This study describes the genetic mechanisms responsible for the de novo occurrence of severe and mild hemophilia A in monozygotic twin females. Both twins were found to carry a previously known factor VIII mutation (Tyr16Cys) in the heterozygous state which most probably arose in the paternal germ line. Both twins showed concordant skewing of X inactivation toward the maternally derived normal X chromosome, the most severely affected twin exhibiting a higher percentage of inactivation of the normal X chromosome. The degree of skewing of X inactivation closely correlated with both the coagulation parameters and the clinical phenotype of the twins. Since these twins were monochorionic, such results suggest that the twinning event in this case has occurred after the onset of the X-inactivation period.
Introduction
Hemophilia A is an X-linked recessive bleeding disorder caused by deficiency of factor VIII (FVIII) which occurs in 1/5000 male births.1 Clinical severity is inversely related to residual factor VIII activity (FVIII:C) such that patients with less than 1%, 1%-5%, and 5%-30% FVIII:C are classified as severe, moderate, or mild, respectively. The molecular basis underlying hemophilia A is well characterized, and about half of severely affected individuals have large genomic inversions disrupting the FVIII gene (F8).2-4 In the remaining cases, different heterogeneous point mutations, insertions, and deletions are found scattered throughout the 26 exons, as well as in the introns ofF8.5 Hemophilia A is transmitted by heterozygous females denoted as carriers, who are generally asymptomatic since random X inactivation results in approximately equal proportions of somatic cells in which either the normal X or the mutated X chromosome is active.6 Even though the disease in females is extremely rare, a few cases have been documented, resulting from different pathophysiologic mechanisms.7-13
Here, we report monozygotic (MZ) twin girls both carrying the sameF8 mutation which results in severe hemophilia A in one twin and mild phenotype in her co-twin. Although they exhibited a different clinical and biologic severity, they both showed concordant skewing of X inactivation toward the normal X chromosome. In this respect, they differed from some pairs of MZ twin females who typically showed a “mirror-image” pattern of X inactivation. As the twins were classified as monochorionic (MC) at birth, our data strongly support the hypothesis that the splitting event in MC-MZ twin pairs occurs after the onset of X-inactivation period.
Study design
Case report
The family is presented in Figure1. Coagulation studies showed FVIII:C of less than 1% for proposita II.2 and 12% for II.3 on repeated measurements, with normal VWF:Ag and VWF:RCo levels in both cases. None of the other family members (I.1, I.2, and II.1) exhibited hemophilia or had abnormal values of plasma FVIII:C and von Willebrand factor (VWF), suggesting an absence of familial inheritance for the disease. Binding of FVIII to VWF was found normal in both twins eliminating a type 2N von Willebrand disease (VWD).14 Patient II.2 suffered from repeated bleeding episodes, that is, hematomas and hemarthroses consistent with a severe hemophilia phenotype for which she was treated with FVIII infusions. The other twin only exhibited a minor hemophilia phenotype concordant with the plasma FVIII:C evaluation. At birth, these twins were documented to be diamniotic and monochorionic after examination of the fetal-placenta anatomy type. At 10 years of age, both were bright students. The study was initiated after an informed consent was obtained according to the Declaration of Helsinki and to the national regulation on genetic analysis.
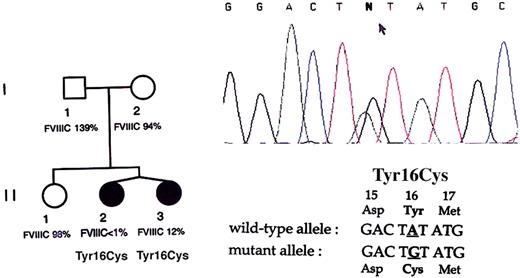
Identification of the Tyr16Cys mutation in the twins.
The family pedigree is presented on the left side with the corresponding plasma FVIII activity for each member. Sequence chromatogram from twin II.1 is shown on the right side with the relevant wild-type and mutant partial sequences of the F8gene noted under the chromatogram. The arrow points to the double peaks of A and G at the second nucleotide position of codon 16, demonstrating a nucleotide transition in the heterozygous state.
Identification of the Tyr16Cys mutation in the twins.
The family pedigree is presented on the left side with the corresponding plasma FVIII activity for each member. Sequence chromatogram from twin II.1 is shown on the right side with the relevant wild-type and mutant partial sequences of the F8gene noted under the chromatogram. The arrow points to the double peaks of A and G at the second nucleotide position of codon 16, demonstrating a nucleotide transition in the heterozygous state.
FVIII binding assay to VWF
FVIII binding to VWF assay was performed as previously described.14
Molecular F8 gene studies
Genomic DNA was extracted from lymphocytes by standard procedures.
X-chromosome inactivation at the HUMARA locus
Assessment of X inactivation was performed using anHpaII polymerase chain reaction (PCR) assay for the X-linked human androgen receptor gene (HUMARA).15 Genomic DNA samples, one predigested with the methylation-sensitive enzymeHpaII (Boehringer Mannheim, Meylan, France) and the other without predigestion, were subjected to PCR amplification with specific fluorescent primers flanking both HpaII sites plus a highly polymorphic (CAG) repeat sequence in the first exon of the HUMARA. Only the methylated allele from the inactive X chromosome which resistsHpaII cleavage was amplified. The PCR products were subjected to automated DNA sequencer analysis (model ABI 377, Applied Biosystems, Courtaboeuf, France) and the results analyzed by GeneScan software (Applied Biosystems). This assay was modified to facilitate quantitation of X inactivation at each allele, as described by Pegoraro et al.16
X-chromosome inactivation at the FMR-1 locus
DNA samples were digested with EcoRI plus the methylation-sensitive enzyme, EagI, and were subjected to Southern blot analysis, as described.17 This assay, which can detect fragments containing the (CGG)n and the CpG island in the 5′ untranslated region (UTR) of the FMR-1 gene, revealed the methylation status of this CpG island and also indicated possible abnormal expansion of the (CGG)n repeat.
Results and discussion
In order to elucidate the genetic mechanisms responsible for hemophilia A in these MZ twin females, molecular investigations were carried out to characterize the F8 molecular defect. First, DNAs from both twins were subjected to BclI Southern blot analysis for F8 intron 22 inversions, and no altered migration pattern was observed (data not shown). All coding sequences and exon-intron boundaries of F8 were then screened by direct sequencing. Exon 1 from both twins was found to contain a single base-pair transition from A to G in codon 16, predicting the replacement of tyrosine (Tyr) by cysteine (Cys) in the factor VIII protein sequence (Figure 1). This alteration was previously reported to be associated with severe hemophilia A (http://europium.csc.mrc.ac.uk). No further F8 sequence alteration was found in the twins. The missense mutation was not detected in the twins' healthy sister or in the parents, suggesting that it had occurred de novo in the germ line of one parent. Both twins carried the mutation in the heterozygous state, indicating that the clinical expression of hemophilia A was not consecutive to homozygosity for the codon 16 mutation.
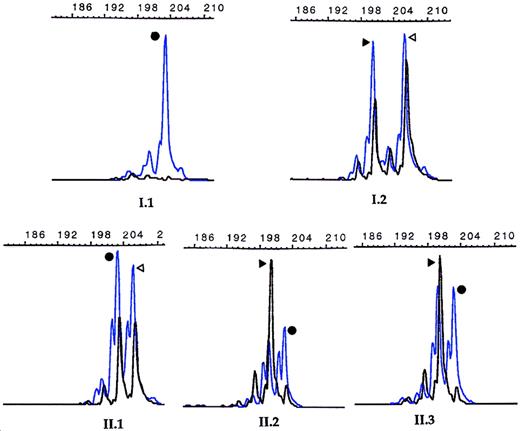
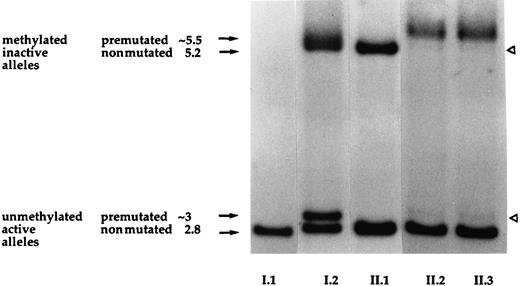
Since the unbalanced X-chromosome inactivation process is currently recognized to be responsible for expression of X-linked recessive disorders in heterozygous females, we examined DNA methylation at the X-linked HUMARA locus. All 4 females (I.2, II.1, II.2, and II.3) were found to be informative as shown in Figure2. The healthy sister (II.1) showed 2 amplification peaks corresponding to approximately an equal methylation of the maternal and paternal X alleles, thus reflecting a random X inactivation. Conversely, only one peak was observed for each twin, which corresponded to inactivation, or methylation, of the maternally derived allele, indicating a skewed X-chromosome inactivation (Figure2). A quantitative evaluation of the degree of intrapair differences indicated that patient II.2 showed an “extremely skewed” profile (ratio 100:0), while the profile for patient II.3 was “skewed” (85:15), suggesting that a small proportion of her mother'sF8 allele remained active. The difference in severity of the disease between the twins correlated with the degree of skewing of X inactivation. Although X-inactivation analysis could not be tested from liver where factor VIII is primarily synthesized, we hypothesized that both twins probably had similar X-inactivation patterns in the liver as suggested by plasma factor VIII values. Since both twins were clinically affected and both showed an inactivation of the maternal X chromosome, we concluded that Tyr16Cys mutation occurred on the paternal X chromosome. We also studied the methylation of the 5′ region of the FMR-1 gene, and the results corroborated those found for the HUMARA locus (Figure 3). This assay, routinely used for diagnosis of the fragile X syndrome, revealed unexpectedly that both twins had inherited from their mother a premutated allele of 80-90 (CGG) repeats. Although this premutation is known to have a high risk of expanding further to a “full” mutation when it is transmitted by a female, the twins carried approximately the same number of copies as the mother. As seen in Figure 3, a band of 2.8 kb was found for the father (I.1), corresponding to an active unmethylated X chromosome (normal male pattern). The healthy sister showed a normal female pattern with random X inactivation since both bands of 2.8 kb and 5.2 kb were present in equal quantities, reflecting the unmethylated and the methylated alleles, respectively. The mother, conversely, showed 2 doubled bands at approximately 2.8 kb and 5.2 kb present in equal quantities. These bands corresponded to the normal and premutated FMR-1 alleles, both being present in the methylated and unmethylated states, typical of a carrier with random X inactivation. Both twins showed 4 bands, as did the mother, but the pattern was further complicated by the fact that X inactivation was skewed, leading to 2 weaker bands representing the methylated paternally derived X chromosome and the unmethylated maternally derived X chromosome carrying the triplet expansion.
X-chromosome inactivation analysis.
Results obtained at the HUMARA locus. The blue peaks and the black peaks correspond to PCR products before and after HpaII digestion, respectively. The length of the PCR fragments are noted on the top of each histogram, and the father had a 203-bp fragment in his only X allele, while the mother had both a 200-bp and a 206-bp fragment in her 2 alleles. The mother's 206-bp fragment was transmitted to the healthy daughter (II.1), while the mother's other X-chromosome containing the 200-bp fragment went to the twins (II.2, II.3). The 2 black peaks in approximately equal proportion are seen in X-chromosomes randomly inactivated from females I.2 and II.1, whereas only one black peak corresponding to the mother's 200-bp inactivated allele is observed in the twins (II.2 and II.3), indicating a skewed X-chromosome inactivation toward the maternally 200-bp–derived allele.
X-chromosome inactivation analysis.
Results obtained at the HUMARA locus. The blue peaks and the black peaks correspond to PCR products before and after HpaII digestion, respectively. The length of the PCR fragments are noted on the top of each histogram, and the father had a 203-bp fragment in his only X allele, while the mother had both a 200-bp and a 206-bp fragment in her 2 alleles. The mother's 206-bp fragment was transmitted to the healthy daughter (II.1), while the mother's other X-chromosome containing the 200-bp fragment went to the twins (II.2, II.3). The 2 black peaks in approximately equal proportion are seen in X-chromosomes randomly inactivated from females I.2 and II.1, whereas only one black peak corresponding to the mother's 200-bp inactivated allele is observed in the twins (II.2 and II.3), indicating a skewed X-chromosome inactivation toward the maternally 200-bp–derived allele.
Results of the southern blot assay at the FMR-1 locus.
Lane numbers correspond to individuals in the pedigree and fragment sizes are indicated in kilobases at the side of the figure. The father (I.1) exhibits a normal pattern with a unique 2.8-kb band corresponding to his unmethylated active X-chromosome, whereas in normal females both bands of 2.8 kb and 5.2 kb are present and correspond to their active and inactive alleles (individual II.1). The mother (I.2) exhibits a pattern of 4 bands, suggestive of a premutated allele in the methylated and unmethylated states (bands of ∼5.5 kb and 3 kb, respectively). Subjects I.2 and II.1 exhibit a random X-chromosome inactivation as indicated by an equal intensity of each fragment. The twins (II.2 and II.3) show a pattern of 4 bands like their mother but 2 bands are weaker (white arrowheads), indicating a skewed X-chromosome inactivation.
Results of the southern blot assay at the FMR-1 locus.
Lane numbers correspond to individuals in the pedigree and fragment sizes are indicated in kilobases at the side of the figure. The father (I.1) exhibits a normal pattern with a unique 2.8-kb band corresponding to his unmethylated active X-chromosome, whereas in normal females both bands of 2.8 kb and 5.2 kb are present and correspond to their active and inactive alleles (individual II.1). The mother (I.2) exhibits a pattern of 4 bands, suggestive of a premutated allele in the methylated and unmethylated states (bands of ∼5.5 kb and 3 kb, respectively). Subjects I.2 and II.1 exhibit a random X-chromosome inactivation as indicated by an equal intensity of each fragment. The twins (II.2 and II.3) show a pattern of 4 bands like their mother but 2 bands are weaker (white arrowheads), indicating a skewed X-chromosome inactivation.
MZ twins may be monochorionic (MC-MZ) or dichorionic (DC-MZ), depending on whether they develop in a single or 2 distinct chorionic sacs. DC-MZ twinning occurs prior to or around the onset of X inactivation and MC-MZ twinning occurs later.18 It might therefore be expected that a discordant X-inactivation pattern would more frequently be seen in DC-MZ twins than in MC-MZ twins. However, anecdotal examples of discordant female MC-MZ twins have been reported where the affected twin exhibited a selective inactivation of the normal X chromosome while her normal co-twin showed either random X inactivation or skewing toward the mutant X chromosome.19-22 Several theoretical explanations for such “mirror-image” inactivation have been proposed. It was initially suggested that random X inactivation followed by asymetric splitting of the inner cell mass would result in only one affected twin because she received a majority of cells in which the normal allele had been inactivated.20 More recently, Monteiro et al23 proposed that in fact MC-MZ twin pairs reflected a heterogeneous group slightly differing in the timing of the twinning event after the onset of X inactivation. MC-MZ pairs indeed split more or less closely after the onset of X inactivation, explaining the X inactivation differences observed between the twins.23 They demonstrated that for each round of replication after the onset of X inactivation, the probability of creating a difference in X inactivation decreased if a twinning event occurred. The genetic profile of MC-MZ twins described here is in accordance with this model. The preferential inactivation of the same X chromosome in both twins likely indicates that the twinning event occurred at a relatively later time than for MC-MZ twins who show discordant X inactivation.
In conclusion, reports of such twins with a correct determination of the fetal-placenta anatomy type provide invaluable information to further understanding of X inactivation and twinning processes in humans.
We would like to thank Dr Claudine Mazurier for the FVIII binding assay to VWF. We also wish to thank professors Cherif Beldjord and Laurent Richard for their technical help with the DNA methylation analysis at the FMR1 gene, and professor Noël Philippe who referred the twins and the family. We would like to express our gratitude to the members of this family for their participation in this study.
Prepublished online as Blood First Edition Paper, June 21, 2002; DOI 10.1182/blood-2002-01-0277.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Claude Negrier, Centre de traitement de l'hémophilie, Laboratoire d'Hémostase, Hôpital Edouard Herriot, Pavillon E, Place d'Arsonval, 69374 Lyon Cédex 08, France; e-mail: claude.negrier@chu-lyon.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal