The molecular mechanisms underlying lymphocyte extravasation remain poorly characterized. We have recently identified junctional adhesion molecule-2 (JAM-2), and have shown that antibodies to JAM-2 stain high endothelial venules (HEVs) within lymph nodes and Peyer patches of adult mice. Here we show that mouse lymphocytes migrate in greater numbers across monolayers of endothelioma cells transfected with JAM-2. The significance of these findings to an understanding of both normal and pathologic lymphocyte extravasation prompted us to clone the human homologue of JAM-2. We herein demonstrate that an anti–JAM-2 antibody, or a soluble JAM-2 molecule, blocks the transmigration of primary human peripheral blood leukocytes across human umbilical vein endothelial cells expressing endogenous JAM-2. Furthermore, we show that JAM-2 is expressed on HEVs in human tonsil and on a subset of human leukocytes, suggesting that JAM-2 plays a central role in the regulation of transendothelial migration.
Introduction
To efficiently protect the body from infectious organisms, cells of the immune system continuously recirculate between the blood and lymphoid tissues.1-4 During this so-called immune surveillance, lymphocytes exit the bloodstream and enter secondary lymphoid organs across the endothelium lining the blood vessels, a process often referred to as extravasation. At the same time as permitting the passage of cells into the underlying tissue, an important role of the endothelium is to limit vascular permeability, which it does via an array of intercellular adhesion molecules, organized into local junctional structures (for reviews, see Dejana et al5 and Johnson-Léger et al6). These junctions are responsible for maintaining an intact endothelium by sealing the paracellular space and thereby allowing separation of apical and basal fluid compartments.7 Thus, the barrier function of the endothelium must somehow be reconciled with its role in allowing the body's cellular traffic. This problem of lymphocyte penetration across the endothelium appears to have been resolved by the evolution of specialized sites in the postcapillary venules within lymph nodes called high endothelial venules (HEVs).8Lymphocytes leave the bloodstream and enter the lymphoid organs at these sites.
The HEVs are composed of modified endothelial cells with a cuboidal or columnar morphology that are very different from the flat endothelium of all other vessels. The structure of the HEVs9 further ensures that lymphocytes are able to infiltrate between adjacent cells, while the leakage of fluid from the blood is limited by the organization of adjacent endothelial cells into an overlapping array (for a review, see Kraal and Mebius10).
Lymphocyte migration across the endothelium involves a multistep cascade11-13: leukocyte rolling on endothelial cells involving the selectins, triggering by rapid activation of integrins via G protein–coupled receptors, tight adhesion to members of the Ig superfamily expressed on endothelial cells, and finally, diapedesis, where the cell crosses the endothelium through the interendothelial junction. This, however, is not the exclusively described route for extravasating lymphocytes and a transcellular route has recently been demonstrated for neutrophils.14 Unlike the early steps of the migration cascade, which are now well understood, the mechanisms governing the final step, whereby the cell passes through the junction between apposing cells, are less clear. It has been reported that the endothelial cells of HEVs are linked together by discontinuous junctional complexes that differ from the tight junctions of arterial endothelium, but that are similar to the nonoccluding junctions found in other postcapillary venules (for a review, see Girard and Springer15). However, the precise molecular components of these junctions remain to be determined.
We have recently identified a novel member of the Ig superfamily, named junctional adhesion molecule-2 (JAM-2),16 which is part of a subfamily of junctional molecules comprising JAM-1,17JAM-2, and vascular endothelial JAM (VE-JAM) or JAM-3.18Following its transfection into Madin Derby canine kidney (MDCK) epithelial cells, JAM-2 was specifically enriched in cell-cell contacts at the level of the tight junction and this localization required homotypic interactions. Interestingly, MDCK monolayers expressing mouse JAM-2, but not JAM-1, show greater paracellular permeability to fluorescein isothiocyanate (FITC)–dextran than nontransfected cells.19 JAM-2 is highly expressed during embryogenesis and is restricted to endothelial subpopulations in adult tissues. Expression of murine JAM-2 is high in lymph nodes and Peyer patches where it is present on HEVs.16 The known involvement of HEVs in lymphocyte recirculation prompted us to investigate whether JAM-2 might play a role during transendothelial migration, perhaps by facilitating the passage of lymphocytes across the endothelium.
Here, we study the effect of JAM-2 overexpression on the transmigration of mouse lymphocytes. Because we additionally wished to study the effect of JAM-2 in a more physiologic context using primary human endothelial cells, we subsequently cloned the human homologue of mouse JAM-2 and studied the transmigration of human lymphocytes across human umbilical vein endothelial cells (HUVECs). Our results suggest that JAM-2 is involved in regulating the transmigration of both murine and human lymphocytes.
Materials and methods
Endothelial cells
The murine thymic (tEnd.1) endothelioma cell line was provided by Dr W. Risau (Max Planck Institute, Bad Nauheim, Germany). The cells were grown in Dulbecco modified Eagle medium (DMEM; Life Technologies, Paisley, United Kingdom), supplemented with 10% fetal calf serum (FCS; PAA Laboratories, Linz, Austria), 2 mM glutamine, 100 U/mL penicillin, and 100 U/mL streptomycin (all from Life Technologies). Adherent cells were detached by washing with phosphate-buffered saline (PBS) and 0.15 mM EDTA (ethylenediaminetetraacetic acid), followed by a 5-minute incubation in trypsin/EDTA at 37°C. Transfected tEnd.1 cells were cultured in the same medium, supplemented with 0.2 mg/mL hygromycin B. HUVECs were isolated by collagenase treatment of umbilical veins.20 HUVECs were maintained in M199 supplemented with 20% FCS (PAA Laboratories), 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), nonessential amino acids, sodium pyruvate, endothelial cell growth supplement (ECGS, 15 μg/mL; Upstate Biotechnology, Lake Placid, NY), and heparin (4 μg/ml; Sigma, Buchs, Switzerland). Cells were used between passages 3 and 5 for all the experiments described in this report.
Primary lymphocytes
Murine lymphocytes were prepared from the mesenteric lymph nodes (MLNs) of C57BL/6 mice aged between 8 and 12 weeks. Human peripheral blood lymphocytes (PBLs) were obtained from fresh blood of healthy donors. Citrated blood was separated by Ficoll-Paque Plus (Pharmacia Biotech, Uppsala, Sweden) gradient centrifugation.
Construction of expression vectors
This has been described previously.16 Briefly, the 3′ restriction sites, HpaI and ScaI, found in the sequence encoding, respectively, the cytoplasmic domain of JAM-2 or JAM-1 were used to fuse the 2 sequences at the N-terminus of the enhanced green fluorescent protein (EGFP) in pcDNA3 vector (Invitrogen, Groningen, The Netherlands). This approach allowed us to select transfected cells prior to the generation of antibodies. The transfection of tEnd.1 cells was carried out using Fugene (Roche Diagnostics, Rotkreuz, Switzerland) or LT1 (Panvera, Merck AG, Zurich, Switzerland) according to the manufacturers' protocols.
Other reagents and antibodies
For fluorescence-activated cell-sorter scanner (FACS) analysis the following monoclonal antibodies were used: antimouse JAM-1 (H2O2.106.7.4, rat IgG1),21 antimouse JAM-2 (CRAM-13 H33, CRAM-19 H36, or CRAM-18 F26, all rat IgG2a).16 The antihuman JAM-1 3D8 was a kind gift from H. Ozaki22 and the anti–VE-JAM/human JAM-3 was a kind gift from Steve Rosen.18 The MECA 79 antibody was a kind gift of Britta Engelhardt. The secondary reagents used were a FITC-labeled antirat antibody (Jackson Immunoresearch, Milan, LaRoche, Switzerland), a phycoerythrin (PE)–labeled antirat antibody, a PE-labeled antimouse antibody, a PE-labeled antirabbit antibody (Pharmingen, Becton-Dickinson, Allschwil, Switzerland) or streptavidin coupled to PE for biotinylated antibodies (all from Southern Biotechnology, BIOREBA, Reinach, Switzerland). For analysis of human PBLs the following antibodies were used: anti-CD3, anti-CD19, and anti-CD14, all directly conjugated to FITC (all from Dako Diagnostics, Postfach 2114, CH-6302 Zug). Mouse stromal cell–derived factor-1 (SDF-1) was from R & D Systems (Abingdon, United Kingdom). Human SDF-1 was a kind gift from RMF Dictagene (Epalinges, Switzerland). The soluble form of JAM-2 was obtained by inserting a polymerase chain reaction (PCR) fragment corresponding to 10 nucleotides of the 5′ untranslated region (UTR) and to the coding region of the human JAM-2 extracellular domain (amino acids 1-238) with the signal peptide but without the transmembrane region into the HindIII and EcoRV restriction sites of a vector system helping secretion (Beghdadi et al, submitted).
To produce soluble JAM-2, 293T cells were transfected using calcium phosphate. The soluble product was purified from supernatant by affinity chromatography with beads coupled to monoclonal antibody directed against the D tag. A synthetic peptide corresponding to the D tag sequence was used as control in transmigration experiments to exclude any role of this sequence. The D tag peptide was from RMF Dictagene.
Flow cytometry
Suspension and trypsinized adherent cells were collected, washed once in DMEM with 10% FCS, then resuspended in PBS containing 0.2% bovine serum albumin (BSA) and 0.1% sodium azide (FACS buffer) with saturating amounts of antibodies. After a 30- to 60-minute incubation on ice, cells were washed 3 times in the same buffer and resuspended in staining solution containing FITC-labeled goat antirat IgG, or a PE-labeled antirat IgG, or streptavidin coupled to PE (for biotinylated antibodies). After another 30 to 60 minutes of incubation on ice, cells were washed 3 times and then resuspended in FACS buffer and analyzed by flow cytometry (FACScan; Becton Dickinson, Mountain View, CA). Control cell suspensions were incubated with secondary antibody alone.
Immunostaining
For immunohistochemistry with anti–JAM-2 (XIX H36 ascites) or MECA 79, samples were fixed for 5 minutes with cooled methanol (−20°C), dried, and rehydrated in PBS, 0.2% gelatin, and 0.05% Tween 20 (PGT). Stainings were visualized using secondary reagent coupled to FITC.
Transendothelial migration assay
A total of 2 × 105 tEnd.1 cells (nontransfected or transfected with JAM-1-EGFP or JAM-2-EGFP) was cultured in Transwell culture inserts in 24-well tissue culture plates (10-mm diameter polycarbonate membranes with 8-μm diameter pores, Life Technologies). They were allowed to grow to confluence on these filters for 48 hours. The cultures were washed once in DMEM with 10% FCS and, where indicated, monoclonal antibodies were added to the tEnd.1 monolayers for 30 minutes at 37°C prior to the addition of lymphocytes and left throughout the duration of the transmigration assay. Antibodies were added at a final concentration of 25 μg/mL. One million washed lymphocytes were added to each filter and incubated for 3 to 4 hours at 37°C, unless otherwise indicated. In all experiments, the chemokine mouse SDF-1 was added to the lower chamber at a final concentration of 300 ng/mL at the start of the transmigration assay. At the end of the assay, the transmigrated cells were collected from the lower chamber and the number of cells that had transmigrated into the lower chamber was determined by light microscopy. For migration assays using HUVECs, the procedure was essentially the same except that 8 × 104 HUVECs were plated into filters previously coated with collagen (collagen G, Seromed, 100 μg/mL in Hanks balanced salt solution for 60 minutes at 37°C) in M199 medium containing 20% FCS and supplemented as described above. Transmigration assays were performed 3 to 4 days later. In some filters anti–JAM-2 reagents were added to the HUVEC monolayers 15 minutes prior to the addition of PBLs and left for the duration of the experiment.
Cloning of human JAM-2
Based on the previously described murine sequence of JAM-2, we identified a human expressed sequence tag (EST) showing high homology with the murine DNA sequence. One IMAGE clone (Acc:H71948) obtained from United Kingdom Human Genome Mapping Project (HGMP) Resource Center encoded the 3′-end sequence of human JAM-2. The remaining 5′-coding sequence was obtained by Rapid Amplification of cDNA Ends (5′RACE-PCR System; Gibco BRL, Paisley, United Kingdom) using cDNA from HUVECs and primers designed on the basis of the EST sequence. Based on the DNA sequences obtained from EST and 5′-RACE products, 2 primers were designed to amplify the full-length sequence with high-fidelity Pfu polymerase from HUVEC cDNA. A PCR product 960 base pairs (bp) in length was obtained and, after subcloning the PCR products in EcoRV site of pcDNA3 (Invitrogen, Leek, The Netherlands), 3 independent clones were sequenced on both strands. The sequence obtained from the 3 clones was identical and is available under accession number AJ344431.
Northern blots with Poly-A(+)
RNA of 12 different human tissues (multiple tissue Northern (MTN) blots, reference 7780-1) was purchased from Clontech (PH Stehelin and Cie, Basel, Switzerland). The amount of RNA on MTN blots has been adjusted so that the β-actin hybridization signal is of comparable intensity in each lane. The riboprobe was prepared from pcDNA3 vector and comprised the full-length coding sequence for human JAM-2. Hybridization was performed at 62°C in buffer containing 50% formamide. The blots were washed twice (0.5× standard sodium citrate [SSC], 0.1% sodium dodecyl sulfate [SDS], 72°C), and autoradiographed on Kodak X-Omat (Kodak SA, Lausanne, Switzerland) at −80°C.
Results
We have previously shown that JAM-2 is expressed by HEVs within the Peyer patches and lymph nodes of adult animals.16 This preferential expression of JAM-2, at sites of high lymphocyte traffic, suggested a potential role for this molecule in lymphocyte extravasation, prompting us to carry out transmigration studies using primary lymphocytes and endothelial monolayers.
Migration of lymphocytes across endothelioma cells is increased on transfection of JAM-2
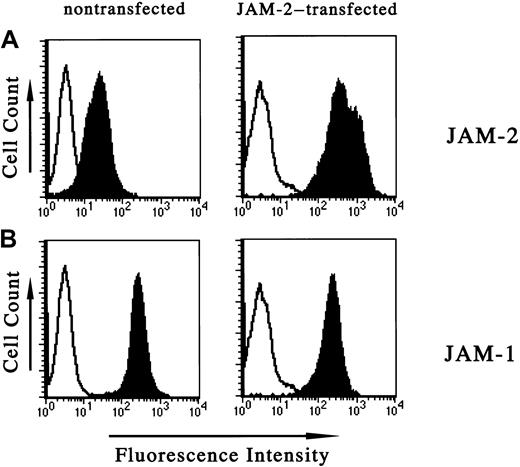
The mouse endothelioma cell line, tEnd.1, expresses very low levels of JAM-2. To mimic the relatively higher expression level of JAM-2 in vivo on HEVs we stably transfected JAM-2 into tEnd.1 cells. The transfected molecule was expressed on the surface of these cells as assessed by flow cytometric analysis (Figure1, upper right panel). JAM-1, by contrast, was highly expressed on untransfected tEnd.1 cells and this level did not change significantly on transfection of JAM-2 (Figure 1, lower panels). Studies by immunofluorescence revealed that the transfected JAM-2 was expressed at intercellular contacts (not shown), whereas the endogenous JAM-2 of wild-type cells could not be detected by this method.
Increased JAM-2 expression on transfection of tEnd.1 endothelial cells.
Cytofluorometric analysis of JAM-2 and JAM-1 expression on endothelial cells with or without transfection of exogenous JAM-2: monoclonal antibodies used for staining were anti–JAM-1 (H202 106.7.4, lower panels), anti–JAM-2 (CRAM-13 H33, upper panels) and are represented by solid histograms. Open profiles represent the negative control obtained using the secondary antibody alone. The left panels show nontransfected cells and the right panels show cells transfected with JAM-2.
Increased JAM-2 expression on transfection of tEnd.1 endothelial cells.
Cytofluorometric analysis of JAM-2 and JAM-1 expression on endothelial cells with or without transfection of exogenous JAM-2: monoclonal antibodies used for staining were anti–JAM-1 (H202 106.7.4, lower panels), anti–JAM-2 (CRAM-13 H33, upper panels) and are represented by solid histograms. Open profiles represent the negative control obtained using the secondary antibody alone. The left panels show nontransfected cells and the right panels show cells transfected with JAM-2.
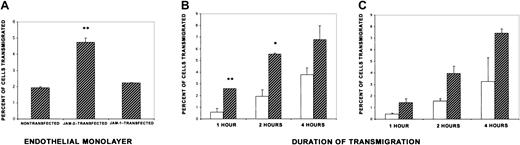
Using monolayers of these JAM-2–transfected endothelioma cells, transmigration of primary lymphocytes, prepared from the mesenteric lymph nodes of normal mice, was assessed. These lymphocytes migrated more efficiently through monolayers expressing high levels of JAM-2 than through nontransfected cells, in a 3-hour assay (Figure2A). This result was obtained with 3 different clones stably expressing high levels of exogenous JAM-2 (not shown). By contrast, when we took clones of tEnd.1 cells expressing exogenous JAM-1 as control, there was no difference in the number of cells that transmigrated the monolayers (Figure 2A). JAM-1 was chosen as a control because it is a member of the same protein subfamily and has been previously demonstrated to play a role in monocyte, but not lymphocyte, migration.17 We subsequently carried out a time course to see whether the difference in transmigration was due to a faster initial migration across JAM-2 transfectants. We observed that the greatest difference in transmigration across transfected and nontransfected endothelioma cells was observed at the earlier time points of the assay, suggesting that lymphocytes may start to migrate sooner across endothelium expressing high levels of JAM-2 (Figure 2B). After 4 hours, we consistently obtained a higher number of cells following transmigration across JAM-2–transfected monolayers, but this was not usually statistically significant.
Increased transmigration of MLN lymphocytes across endothelial monolayers expressing high levels of JAM-2.
(A) Nontransfected tEnd.1 cells and cells transfected with either JAM-1 or JAM-2 were cultured in Transwell culture inserts in 24-well plates. Forty-eight hours later, the cultures were washed and 1 × 106 splenic or MLN lymphocytes were added to each filter and incubated for 3 hours. The chemokine mouse SDF-1 was added to the lower chamber. At the end of the assay, transmigrated cells were collected from the lower chamber and counted by light microscopy. The results are expressed as the percentage of cells transmigrated calculated from the mean of triplicate filters (± SD) and are representative of at least 3 independent experiments. (B,C) A time course of transmigration was carried out on monolayers of tEnd.1 cells, either nontransfected (open bars) or transfected with JAM-2 (hatched bars) as for panel A, except that transmigrated lymphocytes were collected at the times indicated. Two representative experiments are shown *P < .05. **P < .01.
Increased transmigration of MLN lymphocytes across endothelial monolayers expressing high levels of JAM-2.
(A) Nontransfected tEnd.1 cells and cells transfected with either JAM-1 or JAM-2 were cultured in Transwell culture inserts in 24-well plates. Forty-eight hours later, the cultures were washed and 1 × 106 splenic or MLN lymphocytes were added to each filter and incubated for 3 hours. The chemokine mouse SDF-1 was added to the lower chamber. At the end of the assay, transmigrated cells were collected from the lower chamber and counted by light microscopy. The results are expressed as the percentage of cells transmigrated calculated from the mean of triplicate filters (± SD) and are representative of at least 3 independent experiments. (B,C) A time course of transmigration was carried out on monolayers of tEnd.1 cells, either nontransfected (open bars) or transfected with JAM-2 (hatched bars) as for panel A, except that transmigrated lymphocytes were collected at the times indicated. Two representative experiments are shown *P < .05. **P < .01.
Antibodies directed against the extracellular domain of JAM-2 partially inhibit transmigration of lymphocytes
To address whether any of our panel of anti–JAM-2 antibodies would affect lymphocyte migration across JAM-2–transfected monolayers, we carried out a series of experiments using these antibodies at varying concentrations. Two of the antibodies gave consistent results in transmigration assays (CRAM-13 H33, this study, and CRAM-2 D22) blocking the transmigration of lymphocytes by up to 50% (Figure3). Optimal blocking was obtained at an antibody concentration of 25 μg/mL, which was left throughout the course of the transmigration assay.
Anti–JAM-2 blocking of lymphocyte transmigration.
Transmigration assays were carried out as described for Figure 2, except that in some wells, anti–JAM-2 antibody (CRAM-13 H33) was added to the endothelioma monolayers for 30 minutes prior to the addition of lymphocytes. The antibody was left for the duration of the transmigration assay. Results are expressed as the percentage of cells transmigrated, calculated from the mean of triplicate filters (± SD). The degree of inhibition varied between 35% and 50%, and the data presented in this figure are representative of more than 4 experiments; *P < .05.
Anti–JAM-2 blocking of lymphocyte transmigration.
Transmigration assays were carried out as described for Figure 2, except that in some wells, anti–JAM-2 antibody (CRAM-13 H33) was added to the endothelioma monolayers for 30 minutes prior to the addition of lymphocytes. The antibody was left for the duration of the transmigration assay. Results are expressed as the percentage of cells transmigrated, calculated from the mean of triplicate filters (± SD). The degree of inhibition varied between 35% and 50%, and the data presented in this figure are representative of more than 4 experiments; *P < .05.
Cloning of the human homologue of JAM-2
Having demonstrated that monolayers of mouse endothelioma cells overexpressing JAM-2 supported a greater level of transmigration than nontransfected monolayers, we wished to confirm these findings with nontransformed primary endothelial cells expressing endogenous JAM-2. We chose HUVECs because this is a well-established system for studying leukocyte transmigration in vitro. We therefore cloned the homologue of mouse JAM-2 from HUVECs. Human JAM-2 displayed 86% sequence identity at the amino acid level with the mouse protein (Figure 4A). Northern blot revealed that the transcript of human JAM-2 is abundantly expressed in the brain, heart, and placenta (Figure 4B). Apparently, this expression does not overlap entirely with that of the murine homologue where the JAM-2 transcript is highly expressed in the kidney,16 suggesting that there may be both overlapping and distinct roles for human and mouse JAM-2 (discussed further below).
Nucleotide sequence of human JAM-2 cDNA and Northern blot analysis of human JAM-2 transcript.
(A) The putative leader peptide and the putative transmembrane region are, respectively, underlined and boxed. Cysteine residues participating in Ig folds of the membrane distal V domain and membrane proximal C2 domains are boxed in gray. The putative N-glycosylation sites are marked by black boxes. The human cDNA sequence is accessible from sequence database under accession number AJ344431. (B) Multiple tissue blot (Clontech) comprising human poly-A(+) mRNA hybridized with JAM-2 probe. Sizes are indicated on the right.
Nucleotide sequence of human JAM-2 cDNA and Northern blot analysis of human JAM-2 transcript.
(A) The putative leader peptide and the putative transmembrane region are, respectively, underlined and boxed. Cysteine residues participating in Ig folds of the membrane distal V domain and membrane proximal C2 domains are boxed in gray. The putative N-glycosylation sites are marked by black boxes. The human cDNA sequence is accessible from sequence database under accession number AJ344431. (B) Multiple tissue blot (Clontech) comprising human poly-A(+) mRNA hybridized with JAM-2 probe. Sizes are indicated on the right.
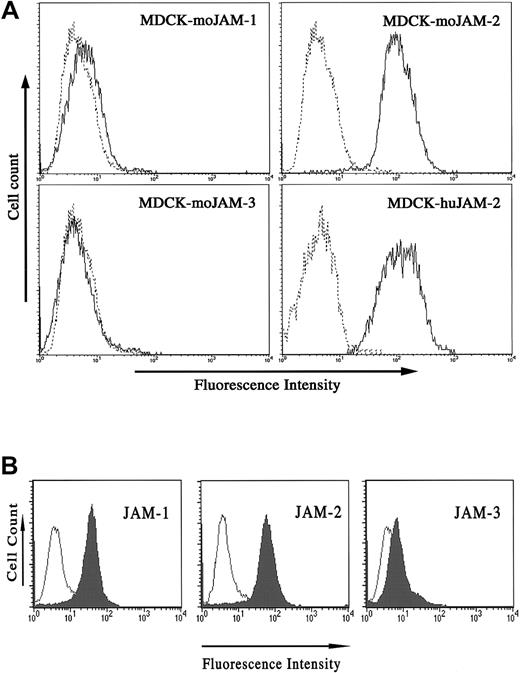
The fact that we cloned JAM-2 from HUVEC cDNA indicated that these endothelial cells do express the protein. To confirm this by flow cytometry, however, we required antibodies that would recognize the human molecule. Because there was a high degree of sequence identity between mouse and human JAM-2, we tested our anti–JAM-2 antibodies for cross-reactivity on the human molecule. To this end we transfected into MDCK cells, human JAM-2, and mouse JAM-1, -2, and -3 and carried out cytofluorometric analysis. The results presented in Figure5A demonstrate that the antimouse JAM-2 antibody used for this analysis binds to human JAM-2, but does not cross-react on either mouse JAM-1 or JAM-3. We have obtained the same result for all our antimouse JAM-2 antibodies (not shown). We then tested HUVECs for the expression of JAM-1, -2, and -3. The results presented in Figure 5B demonstrate that both JAM-1 and JAM-2 are expressed on HUVECs. By contrast, JAM-3 is present at extremely low levels. The result obtained for JAM-1 confirms earlier reports, because HUVECs were previously shown to express JAM-1.23 We carried out immunofluorescence analysis to address the distribution of JAM-2 in HUVEC monolayers, and we observed a partial, but not exclusive, localization at intercellular contacts (Figure6A).
JAM-2 antibodies cross-react on human JAM-2.
(A) MDCK cells stably transfected with the indicated cDNA were stained with anti–JAM-2 antibody (solid profile) or an irrelevant antibody (dashed profiles). (B) HUVECs were tested for expression of the 3 JAM family members using 3D8 against JAM-1, (left panel, filled profile), CRAM-18F26 against JAM-2, (middle panel, filled profile), or anti–VE-JAM against JAM-3, (right panel, filled profile). Open profiles represent the negative control obtained using the appropriate secondary antibodies alone.
JAM-2 antibodies cross-react on human JAM-2.
(A) MDCK cells stably transfected with the indicated cDNA were stained with anti–JAM-2 antibody (solid profile) or an irrelevant antibody (dashed profiles). (B) HUVECs were tested for expression of the 3 JAM family members using 3D8 against JAM-1, (left panel, filled profile), CRAM-18F26 against JAM-2, (middle panel, filled profile), or anti–VE-JAM against JAM-3, (right panel, filled profile). Open profiles represent the negative control obtained using the appropriate secondary antibodies alone.
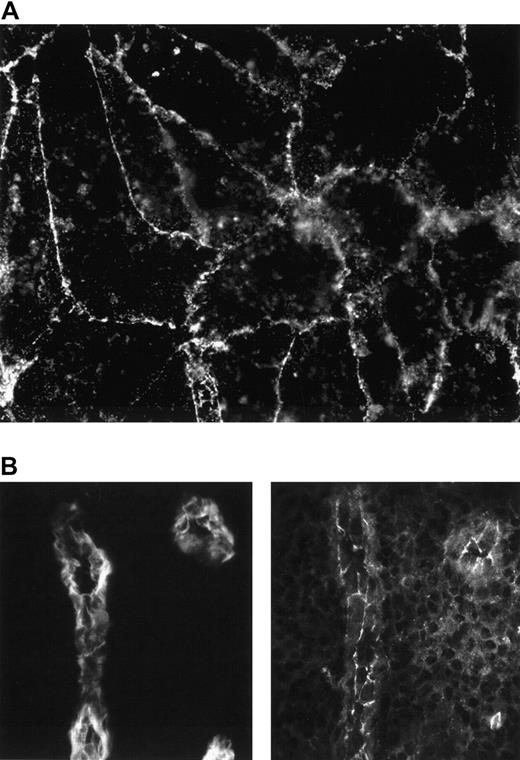
JAM-2 is expressed on HUVECs in monolayers and on human tonsillar HEVs.
(A) Confluent HUVEC monolayers were stained with CRAM-19 H36 ascites and staining was revealed with antirat FITC. (B) Serial sections of human tonsil were stained with either MECA 79 antibody, recognizing carbohydrate epitopes of PNAd on lymphoid tissue HEVs (left panel) or CRAM-19 H 36 ascites, recognizing JAM-2 (right panel) and revealed with antirat FITC. Using the same settings for the CCD, no staining was detectable if primary antibody was omitted.
JAM-2 is expressed on HUVECs in monolayers and on human tonsillar HEVs.
(A) Confluent HUVEC monolayers were stained with CRAM-19 H36 ascites and staining was revealed with antirat FITC. (B) Serial sections of human tonsil were stained with either MECA 79 antibody, recognizing carbohydrate epitopes of PNAd on lymphoid tissue HEVs (left panel) or CRAM-19 H 36 ascites, recognizing JAM-2 (right panel) and revealed with antirat FITC. Using the same settings for the CCD, no staining was detectable if primary antibody was omitted.
JAM-2 is expressed on human tonsillar HEVs
We hypothesize that JAM-2 may facilitate lymphocyte recirculation, and the distribution of the murine molecule is consistent with this. The tissue distribution of the human homologue, however, suggests a broader function for this molecule and so we wished to verify its expression on human HEVs. To this end we prepared serial sections of frozen tonsils and stained them for MECA 79 (a marker of HEVs) and JAM-2. The result, presented in Figure 6B, demonstrates that human tonsillar HEVs do indeed express JAM-2. Although this expression on human HEVs is consistent with a putative role of JAM-2 in lymphocyte recirculation, we also observed some staining on non-HEV vascular structures (not shown). This suggests that JAM-2 may be involved in additional vascular functions to those associated with lymphocyte extravasation.
Chemokine-induced lymphocyte transmigration across HUVECs blocked by an antibody and soluble JAM-2
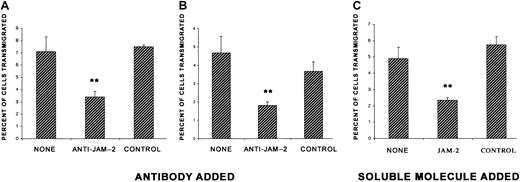
Having established that HUVECs express JAM-2, we carried out transmigration assays across monolayers of these cells in the presence or absence of anti–JAM-2 antibody. Our findings from transmigration assays with monolayers of HUVECs and freshly isolated PBLs were consistent with the results obtained in the murine model. An antibody directed against JAM-2 partially blocked the transmigration of human peripheral blood lymphocytes induced by the chemokine SDF-1 (Figure7A). An isotype-matched control antibody included in the same experiment had no effect on this transmigration. Because JAM-2 interacts homophilically,16 we anticipated that competitive binding of the soluble JAM-2 molecule might block this interaction and thereby modulate the transmigration of the lymphocytes. Therefore, we repeated the assays in the presence of soluble JAM-2. Our results demonstrate that soluble JAM-2, when added at a final concentration of 2 μg/mL, inhibited transmigration by a similar extent to the anti–JAM-2 antibody (Figure 7B). The flag fused to JAM-2 (tag D) was used as a control and had no effect on transmigration.
Transmigration of human PBLs across monolayers of HUVECs is inhibited by anti–JAM-2 antibody and soluble JAM-2.
HUVECs (8 × 104 cells) were cultured per Transwell culture insert. Three days later, the cultures were washed and 8 × 105 PBLs were added to each filter and incubated for 2 hours in the presence of human SDF-1 added to the lower chamber. Fifteen minutes prior to addition of lymphocytes, the indicated reagents were added: panels A and B, anti–JAM-2 (CRAM-18 F26) or isotype-matched control antibody (IgG2a isotype), 2 representative experiments shown, or panel C, soluble JAM-2 or a control soluble peptide (tag D). The transmigrated cells were collected from the lower chamber and counted by light microscopy. Results are expressed as the percentage of cells transmigrated, calculated from the mean of triplicate filters (± SD). The data presented in this figure are representative of 2 experiments. **P < .01.
Transmigration of human PBLs across monolayers of HUVECs is inhibited by anti–JAM-2 antibody and soluble JAM-2.
HUVECs (8 × 104 cells) were cultured per Transwell culture insert. Three days later, the cultures were washed and 8 × 105 PBLs were added to each filter and incubated for 2 hours in the presence of human SDF-1 added to the lower chamber. Fifteen minutes prior to addition of lymphocytes, the indicated reagents were added: panels A and B, anti–JAM-2 (CRAM-18 F26) or isotype-matched control antibody (IgG2a isotype), 2 representative experiments shown, or panel C, soluble JAM-2 or a control soluble peptide (tag D). The transmigrated cells were collected from the lower chamber and counted by light microscopy. Results are expressed as the percentage of cells transmigrated, calculated from the mean of triplicate filters (± SD). The data presented in this figure are representative of 2 experiments. **P < .01.
JAM-2 is expressed on a subset of human lymphocytes and monocytes
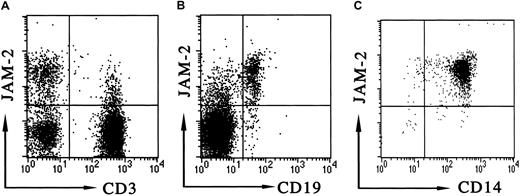
The blocking effect that we observed with antibody and soluble JAM-2 may be due to a direct modulation of the JAM-2 expressed by the endothelial cells, or it may involve the blocking of an interaction between lymphocyte and endothelial monolayer. In the latter scenario, one possibility would be the expression of JAM-2 on lymphocytes, which could then interact homotypically with JAM-2 on the endothelial cell during transmigration. To address the latter possibility we analyzed mouse MLN lymphocytes and human PBLs for the expression of JAM-2. We have been consistently unable to detect JAM-2 on any mouse lymphocytes or circulating cells (not shown). By contrast, we found that JAM-2 was expressed on all human B cells and on a subpopulation of T cells (Figure 8A,B). Furthermore JAM-2 was expressed on human monocytes (Figure 8C). Together, these data suggest that JAM-2 may regulate transmigration via multiple mechanisms and that these may differ for mouse and human systems.
JAM-2 expression on human PBLs.
Cytofluorometric analysis of JAM-2 was performed on human PBLs. Antibodies used for staining were biotinylated anti–JAM-2 in combination with either (A) anti-CD3 directly conjugated to FITC, (B) anti-CD19 directly conjugated to FITC, or (C) anti-CD14 directly conjugated to FITC, as indicated on the dot-plot axes. The appropriate forward and side scatter gates were used to display lymphocytes (A,B) or monocytes (C).
JAM-2 expression on human PBLs.
Cytofluorometric analysis of JAM-2 was performed on human PBLs. Antibodies used for staining were biotinylated anti–JAM-2 in combination with either (A) anti-CD3 directly conjugated to FITC, (B) anti-CD19 directly conjugated to FITC, or (C) anti-CD14 directly conjugated to FITC, as indicated on the dot-plot axes. The appropriate forward and side scatter gates were used to display lymphocytes (A,B) or monocytes (C).
Discussion
We describe here a functional role for a recently identified junctional adhesion molecule, JAM-2. The interesting feature of this molecule is its tissue distribution, being restricted to certain subpopulations of endothelial cells, notably HEVs within lymph nodes in the mouse, suggesting that it may play a role in lymphocyte recirculation in vivo. Human JAM-2 has been detected on vessels of the placenta and on HUVECs, demonstrating that it is expressed on endothelial cells. Furthermore, we have demonstrated the expression of JAM-2 on human tonsillar HEVs, which suggests that at least some of the functions of this molecule overlap with those of mouse JAM-2. However, human JAM-2 expression is clearly not restricted to endothelial cells.
The constitutive transmigration across the HEVs of recirculating lymphocytes is distinct from the transmigration that occurs at sites of inflammation where the damaged tissue triggers new adhesive properties in the adjacent endothelium, resulting in the local extravasation of leukocytes, particularly neutrophils.24,25 The role of HEVs in facilitating transmigration is evidenced by the fact that during chronic inflammation HEV-like vessels are observed in nonlymphoid tissues where they are believed to support lymphocyte recruitment into these sites. Indeed, HEVs were found in pancreatic islets in experimentally induced lymph node–like structures were there was a high recruitment of T and B lymphocytes.26Interestingly, JAM-2 is expressed on these ectopic HEVs (S. Luther, personal communication, April 2001). This suggests that JAM-2 may be a critical structural component of endothelial cells that support a high infiltration of lymphocytes.
In transfected MDCK cells, JAM-2 is restricted to the tight junctional complex and has been shown to regulate paracellular permeability. However, tight junctions are absent from tEnd.1 cells and HUVECs display very few tight junctions unless cultured under special media conditions.27 Furthermore, tight junctions have often been considered absent from HEVs, although this remains controversial and it has been suggested that partial tight junctions may exist for these specialized endothelial cells. This raises an extremely important issue, that the tight-junctional localization of JAM-2 is not a prerequisite for its cellular expression, nor for its role in regulating leukocyte transmigration. Interestingly, JAM-1, the first member of the junctional molecule adhesion family to be identified, is selectively concentrated at the apical region of intercellular junctions and participates as a component of the tight junctional complex.17 However, its expression at areas of intercellular contacts can occur independently of preorganized tight junctions,17,28 where its localization may be mediated by homophilic interactions.29 In addition, JAM-1 has been shown to play a role in leukocyte adhesion and transmigration.17,30 31 The analogies between these 2 family members are striking and clearly justify their inclusion in the same protein subfamily.
A third member of the JAM family has been recently identified in humans and named vascular endothelial junction-associated molecule, or VE-JAM.18 VE-JAM is homologous to JAM-1 and JAM-2 and is expressed on HEVs where it is localized at the intercellular contacts of high endothelial cells, and on the endothelium of other vessels.18 The authors suggest that the localization of VE-JAM may indicate a potential role for this molecule in lymphocyte transmigration across HEVs. We independently cloned VE-JAM, which we named JAM-3.32 JAM-3 has also been cloned in another laboratory33; however, there is some confusion concerning the nomenclature because these authors named this molecule JAM2, although the sequence is identical to the previously published VE-JAM/JAM-3 (see “Note added in proof”). The authors additionally identified a counterreceptor for this molecule on the surface of an immortalized T-cell line.33 While this manuscript was under review, the latter group subsequently published an additional study in which they have also cloned human JAM-2 (our nomenclature) and they demonstrate that JAM-2 is indeed the counterreceptor for JAM-3 expressed on immortal T-cell lines.34 Interestingly, these authors did not detect JAM-2 on freshly isolated peripheral blood cells, and this may reflect differences in the sensitivity of reagents used. A further study has similarly described JAM-2 and JAM-3 as a protein-interacting pair,35 and these authors additionally describe the presence of JAM-2 (our nomenclature) on CD56+ natural killer cells. Neither of these studies demonstrated a functional role for this interaction. JAM-3 appears to be expressed at extremely low levels on HUVECs (Figure 5B), and we have not yet studied the effect of blocking JAM-3 in our system. Hence, although it is unclear whether a JAM-2/JAM-3 interaction plays any functional role in transmigration, it will be important to study this further in the light of our findings.
A role for interendothelial molecules in the regulation of transmigration is becoming the rule rather than the exception. Platelet endothelial cell adhesion molecule 1 (PECAM-1) has long been considered a crucial player in this process. PECAM-1 is a member of the Ig superfamily and is present on endothelial cells, where it is concentrated at intercellular borders.36,37 PECAM-1 has been implicated as a critical mediator of transendothelial migration and an antibody to PECAM-1 blocked the transendothelial migration of monocytes by 70% to 90%.38 Importantly, PECAM-1 is expressed diffusely on the surfaces of most leukocytes. JAM-1 has been shown to play a role in leukocyte adhesion and transmigration17,30 and a recent study demonstrates that JAM-1 is a leukocyte function-associated antigen (LFA-1) ligand.31 More recently CD99, a transmembrane molecule that is concentrated at the intercellular borders of confluent human endothelial cells, has been implicated in the transmigration of monocytes.39 Interestingly, this molecule is also expressed by the monocytes.
The expression of JAM-2 on human lymphocytes contrasts sharply with its expression in the murine system (we have been unable to detect JAM-2 on any mouse leukocytes). Preliminary evidence suggests that the T cells are activated or memory cells, as assessed by expression of CD45 isoforms (not shown). In addition, human monocytes are also positive for JAM-2 and the role of JAM-2 in the transmigration of these cells is being actively pursued in our laboratory. These findings suggest that the mechanisms perturbed in our studies, by antibody and soluble JAM-2, may be severalfold: (1) a homotypic interaction between JAM-2 on lymphocytes and endothelial JAM-2 (for our human system), as has been suggested for PECAM-1, or (2) an interaction between JAM-2 and a counterreceptor (for both our human and mouse systems), as has been suggested for JAM-131 and JAM-3,33,35 or (3) a homotypic interaction between JAM-2 on apposing endothelial cells. This latter might result in a structural modification of the junction. Indeed, our earlier observation that the permeability of MDCK monolayers is increased on JAM-2 transfection19 suggests that JAM-2 can contribute as a structural component of the junction, at least in MDCK cells. These hypotheses are not mutually exclusive and JAM-2 may be a key regulator of lymphocyte transendothelial migration, playing a dual role in lymphocyte-endothelial and interendothelial interactions.
The precise localization of JAM-2 in different endothelia remains to be determined. Perhaps in the absence of tight junctions JAM-2 is targeted to specialized junctional or apical compartment, where it would be well placed to interact with transmigrating lymphocytes. One hypothesis is that its localization is regulated so that, in humans, it has evolved to play a dual role in the regulation of vascular permeability and in leukocyte adhesion and transmigration. These are intriguing issues and understanding the regulation of JAM-2 and particularly its interplay with other junctional molecules such as JAM-1 and JAM-3 should help us piece together the molecular stepping stones used by transmigrating leukocytes.
We are especially grateful to Stephane Jemelin for his expert technical assistance and valuable suggestions. We thank Dr S. Rosen for kindly providing us with polyclonal antibody against JAM-3 and Dr H. Ozaki for the kind gift of antibody 3D8. With thanks also to Claude Magnin and Dominique Gay-Ducrest for their excellent technical assistance.
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2001-11-0098.
Supported by Krebsforschung Schweiz (KFS 981-02-2000) and Foundation Gabriella Giorgio-Cavaglieri. We are grateful to RMF Dictagene S.A. for funding this project.
N.B. is employed by RMF Dictagene (Eplinges, Switzerland), whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Note added in proof
Author notes
Caroline Johnson-Léger, Department of Pathology, University Medical Centre, 1211 Geneva, Switzerland; e-mail:caroline.johnson-leger@medecine.unige.ch.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal