Studies have suggested a pivotal role for free sulfhydryls in platelet integrin function, and enzyme-mediated reduction of disulfide bonds on platelets has been implicated. The platelet fibrinogen receptor αIIbβ3 is the best-studied platelet integrin and serves as a model system for studying the structure-function relation in this family of adhesion receptors. The demonstration of free sulfhydryls on the exofacial domain of purified αIIbβ3, specifically in its activated conformation, prompted us to explore the potential for activation-dependent, enzymatically catalyzed thiol expression on intact platelets and the possible role of surface-associated protein disulfide isomerase (PDI) in αIIbβ3 ligation. Using the membrane-impermeant sulfhydryl blocker para-chloromercuriphenyl sulfonate, the inhibitor of disulfide exchange bacitracin, and the monoclonal anti-PDI antibody RL90, we examined fibrinogen binding to αIIbβ3 as well as ligation-induced allosteric changes in the conformation of αIIbβ3. We sought to distinguish the possible involvement of disulfide exchange in agonist-induced platelet stimulation from its role in integrin ligation. Analysis of the role of free thiols in platelet aggregation suggested a thiol-independent initial ligation followed by a thiol-dependent stabilization of binding. Flow cytometric analysis showed that sustained binding of fibrinogen, as well as expression of ligand-induced binding site epitopes and ligand-bound conformation, depended on free thiols and disulfide exchange. Expression of P-selectin was minimally affected, even with complete inhibition of αIIbβ3function. These data indicate that although agonist-induced platelet stimulation is independent of ecto-sulfhydryls, engagement of integrin αIIbβ3 on the intact platelet depends totally on their enzymatically catalyzed surface expression.
Introduction
The affinity of integrins for their ligands is tightly regulated, both by cellular events and subsequent to ligand binding.1-5 Such cellular control over the interaction of extracellular proteins implies the existence of a mechanism to propagate information back and forth between the extracellular head and the cytoplasmic tail of the integrin receptor. In recent years, the posited mechanism has been the allosteric conformational changes occurring in the integrin receptor, both in response to cellular events that switch the receptor from low to high affinity (inside-out signaling) and external events that relay information about occupancy of the receptor (outside-in signaling).6-9 Although information about the nature of the changes in conformation is slowly emerging (for review, see Woodside et al10) and consequent changes in intracellular interactions are widely documented,10-13 the molecular mechanism regulating such changes, particularly on the exofacial domain, has not been elucidated. We previously reported that extracellular sulfhydryls participate in conformational changes triggered by interaction of the platelet integrin α2β1 with its natural ligand collagen.14 We also found that disulfide exchange is required for platelet adhesion mediated by integrins α5β1 and αIIbβ3as well as α2β1 and that surface-expressed protein disulfide isomerase (PDI) is involved in this exchange.15 Furthermore, involvement of disulfide exchange in receptor function is a feature specific to the integrin-receptor family (J.L. et al, submitted manuscript, 2002). We therefore proposed that conformational changes subsequent to ligand interaction with the integrin lead to exchange of disulfide bonds on the integrin and that these bonds stabilize the conformation induced by ligand binding and enable sustained binding.
Several reports support this hypothesis. Schwartz and Harlan16,17 showed that exposure of free sulfhydryls on the surface of neutrophils promotes their integrin-mediated adhesion to endothelial cells and that blocking naturally occurring free sulfhydryls on the neutrophils with membrane-impermeant reagents inhibits their adhesion to endothelial cells. Expression of free sulfhydryls on the extracellular domain of the purified platelet integrin αIIbβ3 in its active conformation was recently demonstrated.9,18 19
The expression of PDI activity on the surface of cells, including platelets,20,21 indicates the importance of disulfide exchange on the surface of cells in general and platelets in particular. Furthermore, inhibition of PDI was found to block agonist-induced platelet aggregation.22 O'Neill et al23 reported that the platelet integrin αIIbβ3 itself has disulfide-exchange activity and assigned this activity to 9 repeats in β3 of a motif homologous with the active site of enzymes involved in disulfide exchange. The purpose of the study described here was to examine the role of disulfide exchange and surface-associated PDI in direct ligation of the integrin and induced conformational changes in the context of the intact whole cells, using the platelet integrin αIIbβ3 as the model system.
Materials and methods
Materials
Highly purified human fibrinogen (Enzyme Research Laboratories, South Bend, IN) was conjugated with fluorescein isothiocyanate (FITC) by using FITC-celite (Calbiochem-Novabiochem, Bad Soden, Germany) according to the method of Xia et al,24 with the following modifications: incubation was carried out at pH 7.8 for 48 hours at 4°C, resulting in an FITC-to-protein ratio of 5.0 to 5.2. Type I collagen prepared for use in flow cytometry was described previously.25 The triple-helical peptide, collagen-related peptide Gly-Lys-Hyp-(Gly-Pro-Hyp)10-Gly-Lys-Hyp-Gly (CRP), was synthesized and cross-linked by means of its lysyl residues to yield CRP-XL, as described previously.26
Platelet agonists used in aggregation studies were from Bio/Data (Horsham, PA). Para-chloromercuriphenyl sulfonate (pCMPS), dithiobis-nitrobenzoic acid (DTNB), bacitracin, IgG2acontrol ascites, FITC-conjugated anti-mouse IgG (sheep F(ab′)2), and FITC-conjugated mouse isotype-specific IgG1 were from Sigma (Deisenhofen, Germany). All reagents were dissolved directly in phosphate-buffered saline (PBS). FITC–anti-P-selectin (clone CLB-thromb/6) was from Immunotech (Marseilles, France). Quantum 26p FITC-standard beads were from Flow Cytometry Standards (Leiden, The Netherlands).
The monoclonal anti-rat PDI clone RL90, which recognizes human PDI, was obtained from Alexis Biochemicals (Switzerland). FITC-conjugated monoclonal anti-αIIbβ3(PAC-1) was obtained from Becton Dickinson (Heidelberg, Germany). Anti-FcγRII (clone IV.3) was from Medarex (Annandale, NJ). Antibodies anti–ligand-induced binding site (LIBS) 6 and PMI-1 were a gift from Dr Mark Ginsberg, Department of Vascular Biology, Scripps Research institute, La Jolla, CA.
Preparation of platelet-rich plasma (PRP)
Blood was obtained from informed healthy volunteers who had not taken any medication affecting platelet function for at least 2 weeks before the study. Approval was obtained from the University of Muenster institutional review board. Informed consent was provided according to the Declaration of Helsinki. With loose application of a tourniquet, venous blood was drawn from the antecubital vein, anticoagulated with trisodium citrate (0.0108 M/L), and processed within 1 hour of collection to prevent cellular activation. PRP was prepared by differential centrifugation at 200g for 10 minutes at room temperature.
Platelet-aggregation studies
Platelet-aggregation studies were performed in an aggregometer (Chrono-Log, Haverton, PA, or Bio/Data Corporation, Horsham, PA) using PRP adjusted to 2 × 108 platelets/mL with autologous plasma. Platelets were activated by using adenosine diphosphate (ADP; 2-4 μM), epinephrine (0.5-4 μM), thrombin (0.375-0.5 U/mL), arachidonic acid (0.125 mg/mL), type I collagen (0.5-1.25 μg/mL), or ristocetin (1.5 mg/mL) in the presence or absence of pCMPS (125-300 μM) or DTNB (2.5 mM), bacitracin (3-6 mM), or RL90 (in ascites, diluted as indicated).
When the effect of RL90 was tested, the PRP was first incubated for 10 minutes with anti-FcγRII antibody (clone IV.3; 20 μg/mL) to prevent activation by means of the Fc receptor. RL90 serially diluted in PBS was then added, incubation was carried out for 10 minutes, and agonist-induced aggregation was assessed. In parallel, an isotype-matched IgG mouse ascites control was added to PRP in the presence of anti-FcγRII antibody (clone IV.3; 20 μg/mL), and agonist-induced aggregation was assessed.
Flow cytometry
Fibrinogen binding.
PRP diluted in autologous plasma to a concentration of 5 × 107 platelets/mL was preincubated with pCMPS or bacitracin for 10 minutes at room temperature followed by 150 μg/mL fibrinogen-FITC (saturating concentration) for 3 minutes at room temperature as described previously.27 When the effect of RL90 was studied, platelets were preincubated with the Fab fragment of anti-FcγRII (clone IV.3) at a saturating concentration (20 μg/mL) to prevent Fc-receptor–mediated platelet activation by anti-PDI. After preincubation, 100 μL of platelet suspension was added to 10 μL of a solution containing one of the platelet agonists. CRP-XL25 or type I collagen28 were in 0.01 M acetic acid and thrombin was in PBS (pH 7.6) in the presence of 1.25 mM of the peptide Gly-Pro-Arg-Pro (GPRP) to prevent fibrin polymerization29; ADP was in PBS. The reaction was stopped after 3 minutes by fixation with 1% formaldehyde in PBS for 30 minutes. The platelets were washed and resuspended in 500 μL PBS and analyzed with fluorescence-activated cell-sorter scanning (FACS; FACScan flow cytometer; Becton Dickinson); 5000 single platelets were assessed.
Specific binding of monoclonal antibodies was calculated by subtracting nonspecific binding as determined with an FITC-labeled mouse isotype-specific IgG. The nonspecific background labeling was measured by using platelets from patients with thrombasthenia type I and control platelets treated with 10 nM of the inhibitory Gly-Arg-Gly-Asp-Ser-Pro to prevent specific fibrinogen binding.
LIBS antibody binding.
Diluted PRP (5 × 107 platelets/mL) was preincubated with different concentrations of pCMPS, bacitracin, or RL90 as described above. Pretreated platelets (100 μL) were added to 10 μL of the agonists collagen (1 μg/mL), CRP-XL (0.5 μg/mL), or thrombin (0.2 U/mL in the presence of GPRP) at room temperature. After 3 minutes, PAC-1–FITC (5 μg/mL saturating concentration) or PMI-1 was added and incubation was done for 30 minutes at room temperature. If PMI-1 was being used, samples were subsequently labeled with FITC-conjugated anti-mouse IgG. The platelet samples were then diluted with 0.5 mL PBS and analyzed in the flow cytometer.
Expression of CD62P.
Measurement of CD62P expression was performed by using the method of Frenette et al30 with some modifications. Briefly, diluted PRP (5 × 107 platelets/mL) was treated with pCMPS, bacitracin, or RL90 followed by collagen, CRP-XL, or thrombin stimulation. The sample was divided into 3 parts, and FITC-coupled monoclonal anti-CD62P was added at a predetermined saturating concentration to one third of the sample, FITC-fibrinogen to another third, and PAC-1 to the remaining third. After incubation for 30 minutes at room temperature, the samples were analyzed by FACS.
Fibrinogen binding to platelets treated with activating antibody.
FITC-fibrinogen was added to diluted PRP (5 × 107platelets/mL) in the presence of pCMPS, bacitracin, or RL-90. The platelets were incubated with the Fab fragment of anti-FcγRII (clone IV.3) at a saturating concentration (20 μg/mL) to prevent Fc-receptor–mediated platelet activation by anti-LIBS6. Then, activating anti-αIIbβ3 monoclonal antibody anti-LIBS6 was added at the lowest concentration predetermined to trigger maximal binding of FITC-fibrinogen. After 10 minutes at room temperature, the platelets were diluted with 0.5 mL PBS and the amount of bound fibrinogen was determined by FACS.
Results
Effect of extracellular thiol blockers on platelet aggregation
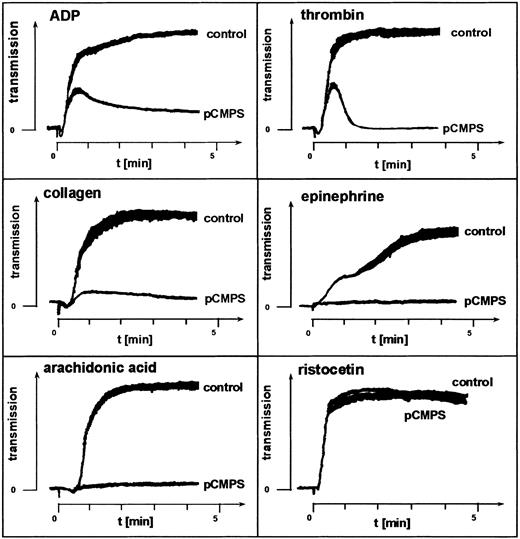
Two inhibitors of free sulfhydryls that do not penetrate the platelet membrane, pCMPS and DTNB, were used to examine the role of ecto-sulfhydryls in platelet aggregation. At concentrations of 125 to 300 μM, pCMPS blocked platelet aggregation induced by the agonists ADP, epinephrine, thrombin, arachidonic acid, or collagen (Figure1). DTNB had the same effect at a concentration of 2 mM (data not shown). Neither pCMPS (Figure 1) nor DTNB (data not shown) inhibited agglutination induced by ristocetin. We observed that, invariably, shape change was not affected by thiol blocking and that thrombin and ADP triggered the first wave of aggregation, which was followed by a rapid disaggregation (Figure 1). These experiments were repeated with PRP obtained from different donors. Similar results were obtained in all experiments, indicating a role for extracellular thiols in fibrinogen-mediated platelet aggregation.
Fibrinogen-mediated platelet aggregation depends on exofacial free thiols.
Effect of membrane-impermeant thiol-blocker pCMPS on agonist-induced platelet aggregation. The pCMPS was added to PRP, and aggregation induced by the agonists indicated was assessed without further incubation. Shown is one representative experiment of 5.
Fibrinogen-mediated platelet aggregation depends on exofacial free thiols.
Effect of membrane-impermeant thiol-blocker pCMPS on agonist-induced platelet aggregation. The pCMPS was added to PRP, and aggregation induced by the agonists indicated was assessed without further incubation. Shown is one representative experiment of 5.
Effect of inhibiting enzymatic catalysis of disulfide isomerization on platelet aggregation
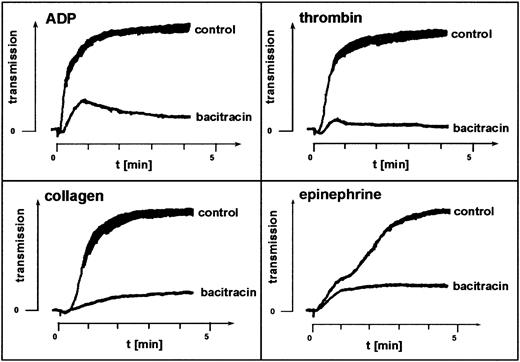
The membrane-impermeant cyclic antibiotic bacitracin inhibits enzymatic catalysis of disulfide exchange.31-33 Platelet aggregation in the presence of 3 to 6 mM bacitracin mimicked the effect of the thiol blockers pCMPS and DTNB. In samples from 5 different donors, bacitracin inhibited agonist-induced aggregation, blocking the second but not the first wave of ADP- or thrombin-induced aggregation and not affecting shape change (Figure2). This suggested involvement of enzymatically catalyzed disulfide exchange in the sulfhydryl-dependent integrin function.
Fibrinogen-mediated platelet aggregation depends on disulfide exchange.
Effect of the disulfide-exchange blocker bacitracin on agonist-induced platelet aggregation. Bacitracin was added to PRP, and aggregation induced by the agonists indicated was assessed without further incubation. Shown is one representative experiment of 5.
Fibrinogen-mediated platelet aggregation depends on disulfide exchange.
Effect of the disulfide-exchange blocker bacitracin on agonist-induced platelet aggregation. Bacitracin was added to PRP, and aggregation induced by the agonists indicated was assessed without further incubation. Shown is one representative experiment of 5.
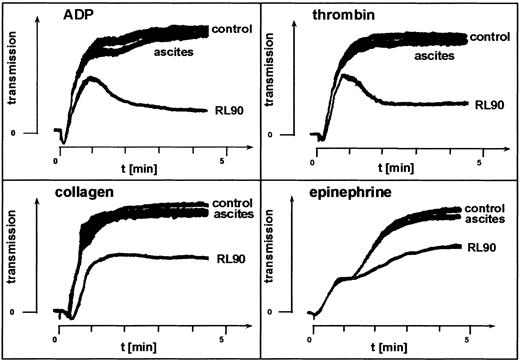
The possible role of membrane-associated PDI in this enzymatic catalysis was examined by directly inhibiting the enzyme using monoclonal anti-PDI clone RL90. This antibody inhibited platelet aggregation, whereas an isotype-matched control mouse IgG had no such effect (Figure 3), thus confirming involvement of PDI in agonist-induced aggregation.
Fibrinogen-mediated platelet aggregation depends on PDI.
Effect of the monoclonal anti-PDI clone RL90 on agonist-induced platelet aggregation. PRP was incubated with anti-FcγRII antibody clone IV.3. Monoclonal anti-PDI, clone RL90, or isotype-matched control mouse IgG in ascites fluid diluted 1:500 in saline was added to the PRP. Aggregation induced by the agonists indicated was assessed after 10 minutes of incubation. Shown is one representative experiment of 3.
Fibrinogen-mediated platelet aggregation depends on PDI.
Effect of the monoclonal anti-PDI clone RL90 on agonist-induced platelet aggregation. PRP was incubated with anti-FcγRII antibody clone IV.3. Monoclonal anti-PDI, clone RL90, or isotype-matched control mouse IgG in ascites fluid diluted 1:500 in saline was added to the PRP. Aggregation induced by the agonists indicated was assessed after 10 minutes of incubation. Shown is one representative experiment of 3.
Binding of fibrinogen
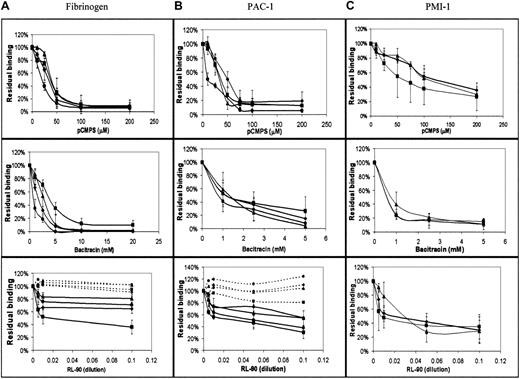
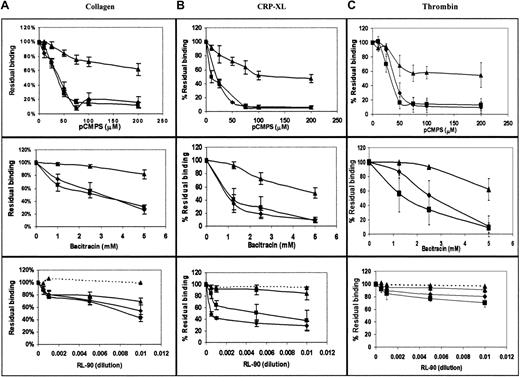
In view of the indicated involvement of extracellular disulfide exchange in platelet-platelet interaction, we measured the effect of blockade of extracellular thiols, inhibition of disulfide exchange, and specific inhibition of PDI on the direct interaction of fibrinogen with its integrin receptor. We used FACS analysis to measure binding of FITC-labeled fibrinogen to agonist-stimulated platelets,25first as a function of agonist and inhibitor concentrations. As expected, we found that the amount of bound fibrinogen was directly proportional to the agonist concentration (data not shown). The level of bound fibrinogen in the presence of membrane-impermeant pCMPS was inversely proportional to the concentration of the thiol blocker and reached complete inhibition at a concentration of 50 to 100 μM pCMPS (Figure 4A). Blocking the extracellular thiols inhibited fibrinogen binding to platelets stimulated by ADP, thrombin, collagen, or the glycoprotein VI (GPVI)–specific agonist CRP-XL (Figure 4A). Thus, blockade of extracellular thiol inhibited sustained interaction between αIIbβ3 and its ligand.
Binding of fibrinogen and expression of the ligand-induced conformation depend on disulfide-exchange–induced expression of free thiols and on PDI.
(A) Concentration-dependent effect of pCMPS, bacitracin, and RL90 on binding of FITC-fibrinogen to platelets in autologous plasma stimulated by ADP (diamonds), collagen (squares), thrombin (triangles), and CRP-XL (circles). The total fluorescence at each concentration of the inhibitor was expressed as the percentage of fluorescence in its absence, defined as 100%. Binding in the presence of the inhibitors is shown in solid lines, and binding in the presence of IgG2aascites (a control for the effect of RL90 in ascites) is shown in dotted lines. Data are mean ± SD from 3 different determinations using samples from 3 different donors. (B) Binding of FITC-conjugated PAC-1 under the same conditions as in panel A. (C) Binding of monoclonal PMI-1, followed by FITC-conjugated anti-mouse IgG, under the same conditions as in panel A.
Binding of fibrinogen and expression of the ligand-induced conformation depend on disulfide-exchange–induced expression of free thiols and on PDI.
(A) Concentration-dependent effect of pCMPS, bacitracin, and RL90 on binding of FITC-fibrinogen to platelets in autologous plasma stimulated by ADP (diamonds), collagen (squares), thrombin (triangles), and CRP-XL (circles). The total fluorescence at each concentration of the inhibitor was expressed as the percentage of fluorescence in its absence, defined as 100%. Binding in the presence of the inhibitors is shown in solid lines, and binding in the presence of IgG2aascites (a control for the effect of RL90 in ascites) is shown in dotted lines. Data are mean ± SD from 3 different determinations using samples from 3 different donors. (B) Binding of FITC-conjugated PAC-1 under the same conditions as in panel A. (C) Binding of monoclonal PMI-1, followed by FITC-conjugated anti-mouse IgG, under the same conditions as in panel A.
Bacitracin also inhibited fibrinogen binding induced by the same agonists as pCMPS, in a concentration-dependent manner (Figure 4A); complete inhibition was reached at the same concentration that inhibits platelet adhesion14 and aggregation (Figure 2). These findings suggest that disulfide exchange is necessary for sustained ligation of αIIbβ3.
To evaluate the possible role of surface-associated PDI, we measured the effect of monoclonal anti-PDI clone RL90 on fibrinogen binding. We found that anti-PDI partially inhibited fibrinogen binding induced by the same agonists as above (Figure 4A). Matched IgG controls had no such effect.
Binding of PAC-1 and PMI-1
Because of the observed role of thiols in the binding of fibrinogen, we studied their possible involvement in the ligand-induced conformational change of its receptor. The effect of thiol blocking and of the inhibition of disulfide exchange on the conformation of the integrin αIIbβ3 was examined by assessing binding of LIBS antibodies PAC-1 and PMI-1 to agonist-stimulated platelets. Binding of either antibody was inhibited by pCMPS, bacitracin, or RL90 but not by a matched IgG control, in a manner very similar to the binding of fibrinogen (Figure 4B,C), thereby indicating that acquisition of the ligand-induced conformation could require disulfide exchange.
Expression of P-selectin
Inhibition of binding of fibrinogen as well as of LIBS antibodies could also imply inhibition of platelet activation by disulfide inhibitors and thiol blockers. To distinguish inhibition of integrin ligation from inhibition of platelet stimulation, we examined the surface expression of P-selectin, a marker of platelet stimulation and vesicular secretion, concomitantly with fibrinogen and PAC-1 binding. Expression of P-selectin on the surface of platelets after agonist-induced stimulation in the presence of pCMPS, bacitracin, and RL90 (Figure 5) was minimally inhibited by either of the inhibitors, whereas binding of fibrinogen and PAC-1, measured in parallel, was markedly inhibited. These findings corroborated our other results indicating thiol involvement in integrin ligation.
Disulfide-exchange–induced thiol expression is necessary for αIIbβ3 ligation but not for agonist-induced expression of P-selectin.
Platelets were stimulated with collagen (A), CRP-XL (B), or thrombin (C) in the presence of FITC-fibrinogen (squares), monoclonal antibody PAC-1 (diamonds), or anti–P-selectin (triangles) and of increasing concentrations of pCMPS, bacitracin, or RL90 (solid lines) or a matched IgG control (broken lines). The total fluorescence at each concentration of inhibitor was expressed as the percentage of fluorescence in the absence of inhibitor, defined as 100%. Data are mean ± SD from 4 different determinations using samples from 4 different donors. Concomitant determination of bound FITC-fibrinogen, PAC-1 and, anti–P-selectin to agonist-stimulated platelets distinguished between inhibition of αIIbβ3activation and platelet stimulation.
Disulfide-exchange–induced thiol expression is necessary for αIIbβ3 ligation but not for agonist-induced expression of P-selectin.
Platelets were stimulated with collagen (A), CRP-XL (B), or thrombin (C) in the presence of FITC-fibrinogen (squares), monoclonal antibody PAC-1 (diamonds), or anti–P-selectin (triangles) and of increasing concentrations of pCMPS, bacitracin, or RL90 (solid lines) or a matched IgG control (broken lines). The total fluorescence at each concentration of inhibitor was expressed as the percentage of fluorescence in the absence of inhibitor, defined as 100%. Data are mean ± SD from 4 different determinations using samples from 4 different donors. Concomitant determination of bound FITC-fibrinogen, PAC-1 and, anti–P-selectin to agonist-stimulated platelets distinguished between inhibition of αIIbβ3activation and platelet stimulation.
Effect of inhibitors on ligation of the high-affinity–state integrin
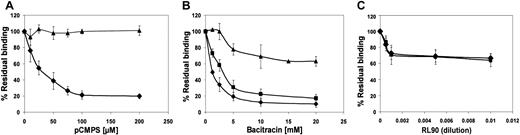
As expected, in the presence of anti-LIBS6, binding of fibrinogen and PAC-1 was much greater than their binding to untreated platelets (data not shown). Exposure of platelets to pCMPS or bacitracin before anti-LIBS6 was added had no effect on binding of the antibody but did inhibit binding of fibrinogen and PAC-1 to the platelets (Figure6). Exposure to RL90 partially inhibited binding of fibrinogen and PAC-1 induced by anti-LIBS6, further suggesting at least some involvement of surface-associated PDI.
Conversion of αIIbβ3 to the high-affinity state does not circumvent dependence on disulfide-exchange–induced thiol expression for binding of fibrinogen and change in conformation.
Platelets were incubated with activating antibody anti-LIBS6 in the presence of FITC-fibrinogen (squares), monoclonal antibody PAC-1 (diamonds), or FITC–anti-mouse IgG (triangles) and of increasing concentrations of pCMPS (A), bacitracin (B), or RL90 (C). The total fluorescence at each concentration of inhibitor was expressed as the percentage of fluorescence in the absence of inhibitor, defined as 100%. Data are mean ± SD from 3 different determinations using samples from 3 different donors. Concomitant determination of bound FITC-fibrinogen and PAC-1 induced by anti-LIBS6, as well as of bound anti-LIBS6 itself, showed that direct activation of αIIbβ3 is not sufficient for thiol-dependent ligation of the receptor.
Conversion of αIIbβ3 to the high-affinity state does not circumvent dependence on disulfide-exchange–induced thiol expression for binding of fibrinogen and change in conformation.
Platelets were incubated with activating antibody anti-LIBS6 in the presence of FITC-fibrinogen (squares), monoclonal antibody PAC-1 (diamonds), or FITC–anti-mouse IgG (triangles) and of increasing concentrations of pCMPS (A), bacitracin (B), or RL90 (C). The total fluorescence at each concentration of inhibitor was expressed as the percentage of fluorescence in the absence of inhibitor, defined as 100%. Data are mean ± SD from 3 different determinations using samples from 3 different donors. Concomitant determination of bound FITC-fibrinogen and PAC-1 induced by anti-LIBS6, as well as of bound anti-LIBS6 itself, showed that direct activation of αIIbβ3 is not sufficient for thiol-dependent ligation of the receptor.
Discussion
We previously established a role for disulfide exchange and PDI in platelet adhesion mediated by integrin αIIbβ3 as well as by integrins α2β1 and α5β1.14 In the study described here, we focused on thiol regulation of the molecular interaction of αIIbβ3 with its ligand on intact platelets. Membrane-impermeant inhibitors of thiols, disulfide exchange, and PDI were used in conjunction with purified fibrinogen, probes specific to LIBSs on the receptor, extracellular activator of αIIbβ3, and a platelet-stimulation reporter.
The first step was to verify involvement of exofacial thiols in fibrinogen-mediated platelet-platelet interaction. We observed that agonist-induced platelet aggregation was inhibited in the presence of the impermeant thiol blockers pCMPS (Figure 1) and DTNB. This finding was in agreement with the reported role of thiols in platelet adhesion.14 We further observed that shape change and ristocetin-induced agglutination were not affected, indicating involvement of extracellular thiols in ligation of αIIbβ3 integrin specifically and not in the nonintegrin receptor GPIb-IX-V or agonist signaling. Moreover, a transient interaction of αIIbβ3 with its ligand sufficient to initiate the first wave of aggregation did occur, but a second wave did not occur, suggesting that ligand-induced conformational changes necessary for outside-in signaling and triggering of the second wave either did not take place or were not stabilized.
Inhibition of catalyzed disulfide exchange by bacitracin also inhibited platelet aggregation, mimicking the effect of the thiol blockers by inhibiting the second but not the first wave of ADP- or thrombin-induced aggregation (Figure 2). This observation was in agreement with previous findings.22 Inhibition was observed at concentrations of bacitracin shown to inhibit other membrane functions32,33 as well as platelet adhesion.14 Surface-associated PDI,20 a principal candidate for such catalysis,22,34 is inhibited by bacitracin.32,33 However, endogenous disulfide-exchange activity of αIIbβ3 is also inhibited by bacitracin,23 thus suggesting possiblecis-isomerization of the ligated receptor. We therefore used monoclonal anti-PDI clone RL90 to focus directly on the role of PDI in agonist-induced aggregation. We found that specific blocking of PDI inhibited platelet aggregation (Figure 3). Thus, our data so far suggested involvement of enzymatic catalysis in disulfide-dependent, integrin-mediated aggregation, possibly by PDI or by both αIIbβ3 and PDI.
We therefore assessed such involvement in fibrinogen binding to αIIbβ3 on intact platelets by directly measuring bound FITC-fibrinogen in the presence of the same inhibitors. We observed that increasing amounts of pCMPS showed concentration-dependent inhibition of agonist-induced binding (Figure4A), with maximal inhibition reached at pCMPS concentrations lower than those needed for maximal inhibition of adhesion.14All agonists used showed similar susceptibility to thiol blocking, thus ruling out any effect of the inhibitor on a specific agonist or on its receptor. Rather, the data suggested that the effect of thiol blockade was mediated by αIIbβ3 itself or associated proteins involved in fibrinogen ligation. Platelets have an integrin and a nonintegrin receptor for collagen, both of which are involved in platelet stimulation. To examine the αIIbβ3activation pathway of nonintegrin GPVI, we stimulated the platelets with the cross-linked collagen-related peptide (CRP-XL), which was previously shown to be GPVI specific.26 Indeed, binding of fibrinogen to αIIbβ3 was also inhibited by pCMPS in the CRP-XL–stimulated platelets.
Bacitracin inhibited fibrinogen binding (Figure 4A), indicating that thiol involvement depends on catalyzed disulfide exchange during the process of αIIbβ3 ligation. RL90 also inhibited fibrinogen binding, although inhibition was incomplete (Figure 4A). These data indicate that surface-associated PDI is involved in αIIbβ3 ligation. Whether the incomplete inhibition by RL90 resulted from an inherently incomplete blocking activity of the antibody32 33 or from involvement of yet another mediator of disulfide exchange (possibly αIIbβ3 itself) remains to be elucidated.
It was previously shown that whereas initial ligation of αIIbβ3 depends on the presence of bound calcium ions, these leave as a consequence of binding,36thereby indicating the existence of changes in conformation in response to ligation of αIIbβ3. Similar observations were made for collagen ligation to platelet α2β1.37 The conformation induced in αIIbβ3 by ligation exposes new epitopes—LIBSs—recognized by specific monoclonal antibodies.35 One of these antibodies, PAC-1, competes with fibrinogen and is thought to bind very near the binding site of the ligand.38 Another LIBS antibody, PMI-1, binds at the carboxy-terminal of the αIIb heavy chain,39does not compete with ligand binding, and reflects an allosteric LIBS on αIIb.35 Use of these antibodies allows detection of conformations that exist only when αIIbβ3 is stably ligated.3 In the study described here, binding of PAC-1 and PMI-1 was inhibited when disulfide exchange or thiol availability was blocked (Figure 4B,C), indicating that transition of αIIbβ3 to its ligated conformation, a process necessary for sustained fibrinogen binding, depends on ecto-sulfhydryls. However, this observation may also indicate that platelet stimulation depends on a similar process and was also inhibited. Therefore, platelet stimulation was followed by assessment of an activation reporter—surface expression of the α-granule protein P-selectin—in parallel with measurement of binding of fibrinogen and PAC-1. We observed that at inhibitor concentrations that totally inhibited binding of fibrinogen or change of conformation, expression of P-selectin was much less affected (Figure 5). In keeping with the reduced level of expression of P-selectin on platelets from patients with Glanzmann thrombasthenia,40 platelet stimulation was only minimally affected by blocked sulfhydryls. Thus, platelet stimulation is independent of thiol exposure.
Is induction of the high-affinity state of the receptor the thiol-dependent step? The presence of the first wave and absence of the second wave of aggregation indicate that the passage from low- to high-affinity state does not depend on free sulfhydryls. To verify this on a molecular level, we used activating antibody anti-LIBS6. Pretreatment of platelets with anti-LIBS6 converts integrin αIIbβ3 to the conformation of high-affinity state, compatible with binding of fibrinogen, without stimulation of the platelets. This antibody does not compete with fibrinogen for αIIbβ3 ligation.41 We found that if ecto-sulfhydryls were blocked or disulfide exchange was inhibited, there was no binding of fibrinogen or PAC-1, even in the presence of anti-LIBS6 (Figure 6). Binding of anti-LIBS6 itself was not affected by these inhibitors, thereby ruling out a simple annulment of its action on the integrin. Thus, a role for free thiols in processes downstream of the initial binding is implied. However, this observation does not rule out the possibility that conversion of the integrin to its high-affinity state by anti-LIBS6 involves disulfide exchange, and in the absence of the ability to induce disulfide exchange, anti-LIBS6 could not convert αIIbβ3 to its high-affinity state, a distinction that requires further exploration.
The involvement of thiols described above, although clearly associated with integrin ligation, does not necessarily indicate that disulfide exchange occurs in the integrin itself. Strong circumstantial evidence, however, supports the presence of thiol function in the αIIbβ3 molecule. Yan and Smith18 recently showed that αIIbβ3 purified in the active compared with the nonactive form shows distinct free thiols in the former but not in the latter form and that the difference in conformation between the 2 states encompasses the cysteine-rich domain on the extracellular domain of the molecule.18 However, further work is necessary to define the role of ligand binding in triggering these molecular events.
On the basis of the data presented here, we suggest that binding of fibrinogen to αIIbβ3 is a multistep process in which, after initial binding, enzymatically catalyzed disulfide exchange is necessary for a step of conformational change that enables sustained binding. This multistep process could be a general mechanism of integrin ligation.
We thank Dr Mark H. Ginsberg of the Department for Vascular Biology, Scripps Institute, La Jolla, CA, for the kind gifts of anti-LIBS antibodies PMI-1 and anti-LIBS6, and Coren Lahav for diligent work on the graphical presentation.
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2001-12-0339.
Supported in part by grants from the Chief Scientist's office of the Ministry of Health, Israel, and The Interdisciplinary Center for Clinical Research, Muenster, Germany (projects Fo.01KS9604/0 and IZKF C21).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Judith Lahav, Coagulation Laboratory, Rabin Medical Center, Beilinson Campus, Zabotinski Street, Petah-Tiqva 49100, Israel; e-mail: jlahav@netvision.net.il.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal