inhibitory receptors for donor class I antigens, which would be predicted to have no effect on the BMC rejection capability, resulted in the marked rejection of BMCs where no resistance was normally seen. These results extend the “missing self” hypothesis to suggest that NK Ly49 inhibitory receptors can both inhibit activation and killing by those cells, but also can in some way influence the function of NK cells that do not express that inhibitory receptor in a cell-cell interaction. This suggests that caution must be exercised before removal of host NK cell subset is applied clinically because enhanced BMC rejection may result. Altering the balance of Ly49 NK subsets may also affect other in vivo activities of these cells.
Introduction
Natural killer (NK) cells are large granular lymphocytes capable of spontaneously killing various tumor cells and virally infected cells.1-3 Murine NK cells can also mediate the acute rejection of allogeneic bone marrow cells (BMCs) in lethally irradiated mice but not solid tissue allografts.3-7 They also exhibit a phenomenon called “hybrid resistance” in which H2 homozygous parental BMC grafts are rejected by irradiated H2 heterozygous F1 hybrid mice.7 The recognition of major histocompatibility complex (MHC) class I molecules by murine NK cells is mediated by 2 families of cell surface receptors and has at least partially explained how NK cells can detect BMC allografts.6,8-18 One receptor family is called Ly49, which is composed of C-lectin type II membrane glycoproteins.6,8-18 Individual members of these Ly49 receptors have an affinity for particular MHC class I determinants.16,17 Several Ly49 receptors have been identified and cDNAs have been sequenced in mice with strain-specific allelic forms being reported.18-21 Most Ly49 molecules are “inhibitory receptors,” because binding to their specific targets transmits inhibitory intracellular signals through an immunoreceptor tyrosine-based inhibitory motif (ITIM) in their cytoplasmic domain.9,10,16,17 The ITIM recruits tyrosine phosphatase, which results in a potent inhibitory signal being sent to the NK cell.15-17 Three Ly49 receptors (Ly49D, Ly49H, and Ly49P) are characterized as “activating receptors,” because they lack the inhibitory motif (ITIM) in their cytoplasmic domain and instead are dependent on a noncovalently associated molecule “DAP12,” which contains an immunoreceptor tyrosine-based activating motif (ITAM).15,22,23 Recent studies have shown that NK cytotoxicity is regulated by a balance between expression of specific activating and inhibitory receptors.17 A second group of MHC class I–specific receptors on NK cells is composed of disulfide bonded heterodimers termed CD94/NKG2.16,17 CD94 has a short cytoplasmic tail with no signaling function and it forms a heterodimer with various NKG2 proteins A, B, C, D, and E.10 17
We and others have shown the role of inhibitory Ly49A, C/I, and G2 NK subsets as well as the activating Ly49D NK subset in bone marrow allograft rejection in lethally irradiated mice.22-30Although a small percentage of T cells also express Ly49 receptors, mice with severe combined immunodeficiency (SCID), deficient in T and B cells, also mediate class I–specific bone marrow allograft rejection, indicating that NK cells alone can mediate the specificity seen in BMC rejection.5
Our previous studies were designed to delineate the role of various Ly49 NK subsets in mediating BMC rejection. These studies involved infusing relatively small numbers of allogeneic BMCs that were rejected by untreated irradiated mice unless specific depletion of a Ly49 NK subset directly mediating the rejection was performed, which instead resulted in BMC engraftment.26,27,29 During those studies using some Ly49 monoclonal antibodies (mAbs) as controls for examining specificity of functions of other Ly49 subsets in mediating rejection, we made a novel and unexpected preliminary observation in mice. We observed that depletion of large subsets of NK cells expressing inhibitory receptors for a given H2 type of BMC had 2 effects: (1) increased resistance to the H2 type BMCs that these receptors recognized to receive negative signals and (2) weakening of rejection against BMC grafts that were not recognized by the particular NK receptor. For example, depletion of Ly49G2+ NK cells, which receive negative signals from H2d but not H2bclass I antigens, led to decreased ability of H2dhost mice to reject H2b BMCs.27 We describe here in more detail the in vivo effect of depletion of Ly49 NK subsets, which do not directly mediate the rejection of H2d or H2b haplotype BMCs, on the rejection of higher numbers of BMCs and discuss the potential relevance of these findings in clinical bone marrow transplantation (BMT).
Materials and methods
Mice
BALB/c (H2d), C.B-17 SCID (H2d), C57BL/6 (H2b), and BALB/c × C57BL/6 F1(CB6F1, H2bxd), mice were obtained from the Animal Production Area, National Cancer Institute (Frederick, MD). All mice were kept under specific pathogen-free conditions until use at 8 to 12 weeks of age.
NK cell isolation and flow cytometric analysis
Groups of 5 to 10 C57BL/6 (H2b) mice were injected intraperitoneally with 500 μg mAb 5E6 (anti-Ly49C/I) or 200 μg mAb 4D11 (anti-Ly49G2) or 0.2 mL normal rat serum (NRS). Seventy-two hours later spleens were harvested and single-cell suspensions were prepared. Four-color flow cytometric analysis was performed to determine the percentage of Ly49C/I+, Ly49G2+, and Ly49D+ NK cells. Cells were incubated with phycoerythrin (PE)–conjugated mAbs 5E6 (anti-Ly49C/I) or 4E5 (anti-Ly49D), fluorescein isothiocyanate (FITC)–conjugated mAb 4D11 (anti-Ly49G2) or goat-antimouse/rat IgG, allophycocyanin (APC)–conjugated NK pan marker NK1.1 and peridinin chlorophyll protein (PCP)–conjugated anti-CD3 for 30 minutes at 4°C. After 2 washes, the cells were analyzed on an LSR flow cytometer (Becton Dickinson, San Jose, CA). All secondary antibodies were titered and tested for their ability to stain for primary antibodies.
Assays for BMC engraftment
Groups of 4 to 5 conventionally housed recipient mice were injected intraperitoneally with 500 μg mAb 5E6 (anti-Ly49C/I), 200 μg mAb 4D11 (anti-Ly49G2), 500 μg of mAb PK136 (anti-NK 1.1), 20 μL antiasialo GM1 (anti-ASGM1; Wako Chemicals, Richmond, VA), 0.2 mL NRS, or 120 μg polyinosinic/polycytidylic (poly I:C), 2 days before irradiation. The engraftment of donor BMCs was assessed by an in vitro colony assay for hematopoietic cell growth.26 Two days after antibody injection, mice were lethally irradiated with a137Cs source (BALB/c at 850 cGy, C57BL/6 at 950 cGy, C.B-17 SCID at 350 cGy, and CB6F1 at 1000 cGy) and later injected intravenously with 0.5 × 106 to 25 × 106BMCs in 0.5 mL phosphate-buffered saline (PBS). At day 8, spleens were gently crushed in medium and single-cell suspensions were prepared. Then, 106 spleen cells were plated in 0.3% sea plaque agar in 35-mm dishes containing optimal concentrations of recombinant murine cytokines (interleukin 3 [IL-3; 10 ng/mL] and granulocyte-macrophage colony-stimulating factor [GM-CSF; 10 ng/mL]). Cytokines were obtained from the Biological Resources Branch (Frederick, MD). Plates were incubated at 37°C for 7 days and the colonies with more than 50 cells were counted (colony-forming unit-culture [CFU-c]). The data are presented as mean ± SD total CFU-c per spleen, which was obtained by multiplying number of colonies by the cellularity. The Student t test was performed to determine if the mean values were significantly different (P < .05). Each experiment was performed 3 to 4 times with at least 4 mice per group.
Results
We have reported earlier that in BALB/c (H2d) mice, the Ly49G2+ NK subset can mediate the rejection of allogeneic H2b BMCs and conversely Ly49C/I+ NK cells are responsible for the rejection of H2d BMCs in C57BL/6 (H2b) mice as ascertained by the in vivo depletion of these subsets.25,26,29 While studying the in vivo role of Ly49G2+ NK subset in BALB/c (H2d) mice, we observed an interesting phenomenon by using antibodies directed against the Ly49C/I+ NK cell subset as control for the specificity of Ly49G2+ NK subset removal.26 Depletion of Ly49C/I+ NK cells in BALB/c (H2d) mice, which is the NK subset that receives negative signals from H2Kband therefore cannot reject H2b BMCs, resulted in the ability of the recipient mice to reject higher numbers of allogeneic H2b BMCs.26 Therefore, depletion of a population of NK cells that were considered indifferent to H2b BMCs nevertheless resulted in enhanced rejection by H2d mice.
We carried out further transplantation studies to determine the effects of in vivo depletion of this and other Ly49 NK subsets that are not responsible for the rejection of a particular BMC allograft. To do this, we wanted to assess the effect of NK subset removal in instances where increasing BMC numbers could overcome resistance and result in engraftment. The number of allogeneic BMCs transplanted varied because mice differ in their BMC rejection capability.
Depletion of Ly49C/I+ NK subset in H2d mice enhances their ability to reject H2b BMCs
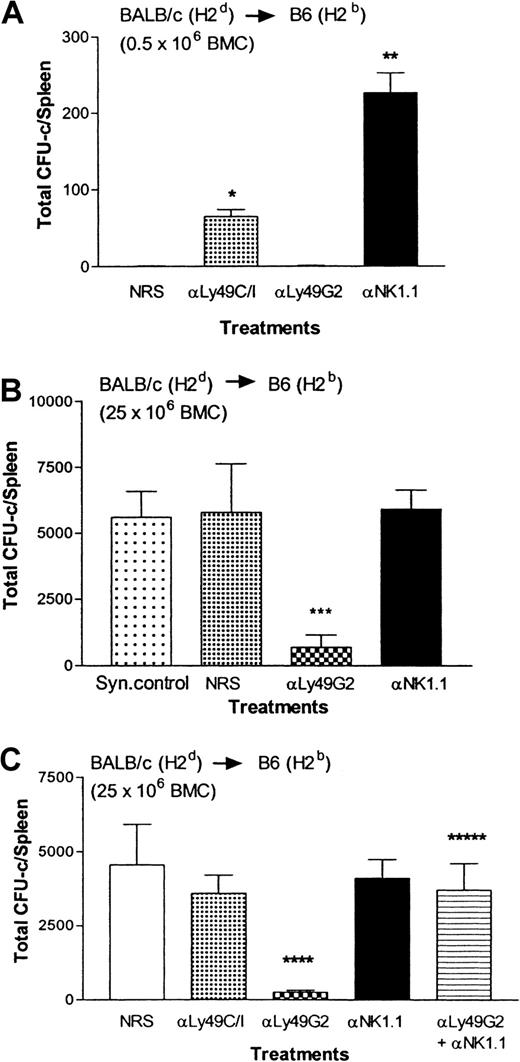
We examined the effect of in vivo depletion of Ly49C/I+ or G2+ NK subsets on allograft rejection in BALB/c (H2d) mice. Mice were injected with 200 μg mAb 4D11 (anti-Ly49G2) or 500 μg mAb 5E6 (anti-Ly49C/I) to deplete the respective Ly49 NK subset and 48 hours later mice were lethally irradiated and allogeneic BMCs were infused. After 8 days, the splenic CFU-c assay, which quantitates hematopoietic progenitors as reflected by growth in agar, was performed to assess BMC engraftment in mice. The CFU-c assay was chosen over the (125I) UdR radioisotope incorporation assay because though both assays detect progenitor cell function, the CFU-c assay, in addition, detects the production of CFU-c precursor/progenitor cells derived from the inoculum, whereas the other detects any proliferating marrow-derived cells in S-phase of the cycle. The results presented in Figure1A demonstrate that irradiated BALB/c mice were capable of resisting 0.5 × 106 H2bBMCs, whereas the syngeneic control H2b C57BL/6 mice engrafted H2b BMCs. When the total NK cell population was removed by injecting ASGM1 antibodies, engraftment of allogeneic H2b BMCs was enhanced (P < .0001; Figure 1A). Removal of Ly49C/I+ NK subset, which does not mediate the rejection of allogeneic H2b BMCs as they receive negative signals from H2Kb BMCs, led to rejection of the BMCs but depletion of Ly49G2+ NK cells (which receive negative signals from H2Dd BMCs and therefore can mediate the rejection of H2b BMCs) significantly increased the engraftment of allogeneic H2b BMCs compared with the control group (NRS treated).26 However, when rejection was overridden by infusing larger numbers of (8 × 106) allogeneic H2b BMCs (Figure 1B-C), engraftment of the infused BMCs was noted in the control group (NRS treated), similar to syngeneic control (Figure 1B) and anti-ASGM1–treated group (Figure 1B-C), which had all of the NK cells depleted, suggesting that no resistance was occurring. Removal of Ly49G2+ NK cells, which mediates the rejection of H2b BMCs, did not increase the engraftment of allogeneic H2b BMCs compared with the control group (NRS treated) also indicating that no appreciable resistance was occurring (Figure 1B-C). In vivo activation of NK cells by poly I:C, which involves interferon α/β (IFN-αβ) production and increases BMC rejection ability of mice, prior to BMC infusion resulted in inhibition of engraftment (P < .007) compared with the control group (NRS treated; Figure 1C). However, in vivo removal of the supposedly irrelevant Ly49C/I+ NK subset (which does not mediate the rejection of allogeneic H2bBMCs because it receives negative signals from H2Kb BMCs) resulted in significant rejection (P < .0001) of H2b BMCs compared with either of the control groups (NRS or ASGM1 treated; Figure 1B-C) or poly I:C-treated group (P < .002; Figure 1C). Thus, removal of Ly49C/I+ NK cells in H2d mice enhances the capability of the mice to reject H2b BMCs and even greater than if agents commonly used to activate NK cells (ie, poly I:C) are used, which is not noticeable when low numbers of allogeneic H2b BMCs are transplanted. This suggests that in H2d (BALB/c) mice, Ly49C/I+ NK cells exert an inhibitory effect on the cells responsible for the rejection of H2b BMCs.
Effect of depletion of Ly49G2+ or Ly49C/I+ NK subsets in H2d BALB/c mice on the engraftment of H2b allografts.
Mice were given various mAb treatments or NRS 2 days before irradiation. Irradiated mice (4 mice/group) received 0.5 × 106 (A) or 8 × 106 (B,C) allogeneic BMCs. On the eighth day a soft agar colony assay was done to assess hematopoietic progenitor content in the spleens (mean ± SD). *Values significantly (P < .0001) greater than mice receiving NRS in experiment A; **values significantly (P < .0001) greater than mice receiving NRS in experiment A; ***values significantly (P < .0001) lower than mice receiving NRS in experiment B; ****values significantly (P < .0001) lower than mice receiving NRS in experiment C; *****values significantly (P < .002) greater than mice receiving anti-Ly49C/I (mAb 5E6) in experiment C.
Effect of depletion of Ly49G2+ or Ly49C/I+ NK subsets in H2d BALB/c mice on the engraftment of H2b allografts.
Mice were given various mAb treatments or NRS 2 days before irradiation. Irradiated mice (4 mice/group) received 0.5 × 106 (A) or 8 × 106 (B,C) allogeneic BMCs. On the eighth day a soft agar colony assay was done to assess hematopoietic progenitor content in the spleens (mean ± SD). *Values significantly (P < .0001) greater than mice receiving NRS in experiment A; **values significantly (P < .0001) greater than mice receiving NRS in experiment A; ***values significantly (P < .0001) lower than mice receiving NRS in experiment B; ****values significantly (P < .0001) lower than mice receiving NRS in experiment C; *****values significantly (P < .002) greater than mice receiving anti-Ly49C/I (mAb 5E6) in experiment C.
Depletion of the Ly49G2+ but not the Ly49C/I NK+ subset in H2b mice enhances the rejection of H2d BMCs
Next, we performed the reciprocal experiment. We examined the effect of depletion of the Ly49C/I+ or Ly49G2+NK subset on the rejection of higher numbers of allogeneic H2d BMCs in irradiated C57BL/6(H2b) mice to assess whether Ly49G2+ NK cells were able to inhibit the ability of mice to reject H2d BMCs.23,24 In lethally irradiated H2b mice, the Ly49C/I+ NK subset has been shown to mediate the rejection of allogeneic H2d BMCs when transplanted in limited (0.5 × 106) numbers (Figure2A).25 29 However, when allogeneic H2d BMCs were infused in larger numbers (25 × 106 BMCs), the control group (NRS treated) could no longer reject the bone marrow allograft because the CFU-c content was similar to mice depleted of all NK cells by NK1.1 mAb and syngeneic control (Figure 2B-C). Under such circumstances, depletion of the Ly49C/I+ NK subset (containing Ly49D+ cells) also did not affect the engraftment of H2d bone marrow allografts when compared with the control group (NRS-treated group; Figure 2C). However, the C57BL/6 mice treated with the anti-Ly49G2 mAb 4D11 demonstrated significant augmentation of H2d bone marrow allograft rejection (Figure 2B-C; P < .003) compared with the control recipients and this could be reversed if all the NK1.1+ cells were removed by injecting pan NK mAb, PK136 (αNK1.1) along with anti-Ly49G2 mAb 4D11, suggesting that BMC graft rejection was mediated by NK1.1+ cells (Figure2C).
Effect of depletion of Ly49G2+ or Ly49C/I+ NK subsets in H2b C57BL/6 mice on the engraftment of H2d allografts.
Mice were given various mAb treatments or NRS 2 days before irradiation. Irradiated mice (4 mice/group) received 0.5 × 106 (A) or 25 × 106 (B,C) allogeneic BMCs. On the eighth day a soft agar colony assay was done to assess hematopoietic progenitor content in the spleens (mean ± SD). *Values significantly (P < .0001) greater than mice receiving NRS in experiment A; **values significantly (P < .0001) greater than mice receiving NRS in experiment A; ***values significantly (P < .006) lower than mice receiving NRS in experiment B; ****values significantly (P < .003) lower than mice receiving NRS in experiment C; *****values significantly (P < .001) greater than mice receiving anti-Ly49G2 (mAb 4D11) only in experiment C.
Effect of depletion of Ly49G2+ or Ly49C/I+ NK subsets in H2b C57BL/6 mice on the engraftment of H2d allografts.
Mice were given various mAb treatments or NRS 2 days before irradiation. Irradiated mice (4 mice/group) received 0.5 × 106 (A) or 25 × 106 (B,C) allogeneic BMCs. On the eighth day a soft agar colony assay was done to assess hematopoietic progenitor content in the spleens (mean ± SD). *Values significantly (P < .0001) greater than mice receiving NRS in experiment A; **values significantly (P < .0001) greater than mice receiving NRS in experiment A; ***values significantly (P < .006) lower than mice receiving NRS in experiment B; ****values significantly (P < .003) lower than mice receiving NRS in experiment C; *****values significantly (P < .001) greater than mice receiving anti-Ly49G2 (mAb 4D11) only in experiment C.
Thus, in H2b mice, the converse of the effect seen with NK subset removal in H2d mice (Figure 1B) occurs. Removal of the Ly49 NK subset that receives negative signals from H2dsignificantly enhances the ability of the remaining NK1.1+cells to reject H2d BMCs.
In F1 (H2bxd) mice, rejection of higher numbers of either parental bone marrow allografts (H2b or H2d) is dependent on specific Ly49 NK subset depletion
NK cells in F1 (H2bxd) hybrid mice can reject both parental bone marrow allografts when transplanted in limited numbers, by a phenomenon called “hybrid resistance.”7 In F1 (H2bxd) mice, Ly49C/I and G2 receptors are also exposed to their “self-ligands” H2b and H2d, respectively, in vivo, which transmit inhibitory signals to NK cells. Therefore, it was of interest to examine the effect of depletion of Ly49 NK subsets in a hybrid resistance model, that is, depleting the NK subset that does not mediate the rejection of a particular haplotype BMC (Ly49C/I for H2b and Ly49G2 for H2d BMCs) on the rejection of higher numbers of those parental BMCs in F1(H2bxd) mice.
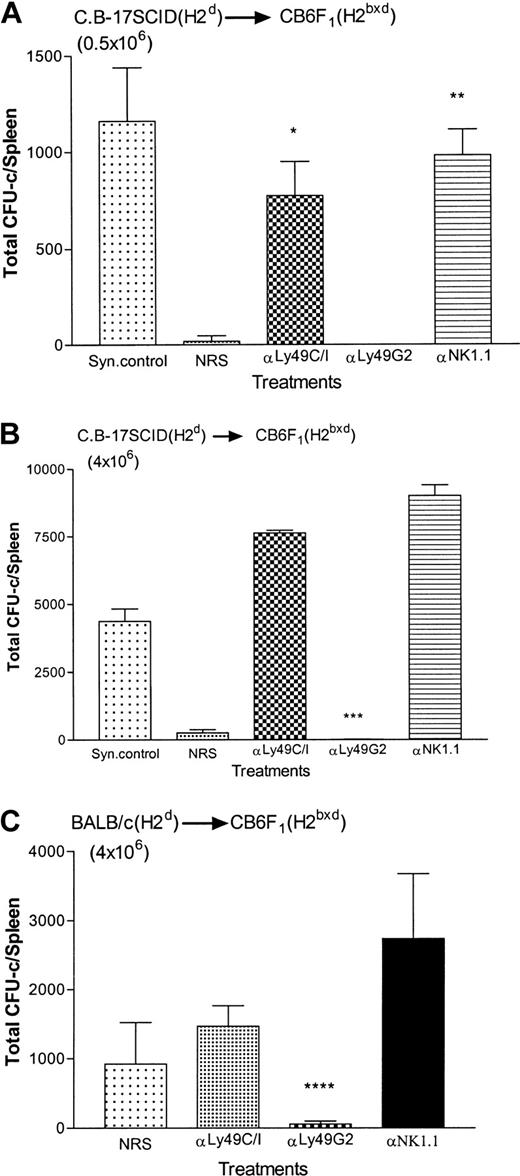
We have reported earlier, and also show in Figure3A, that in CB6F1(H2bxd) hybrid mice NK cells expressing inhibitory Ly49G2 receptors mediate the rejection of parental H2b BMCs (0.5 × 106) because depletion of the Ly49G2+NK subset in vivo results in the engraftment of the BMCs.26 However, when higher numbers of H2bBMCs (20 × 106) were transplanted to override the resistance, removal of Ly49G2+ NK cells (which plays a role in mediating the rejection of H2b BMCs in heterozygous F1 [H2bxd] and homozygous H2dmice) did not augment allograft engraftment when compared to the control group (NRS-treated group; Figure 3B). Interestingly, similar to the results in homozygous H2d mice (Figure 1B), significant rejection of parental H2b bone marrow allografts occurred (P < .006) in Ly49C/I+ NK subset–depleted group when compared with anti-NK1.1–treated group, where all NK cells were depleted.
Effect of depletion of Ly49G2+ or Ly49C/I+ NK subsets in H2bxd F1mice on the engraftment of H2b allografts.
Mice were given various mAb treatments or NRS 2 days before irradiation. Irradiated mice (4 mice/group) received either 0.5 × 106 (A) or 20 × 106 parental-strain H2b BMCs (B). On the eighth day a soft agar colony assay was done to assess hematopoietic progenitor content in the spleens (mean ± SD). *Values significantly (P < .006) greater than mice receiving NRS in experiment A; **values significantly (P < .0004) greater than mice receiving NRS in experiment A; ***values significantly (P < .006) lower than mice receiving NRS in experiment B.
Effect of depletion of Ly49G2+ or Ly49C/I+ NK subsets in H2bxd F1mice on the engraftment of H2b allografts.
Mice were given various mAb treatments or NRS 2 days before irradiation. Irradiated mice (4 mice/group) received either 0.5 × 106 (A) or 20 × 106 parental-strain H2b BMCs (B). On the eighth day a soft agar colony assay was done to assess hematopoietic progenitor content in the spleens (mean ± SD). *Values significantly (P < .006) greater than mice receiving NRS in experiment A; **values significantly (P < .0004) greater than mice receiving NRS in experiment A; ***values significantly (P < .006) lower than mice receiving NRS in experiment B.
Weak resistance to parental BALB/c(H2d) bone marrow allografts has been reported to occur by F1(H2bxd) hybrid mice and this rejection can be enhanced by using BMCs that are depleted of T cells.31 We have used C.B-17 SCID (H2d) BMCs as donors because it has been shown that the rejection pattern of congenitally T cell-deficient C.B-17 SCID BMCs is identical to BALB/c BMCs that were depleted of T cells.31 The BMT results presented in Figure4A demonstrate that as reported earlier, in CB6F1 (H2bxd) hybrid mice, NK cells expressing inhibitory Ly49C/I receptors mediate the rejection of parental H2d BMCs (0.5 × 106) as depletion of Ly49C/I+ subset in vivo results in the engraftment of the C.B-17 SCID (H2d) BMCs.26 Removal of the other Ly49 NK subset, that is, the Ly49G2 NK subset that does not mediate the rejection of H2d BMCs instead resulted in the rejection of the bone marrow allograft, similar to the control group, whereas syngeneic controls engrafted the BMCs (Figure 4A). Increasing the C.B-17 SCID (H2d) BMCs to 4 × 106 also resulted in rejection by the host (Figure 4B). However, when parental 4 × 106 BALB/c (H2d) BMCs were transplanted there was engraftment of H2d BMCs in the control group (NRS treated) and the reason is weak resistance to parental BALB/c (H2d) bone marrow allografts by F1(H2bxd) hybrid mice.31 Removal of Ly49C/I+ NK cells (H2d rejecting) did not augment allograft engraftment when compared with the control group (NRS-treated group; Figure 4C). Interestingly, as observed in homozygous H2b mice (Figure 2B-C), removal of Ly49G2+ NK cells, which does not mediate the rejection of H2d BMCs, resulted in enhanced rejection of H2dbone marrow allografts (P < .02) by the irradiated F1 recipients (Figure 4C).
Effect of depletion of Ly49G2+ or Ly49C/I+ NK subsets in H2bxd F1mice on the engraftment of H2d allografts.
Mice were given various mAb treatments or NRS 2 days before irradiation. Irradiated mice (4 mice/group) received either 0.5 × 106 (A) or 4 × 106 (B) parental strain C.B-17 SCID (H2d) BMCs or 4 × 106BALB/c (H2d) BMCs (C). On the eighth day a soft agar colony assay was done to assess hematopoietic progenitor content in the spleens (mean ± SD). *Values significantly (P < .001) greater than mice receiving NRS in experiment A; **values significantly (P < .0002) greater than mice receiving NRS in experiment A; ***values significantly (P < .0001) lower than mice receiving NK1.1 in experiment B; ****values significantly (P < .02) lower than mice receiving NRS in experiment C.
Effect of depletion of Ly49G2+ or Ly49C/I+ NK subsets in H2bxd F1mice on the engraftment of H2d allografts.
Mice were given various mAb treatments or NRS 2 days before irradiation. Irradiated mice (4 mice/group) received either 0.5 × 106 (A) or 4 × 106 (B) parental strain C.B-17 SCID (H2d) BMCs or 4 × 106BALB/c (H2d) BMCs (C). On the eighth day a soft agar colony assay was done to assess hematopoietic progenitor content in the spleens (mean ± SD). *Values significantly (P < .001) greater than mice receiving NRS in experiment A; **values significantly (P < .0002) greater than mice receiving NRS in experiment A; ***values significantly (P < .0001) lower than mice receiving NK1.1 in experiment B; ****values significantly (P < .02) lower than mice receiving NRS in experiment C.
Hence, the ability of F1 (H2bxd) hybrid mice to reject larger numbers of either parental BMCs (H2d or H2b) is enhanced on the depletion of a specific Ly49 NK subset, Ly49G2+ and Ly49C/I+, respectively.
Augmentation of the ability of C.B-17 SCID (H2d) mice to reject H2b BMCs following depletion of Ly49C/I+ NK subset
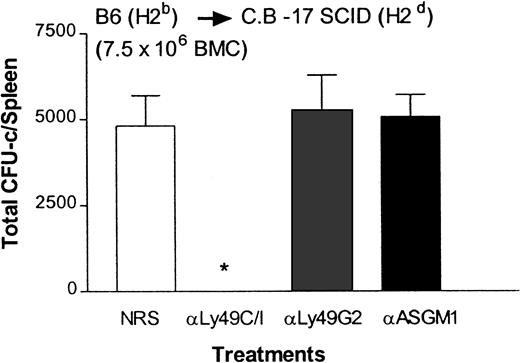
To ascertain whether enhanced rejection of allogeneic BMCs is indeed due to NK cells versus NK/T or T cells, we performed the BMT experiment in C.B-17 SCID (H2d) mice, which are deficient in T and B cells.5 Results presented in Figure5 demonstrate that depletion of Ly49C/I+ NK subset in C.B-17 SCID (H2d) mice enhanced their ability to reject large numbers of allogeneic H2b BMCs when compared with the control group treated with NRS (P < .0001). This is consistent with results also observed in BALB/c (H2d) mice (Figure 1B). Removal of Ly49G2+ NK cells that mediate the rejection of H2b BMCs in C.B-17 SCID (H2d) mice did not augment the engraftment further compared with the control group because large numbers (7.5 × 106) of allogeneic BMCs were transplanted. The results with C.B.-17 SCID (H2d) mice suggest that NK cells, and not T cells, are responsible for the increased marrow rejection as a result of Ly49 NK subset depletion.
Effect of depletion of Ly49G2+ or Ly49C/I+ NK subsets in C.B-17 SCID (H2d) mice on the engraftment of H2b allografts.
Mice were given various mAb treatments or NRS 2 days before irradiation. Irradiated mice (4 mice/group) received 7.5 × 106 allogeneic BMCs. On the eighth day a soft agar colony assay was done to assess hematopoietic progenitor content in the spleens (mean ± SD). *Values significantly (P < .0001) lower than mice receiving NRS.
Effect of depletion of Ly49G2+ or Ly49C/I+ NK subsets in C.B-17 SCID (H2d) mice on the engraftment of H2b allografts.
Mice were given various mAb treatments or NRS 2 days before irradiation. Irradiated mice (4 mice/group) received 7.5 × 106 allogeneic BMCs. On the eighth day a soft agar colony assay was done to assess hematopoietic progenitor content in the spleens (mean ± SD). *Values significantly (P < .0001) lower than mice receiving NRS.
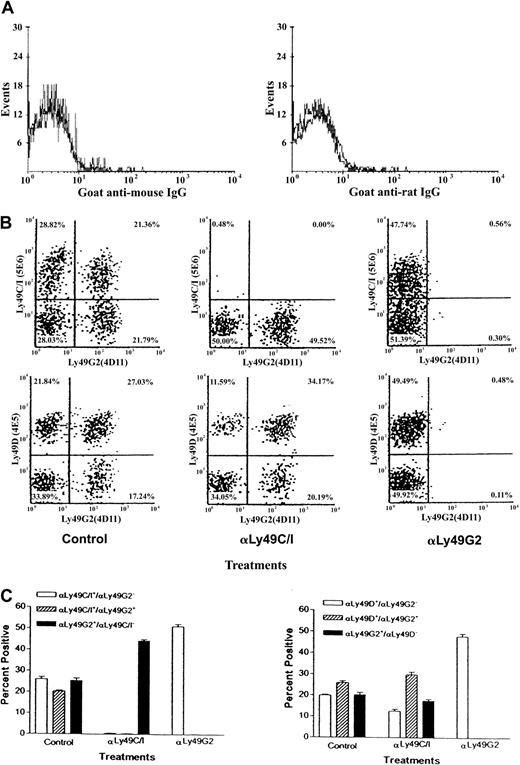
Depletion of Ly49G2 NK cells leads to enrichment of Ly49C/I+ and Ly49D+ NK+ cells in H2b mice
We wondered whether the enhanced allograft resistance in the NK subset–depleted mice might be due to an expansion of the Ly49 NK subset, capable of mediating rejection after depletion of the nonrejecting subset. C57BL/6 (H2b) mice were injected with 200 μg anti-Ly49G2 (4D11 mAb) or 500 μg anti-Ly49C/I (5E6 mAb) and 48 hours later half the mice were lethally irradiated. Seventy-two hours after mAb treatment (24 hours after irradiation) spleen cells were stained from nonirradiated and irradiated mice with various Ly49 fluoresceinated mAbs. Staining with a goat–anti-mouse antibody (to detect mAb 5E6 bound to NK cells in mAb 5E6-treated mice) or goat–anti-rat antibody (to detect mAb 4D11 bound to NK cells in mAb 4D11–treated mice) was completely negative, suggesting that antibody treatment resulted in depletion of the subsets (Figure6A). Results presented in Figure 6B and6C, demonstrate that in vivo treatment of C57BL/6 mice with the mAb 4D11 (anti-Ly49G2) or mAb 5E6 (anti-Ly49C/I) depleted 90% or more of splenic Ly49G2+ and Ly49C/I+ NK cells, respectively. However, no increase in absolute numbers of splenic Ly49C/I+ NK cells was detected in Ly49G2 NK subset–depleted C57BL/6 (H2b) mice and vice versa. A slight decrease in the total Ly49C/I+ NK cell numbers was noted, most likely due to removal of double-positive Ly49C/I+G2+ cells. Therefore, these data suggest that no compensatory expansion of surviving subsets occurred, at least within the time frame of 3 days.
Effect of anti-Ly49G2 (mAb 4D11) treatment on Ly49C/I+, Ly49G2+, and Ly49C/I+xLy49G2+ NK cell numbers in the spleens of C57BL/6 (H2b) mice.
Mice were treated as described in “Materials and methods.” Flow cytometric analysis of fresh spleen cells of control mice, mice treated with mAb 5E6 (anti-Ly49C/I) or mAb 4D11 (anti-Ly49G2), stained with goat–anti-mouse or goat–anti-rat IgG, anti-Ly49C/I (mAb 5E6), anti-Ly49G2 (mAb 4D11), or anti-Ly49D (mAb 4E5), anti-CD3 and pan NK mAb NK1.1. Panel A represents staining flow profile of NK1.1+ CD3− gated spleen cells stained with goat–anti-mouse IgG (gray line) for in vivo mAb 5E6–treated mice and with goat–anti-rat IgG (gray line) for in vivo mAb 4D11–treated mice. The black lines in panel A represent staining of NK1.1+ CD3− gated spleen cells of control mice with goat–anti-mouse IgG or goat–anti-rat IgG. Panel B represents staining flow profile from a single mouse and panel C represents average percentages (mean ± SD, 5 mice per group) of NK1.1+ gated Ly49C/I+(5E6+), Ly49G2+(4D11+), Ly49D+(4E5+), and Ly49C/I+(5E6+) × Ly49G2+(4D11+) or Ly49D+(4E5+) × Ly49G2+(4D11+) NK cell population of the same experiment. The experiment was done 2 times.
Effect of anti-Ly49G2 (mAb 4D11) treatment on Ly49C/I+, Ly49G2+, and Ly49C/I+xLy49G2+ NK cell numbers in the spleens of C57BL/6 (H2b) mice.
Mice were treated as described in “Materials and methods.” Flow cytometric analysis of fresh spleen cells of control mice, mice treated with mAb 5E6 (anti-Ly49C/I) or mAb 4D11 (anti-Ly49G2), stained with goat–anti-mouse or goat–anti-rat IgG, anti-Ly49C/I (mAb 5E6), anti-Ly49G2 (mAb 4D11), or anti-Ly49D (mAb 4E5), anti-CD3 and pan NK mAb NK1.1. Panel A represents staining flow profile of NK1.1+ CD3− gated spleen cells stained with goat–anti-mouse IgG (gray line) for in vivo mAb 5E6–treated mice and with goat–anti-rat IgG (gray line) for in vivo mAb 4D11–treated mice. The black lines in panel A represent staining of NK1.1+ CD3− gated spleen cells of control mice with goat–anti-mouse IgG or goat–anti-rat IgG. Panel B represents staining flow profile from a single mouse and panel C represents average percentages (mean ± SD, 5 mice per group) of NK1.1+ gated Ly49C/I+(5E6+), Ly49G2+(4D11+), Ly49D+(4E5+), and Ly49C/I+(5E6+) × Ly49G2+(4D11+) or Ly49D+(4E5+) × Ly49G2+(4D11+) NK cell population of the same experiment. The experiment was done 2 times.
Interestingly, in C57BL/6 (H2b) mice in which the Ly49G2 NK subset was removed by mAb 4D11, (Figure 6B,C) a significant increase in the percentage of Ly49C/I+ (P < .0001) and Ly49D+ NK cells (P < .0001) was noted (control, 25.9% ± 2.4% Ly49C/I+ and 19.8% ± 1.0% Ly49D+ cells; mAb 4D11 treated, 51.1% ± 2.3% Ly49C/I+ and 47.8% ± 2.8% Ly49D+ cells). Therefore, enhanced rejection of H2d BMCs observed in Ly49G2 subset–depleted C57BL/6 (H2b) mice (Figure 2B-C) may be due at least in part to an increased likelihood of allogeneic BMCs coming in contact with NK cells responsible for rejection.
In agreement with these data, mice receiving the mAb 5E6 (anti-Ly49C/I) also had a significant increase in the percentage of Ly49G2+ NK cells (Figure 6B-C), which correlated with the increased ability of H2d mice to reject H2b BMC allograft after Ly49C/I+ NK subset depletion (Figure 1B-C). The activating receptor for H2b BMC grafts has not been identified but Ly49H is a candidate because it is similar to Ly49C and Ly49I in the extracellular domain.32
Next, we did the flow cytometric analysis of Ly49 NK subsets 72 hours following depletion in the spleen cells of lethally irradiated mice. The results presented in Figure 7indicate that the percentage of NK cells following irradiation increased in the untreated (nonirradiated, 3.3%; irradiated, 23.4%) as well as Ly49C/I NK subset–depleted (nonirradiated, 1.4%; irradiated, 6.9%) or Ly49G2 NK subset–depleted group (nonirradiated, 2.6%; irradiated, 15.5%) because of killing of radiosensitive cells by irradiation and removal of NK subsets by Ly49 antibody treatments (Figure 7A). However, the total number of NK cells also decreased in the untreated control group following irradiation (nonirradiated, 2.09 × 106; irradiated, 0.86 × 106) and even further in mAb-treated groups (Figure 7B).
Effect of anti-Ly49G2 (mAb 4D11) or anti-Ly49C/I (mAb 5E6) treatment and irradiation on NK percentage and cell numbers in the spleens of C57BL/6 (H2b) mice.
Groups of 10 C57BL/6 (H2b) mice were injected intraperitoneally with 500 μg mAb 5E6 (anti-Ly49C/I) or 200 μg mAb 4D11 (anti-Ly49G2) or 0.2 mL NRS. Forty-eight hours later, 5 mice from each group were lethally irradiated at 950 cGy and 24 hours after irradiation (total 72 hours since antibody treatments) spleens from all the mice were harvested, each group of spleens pooled, and single-cell suspension prepared. Four-color flow cytometric analysis was performed as mentioned in “Materials and methods.” The figure represents NK percentage (A) and total NK cell numbers (B) in the spleens of nonirradiated and irradiated mice.
Effect of anti-Ly49G2 (mAb 4D11) or anti-Ly49C/I (mAb 5E6) treatment and irradiation on NK percentage and cell numbers in the spleens of C57BL/6 (H2b) mice.
Groups of 10 C57BL/6 (H2b) mice were injected intraperitoneally with 500 μg mAb 5E6 (anti-Ly49C/I) or 200 μg mAb 4D11 (anti-Ly49G2) or 0.2 mL NRS. Forty-eight hours later, 5 mice from each group were lethally irradiated at 950 cGy and 24 hours after irradiation (total 72 hours since antibody treatments) spleens from all the mice were harvested, each group of spleens pooled, and single-cell suspension prepared. Four-color flow cytometric analysis was performed as mentioned in “Materials and methods.” The figure represents NK percentage (A) and total NK cell numbers (B) in the spleens of nonirradiated and irradiated mice.
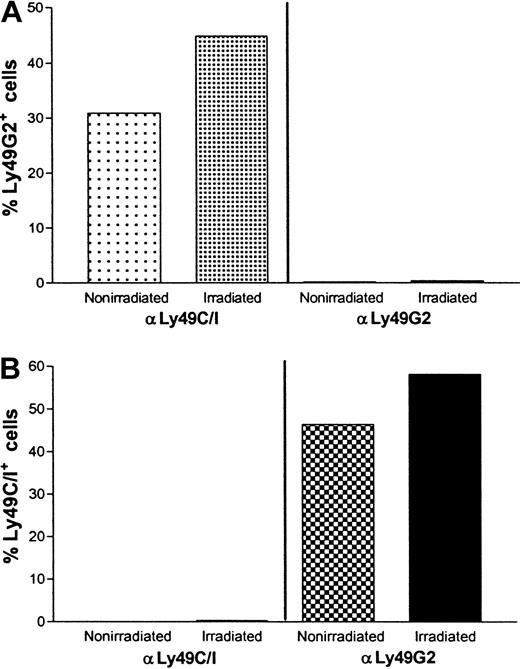
Similar to the effects observed in nonirradiated C57BL/6 (H2b) mice (Figure 6), in lethally irradiated C57BL/6 (H2b) mice, Ly49G2 NK subset removal by mAb 4D11 led to a significant increase in the percentage of Ly49C/I+ cells, whereas the Ly49C/I NK subset removal by 5E6 mAb treatment led to a significant increase in the percentage of Ly49G2+ NK cells (Figure 8A-B). These results indicate that antibody depletion of the subsets was not altered when irradiation was performed.
Effect of anti-Ly49G2 (mAb 4D11) or anti-Ly49C/I (mAb 5E6) treatment and irradiation on Ly49C/I+, Ly49G2+ NK cell numbers in the spleens of C57BL/6 (H2b) mice.
Groups of 10 C57BL/6 (H2b) mice were injected intraperitoneally with 500 μg mAb 5E6 (anti-Ly49C/I) or 200 μg mAb 4D11 (anti-Ly49G2) or 0.2 mL NRS. Forty-eight hours later, 5 mice from each group were irradiated at 950 cGy and 24 hours after irradiation spleens from all the mice were harvested (total 72 hours since antibody treatments), each group of spleens pooled and single-cell suspension was prepared. Four-color flow cytometric analysis was performed as mentioned in “Materials and methods.” Panels A and B represent NK1.1+ CD3−-gated Ly49C/I+(5E6+) and Ly49G2+(4D11+) NK cell populations in the spleens of nonirradiated and irradiated mice.
Effect of anti-Ly49G2 (mAb 4D11) or anti-Ly49C/I (mAb 5E6) treatment and irradiation on Ly49C/I+, Ly49G2+ NK cell numbers in the spleens of C57BL/6 (H2b) mice.
Groups of 10 C57BL/6 (H2b) mice were injected intraperitoneally with 500 μg mAb 5E6 (anti-Ly49C/I) or 200 μg mAb 4D11 (anti-Ly49G2) or 0.2 mL NRS. Forty-eight hours later, 5 mice from each group were irradiated at 950 cGy and 24 hours after irradiation spleens from all the mice were harvested (total 72 hours since antibody treatments), each group of spleens pooled and single-cell suspension was prepared. Four-color flow cytometric analysis was performed as mentioned in “Materials and methods.” Panels A and B represent NK1.1+ CD3−-gated Ly49C/I+(5E6+) and Ly49G2+(4D11+) NK cell populations in the spleens of nonirradiated and irradiated mice.
Discussion
We have previously described the roles of various Ly49 NK subsets (inhibitory and activating) in bone marrow allograft rejection in lethally irradiated mice.26,27,29 However, all previous studies involved transplanting limiting numbers of allogeneic BMCs, which were rejected by the control (untreated) irradiated mice. During those studies, we observed that in H2d mice, removal of the inhibitory Ly49C/I NK subset, which played no role in the rejection of allogeneic H2b grafts, augmented rejection of larger numbers of H2b BMCs.26 This suggested that such NK subsets are potentially “immunoregulatory” and may functionally regulate the NK cells that mediate marrow graft rejection.
Our previous studies examined the ability of Ly49 NK subset removal to abrogate bone marrow allograft rejection and the work presented here suggests that NK subset modulation can also enhance allograft rejection. We have demonstrated that large numbers of allogeneic BMCs can be strongly rejected by mice of a particular haplotype if the appropriate inhibitory Ly49 NK cells are depleted in vivo. Thus, in homozygous H2d or heterozygous H2bxd mice, removal of inhibitory Ly49C/I+ NK cells, which do not mediate the rejection of allogeneic H2b bone marrow grafts, significantly augmented the capability of the mice to reject H2b BMCs. The opposite effect was observed with homozygous H2b or heterozygous H2bxd mice, where removal of inhibitory Ly49G2+ NK cells, which do not mediate the rejection of allogeneic H2d bone marrow grafts, significantly augmented the capability of the mice to reject allogeneic H2d BMCs. Although in F1 (H2bxd) mice, both Ly49 receptors (Ly49C/I and G2) are exposed to their “self-ligands” (parental MHC class I, H2d and H2b, respectively) during development, which on interaction transmits inhibitory signals to NK cells, the NK cells were strongly capable of mediating allograft rejection despite living in an environment expressing inhibitory MHC.
It is possible that the enhanced rejection observed by removal of either Ly49G2+ or C/I+ NK cells is due to either the expansion in cell numbers or the enrichment of the NK subset directly mediating the rejection. Depletion of the Ly49G2+NK subset, in H2b mice (Figures 6 and 7) or the Ly49C/I+ NK subset, in H2d mice (data not shown) did not result in expansion of cell numbers of the other NK subset but did result in enrichment of Ly49C/I+ and Ly49D+ NK cells (Figures 6 and 8). Presumably, Ly49C/I+D+ NK cells mediate rejection of H2d BMCs. Conceivably, the alterations induced by depletion of NK cells expressing a given inhibitory receptor(s) increases the frequency of activating receptor-positive cells or removes inhibition by those inhibitory receptor-positive cells in NK cells capable of rejection.
It is well documented that BMC rejection in mice can be enhanced by treatment with poly I:C in vivo.33,34 Poly I:C treatment activates the macrophages, which produce IFN-α/β, and that enhances NK cell cytotoxicity.33,34 Interestingly, removal of the appropriate Ly49 NK subset enhances the BMC rejection capability of the remaining NK1.1+ cells significantly greater than poly I:C–activated cells. NK cells also produce a variety of cytokines on activation, for example, GM-CSF, IFN-γ, tumor necrosis factor α, transforming growth factor β, and so forth.35 Therefore, one mechanism may be novel NK-NK cell immunoregulation whereby one Ly49 NK subset influences another Ly49 NK subset(s) mediating rejection possibly through the production of cytokines or other factors. Removal of that subset relieves the inhibition of NK cells mediating the rejection and results in enhanced rejection of allogeneic BMCs. These studies suggest that caution must be exercised when using other Ly49 mAbs because negative controls in in vivo assays examining the role or function of a particular NK subset because activity of the remaining subsets may be heightened.
We have previously observed that in H2d mice, Ly49C/I+ NK cells produce larger amounts of the growth-promoting cytokine (GM-CSF) than the growth inhibiting cytokine (IFN-γ).36 Similarly, in H2b mice, Ly49G2+ NK cells produce larger amounts of the growth-promoting cytokine (GM-CSF) than growth inhibiting cytokine (IFN-γ; unpublished data, January 1994). Therefore, depletion of Ly49C/I+ or Ly49G2+ NK cells in a particular haplotype strain of mice may create condition(s) unfavorable for the engraftment of the BMCs.
It is conceivable that following removal of either Ly49 NK subset (Ly49C/I or G2) more than one mechanism may be involved in augmenting the ability of the mice to reject larger numbers of BMCs. Our data suggest that NK subsets are complex in that a particular inhibitory Ly49 NK subset known to play no role in rejection of BMCs of a particular haplotype can apparently prevent rejection of allogeneic marrow. Human NK cells recognizing HLA-C may play a role in marrow graft rejection.37 This study has implications for human NK subset depletion for BMT. Removal of inappropriate NK subsets could have disastrous consequences by enhancing the recipient's ability to reject bone marrow allografts. It is perhaps imperative to create reagents that deplete or inhibit activating but not inhibitory NK cell receptors.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. Animal care was provided in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals (NIH publication no. 86-23, 1985).
We thank Drs. Joost Oppenheim, Frank W. Ruscetti,and Crystal Y. Koh for critically reviewing the manuscript and Steve Stull for the superb technical assistance. We are especially grateful to Ms Laura Knott for her excellent secretarial assistance.
We thank Dr Llewellyn Mason for providing 4D11 and PK136 monoclonal antibodies.
Supported in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health (NIH), under contract no. N01-CO-12400.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
William Murphy, SAIC-Frederick, Building 567, Room 210, Frederick, MD 21702; e-mail: murphyw@ncifcrf.gov.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal