Although the SDF-1 (CXCL12)/CXCR4 axis is important for B-cell development, it is not yet clear to what extent CC chemokines might influence B lymphopoiesis. In the current study, we characterized CC chemokine receptor 5 (CCR5) expression and function of primary progenitor B-cell populations in human bone marrow. CCR5 was expressed on all bone marrow B cells at levels between 150 and 200 molecules per cell. Stimulation of bone marrow B cells with the CCR5-binding chemokine macrophage inflammatory protein 1β (MIP-1β; CCL4) did not cause chemotaxis, but CCL4 was able to trigger potent calcium mobilization responses and activation of the mitogen-activated protein kinase (MAPK) pathway in developing B cells. We also determined that CCR5-binding chemokines MIP-1α (CCL3), CCL4, and RANTES (CCL5), specifically by signaling through CCR5, could affect all progenitor B-cell populations through a novel mechanism involving heterologous desensitization of CXCR4. This cross-desensitization of CXCR4 was manifested by the inhibition of CXCL12-induced calcium mobilization, MAPK activation, and chemotaxis. These findings indicate that CCR5 can indeed mediate biologic responses of bone marrow B cells, even though these cell populations express low levels of CCR5 on their cell surface. Thus, by modulation of CXCR4 function, signaling through CCR5 may influence B lymphopoiesis by affecting the migration and maturation of B-cell progenitors in the bone marrow microenvironment.
Introduction
Chemokines were initially defined as molecules abundantly produced at sites of inflammation and responsible for the recruitment of cells to participate in local immune responses.1 Indeed, chemokines have been shown to direct the movement of cells between intravascular and extravascular compartments, within specific zones of secondary lymphoid organs, and to drive cells to recirculate between tissues and lymphoid organs.2,3 It has recently become clear that this family of molecules also controls multiple cell functions involving angiogenesis, embryogenesis, lymphocyte development, and hematopoiesis.4 5
The growth and differentiation of progenitor B cells are dependent on factors such as chemokines and cytokines, which are either bound or secreted by bone marrow stromal cells.6 Within the extravascular compartment of bone marrow, there are several well-known movements of progenitor B cells in which chemokines might be involved. The earliest progenitor B cells (eg, pro-B cells), are located within or near the endosteum.7,8 In subsequent stages of development, the differentiating B cells move toward sinusoids along stromal cell processes.8 9 After beginning to express surface IgM (sIgM), the immature and mature B cells migrate into the sinusoid segments and are released into the circulation.
The CXCL12/CXCR4 axis appears essential for B-cell development during early ontogeny. Mice in which the gene for CXCR4 or CXCL12 is disrupted show impaired B lymphopoiesis in the bone marrow.10,11 In other experiments, the transfer of CXCR4-deficient stem cells into wild-type mice results in high circulating levels of progenitor B cells, suggesting that the CXCL12/CXCR4 axis is important for the retention/positioning of early-lineage B cells in the bone marrow cavity.12,13 Furthermore, in support of this hypothesis is the observation that in both mice and humans CXCL12-mediated responsiveness decreases with B-cell maturation, despite comparable high levels of CXCR4 expression at all developmental B-cell stages.14-16 In contrast, little data are available as to the role of CC chemokine receptors during B-cell development. We reasoned that CC chemokines and corresponding receptors might also participate in the positioning of progenitor B cells in the bone marrow.
In the current study we therefore characterized CCR5 expression and function on primary progenitor B-cell populations (pro-, pre-, immature, and mature B cells) in human bone marrow. Cell surface expression of CCR5 on all bone marrow B cells was low compared with CXCR415 and calculated to be between 150 and 200 molecules per cell. Although CCL4 did not elicit chemotaxis of bone marrow B cells, CCL4 was capable of triggering potent calcium mobilization responses of pro-B and pre-B cells and mitogen-activated protein kinase (MAPK) activation of all progenitor B-cell populations. Moreover, we determined that CCL4 as well as other CCR5 ligands (ie, CCL3 and CCL5) could desensitize primary bone marrow B cells to subsequent CXCR4/CXCL12-mediated calcium mobilization, extracellular signal–regulated kinase 1 (ERK1) and 2 (ERK2) and p38 phosphorylation as well as chemotaxis responses. We also established that this inhibitory effect was dependent on the specific interaction of these chemokines with CCR5. To our knowledge, the present data represent the first example of heterologous desensitization of CXCR4 by signaling through CCR5.
Thus, CCR5-binding chemokines can trigger biologically significant responses of bone marrow B cells. The negative regulation of the CXCL12/CXCR4 axis by CCL4 may be important in the migration of progenitor B cells within the bone marrow compartment prior to their egress into the circulation.
Materials and methods
Cells
Heparinized bone marrow was obtained by iliac crest aspiration from healthy adult volunteers after informed consent was given and in accordance with the guidelines approved by the Institutional Review Committees of the Dana Farber Cancer Institute. Mononuclear cells were isolated by Ficoll-Hypaque (Pharmacia, Piscataway, NJ) gradient centrifugation (density 1.077 g/mL). Pelleted cells were collected and washed 3 times in 1 times phosphate-buffered saline (PBS), pH 7.4. After the third wash, cells were resuspended in ice-cold staining buffer (1 × PBS with 2% fetal bovine serum [FBS]) for further analysis.
Isolation of total RNA and RT-PCR of CCR5 chemokine receptor
mRNA from sorted bone marrow B cells was denatured in the presence of 5 μM oligo(dT)12-18 primer and then reverse transcribed using Moloney murine leukemia virus reverse transcriptase (Clontech Laboratories, Palo Alto, CA) at 42°C for 1 hour. One-tenth volume cDNA was amplified by polymerase chain reaction (PCR) in 1 times PCR buffer, 0.5 μM sense and antisense primers, and 0.5 U DNA Taq polymerase (New England Biolabs, Beverly MA).
The following primers were synthesized by Gibco BRL (Grand Island, NY). For CCR5, sense primer sequence was 5′-GTTTGCGTCTCTCCCAGGAATCA-3′ and antisense primer was 5′-CTTGCTCGCTCGGGAGCCTCTT-3′. The product length was 600 bp. For control gene GAPDH, sense primer was 5′-GGTGAAGGTCGGAGTCAACG-3′ and antisense primer was 5′-CAAAGTTGTCATGGATGACC-3′. The product length from cDNA was 500 bp. The conditions for amplification were 5 minutes at 94°C, followed by 35 cycles of 30 seconds at 94°C, 30 seconds at 62°C, 40 seconds at 72°C, followed by an extension for 4 minutes at 72°C. PCR products were resolved by electrophoresis on 1.2% agarose gels and visualized by the ethidium bromide staining. The appropriate length of the GAPDH product and the lack of a detectable band in the control without reverse transcriptase (no-RT), indicated that there was no contamination of gDNA.
Antibodies
Surface staining of bone marrow cells was performed with the following antibodies (all from Pharmingen, San Diego, CA, unless otherwise stated): fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)-labeled anti-κ light chain, FITC- or PE-labeled anti-λ light chain, PE-labeled anti-CD19, Cy-chrome–labeled anti-CD19, Cy-chrome–labeled anti-CD34, FITC- or PE-labeled anti-IgD, Red 613-labeled anti-CD19 (Caltag Laboratories, Burlingame, CA), allophycocyanin (APC)-labeled anti-CD19 (Caltag Laboratories), PE-labeled anti-CCR5 antibody (clone 2D7).
The secondary antibody was goat antimouse IgG-Cy5 labeled (Jackson Immunoresearch Laboratories, West Grove, PA). Nonconjugated mouse monoclonal antibodies were anti-CCR5 clone 2D7, clone 45529 (R & D Systems, Minneapolis, MN), anti-CXCR4 antibody (clone 12G5; R & D Systems).
Cell surface staining of bone marrow B cells
Four stages of B-cell differentiation were isolated from bone marrow cells as previously described.15 The earliest B-cell population, designated here as pro-B cells, was κ−/λ−, CD19+, CD34+. The next developmental B-lineage populations were designated as pre-B cells, identified as κ−/λ−, CD19+, CD34−; followed by immature B cells identified as κ+/λ+, CD19+, IgD−; and mature B cells identified as κ+/λ+, CD19+, IgD+.
Cell sorting
The Ficoll-Hypaque–purified bone marrow cells were suspended in ice-cold staining buffer (1 times PBS, 2% FBS) and incubated for 30 minutes with monoclonal antibodies. Cells were washed 3 times and resuspended in buffer (1 times PBS, 0.5% FBS).
The sorting into highly purified populations was performed on a MoFlo sorter (Cytomation, Fort Collins, CO) with the purity being more than 98.5%.
Cell surface staining for CCR5
Five-color immunofluorescence analysis was used to examine the expression of chemokine receptors on the surface of different B-cell populations. The different subsets of B cells were identified as described above. The fifth channel was used to define cell surface expression of CCR5. Ten thousand events were acquired in the fifth channel after triple gating for B-cell subsets. The threshold line was based on the maximum staining of a matched isotypic antibody with irrelevant specificity (mouse IgG2a) used in the same concentration as the anti-CCR5 antibody (4 μg/50 μL staining buffer). CCR5− cells were defined such that less than 1% of cells stained positive with control antibodies. Anti-CCR5–labeled cells that were brighter than those stained with the isotypic antibody (present to the right of isotype control histogram) were defined as positive for CCR5.
Data were acquired using a MoFlo flow cytometer (Cytomation) and analyzed with WinMDI 2.8, kindly provided by Dr Joseph Trotter (Scripps Research Institute, La Jolla, CA), or Summit (Cytomation) software.
QFACS
Quantitative flow cytometric analysis (QFACS) was performed by converting the mean fluorescence intensity of the cell subset into antibody-binding sites (ABSs) using a standardized microbeads kit (Sigma, St Louis, MO).
Beads (105/sample) were incubated with the same saturating concentration of 2D7-PE–labeled anti-CCR5 antibody (Pharmingen) as the samples and processed identically to those being quantified. The binding capacities of the stained beads were then regressed against the corresponding geometric mean of each bead population. The mean fluorescence intensity of CCR5 staining of the cells was converted to ABSs per cell. The mean fluorescence intensity of the isotype control (68-70 ABSs) for each B-cell subset also was converted to ABSs and subtracted from ABS values obtained with the experimental sample. The threshold of detection was 110 ABSs per cell. Additional details on the relationship between ABSs and mean fluorescence intensity values can be obtained from the manufacturer (Sigma).
Measurement of calcium signaling using FACS
Cells used in the calcium flux assay were stored overnight at 37°C in serum-free medium (StemSpan H2000, Stem Cell Technologies, Vancouver, BC, Canada).
Cells were suspended in 1 mL 30°C Hanks balanced salt solution containing 1.3 mM CaCl2, 0.5 mM MgCl2, 1 μM Indo-1am (Molecular Probes, Eugene, OR), and 0.01% F-127 Pluronic detergent (Molecular Probes) and incubated for 45 minutes at 30°C.
After stimulation with 1000 ng/mL CCL4 (R & D Systems) or 100 ng/mL CXCL12 (kindly provided by Dr Bill Holmes, GlaxoWellcome, Research Triangle Park, NC), calcium mobilization was analyzed with a Becton Dickinson FACStar Plus flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) equipped with Time Zero Injection Module (Cytek, Fremont, CA). UV excitation wavelengths were 350 to 364 nm, emission 405 nm and 510 nm. Cells were incubated at 37°C for 5 minutes before analysis.
Chemotaxis assay
Total bone marrow Ficoll-purified lymphocytes were stored overnight at 37°C in serum-free medium (StemSpan H2000) to remove adherent cells. Total lymphocytes were put into the upper well (106 cells/well) of Transwell inserts (6.5 mm diameter, 5 μm filter pore size; Costar, Cambridge, MA); the lower well contained 600 μL serially diluted (1-10 000 ng/mL) CCL4 or, as a positive control, 100 ng/mL CXCL12 (all from Peprotech, Rocky Hill, NJ) in 0.5% bovine serum albumin (BSA), RPMI 1640.
Cells were allowed to migrate for 2 to 4 hours at 37°C. Cells that passed through the membrane to the lower well were collected and stained with antibodies to B-cell determinants, as described above. Cells were counted by timed acquisition (120 seconds each sample, at 2 psi sample pressure differential) on a MoFlo flow cytometer (Cytomation).
CCR5-mediated inhibition of CXCL12-induced chemotaxis
The Ficoll-purified lymphocytes (106 cells in 0.5% BSA, RPMI-1640) along with 1000 ng/mL CCL3, CCL4, CCL5, macrophage inflammatory protein 3β (MIP-3β; CCL19), MIP-3α (CCL20), or BCA-1 (CXCL13; all from Peprotech) were placed into the upper well of Transwell inserts (6.5 mm diameter, 5 μm filter pore size; Costar). The lower well contained either 600 μL 100 ng/mL CXCL12 or 600 μL 100 ng/mL CXCL12 together with 1000 ng/mL CCL3, CCL4, CCL5, CCL19, CCL20, or CXCL13 in 0.5% BSA, RPMI 1640. The cells were allowed to migrate for 15 or 30 minutes.
To determine if CCL4-induced inhibition of CXCL12 chemotaxis was concentration dependent, increasing concentrations of CCL4-1, 10, 100, 1000, and 10 000 ng/mL were placed in both upper and lower wells, whereas 100 ng/mL CXCL12 was present in the lower well.
To assess whether inhibition of chemotaxis to CXCL12 was specific for CCL4, we used the following controls: (1) 1000 ng/mL heat-denatured CCL4 (boiled for 10 minutes at 100°C), (2) 1000 ng/mL CCL4 preincubated for 30 minutes with CCL4 neutralizing antibody (40 μg; R & D Systems), (3) cells were preincubated at 37°C with 1000 ng/mL CCL4 for 30 minutes before placement into upper well; this latter measure causes internalization of CCL4-binding chemokine receptors, thus abrogating the inhibition of CXCL12-mediated chemotaxis.
We also used controls to assess whether inhibition of CXCL12-induced chemotaxis was CCR5 dependent. Lymphocytes were preincubated for 30 minutes with either: (1) 10 μg/mL isotype control antibody (IgG2a, Pharmingen); (2) 10 μg/mL nonneutralizing anti-CCR5 monoclonal antibody (clone 45529, recognizing the second half of the second extracellular loop of the CCR5 receptor17; R & D Systems); or (3) 10 μg/mL neutralizing anti-CCR5 monoclonal antibody (clone 2D7, recognizing the first half of the second extracellular loop of the CCR5 receptor17; Pharmingen). The lymphocytes were subsequently placed into the upper well (106 cells/well) of Transwell inserts along with 1000 ng/mL CCL4. The lower well contained 600 μL 100 ng/mL CXCL12 along with 1000 ng/mL CCL4 in 0.5% BSA, RPMI 1640. The cells were allowed to migrate for 15 minutes.
Cells that passed through the membrane to the lower well were collected, stained with antibodies to B-lineage determinants and analyzed as described above.
Influence of CCL4 on the expression of CXCR4
To detect internalization of CXCR4, cells were incubated for 30 minutes at 37°C with 100, 1000 ng/mL CCL4 or 1000 ng/mL CXCL12 (Peprotech). After incubation, cells were immediately put on ice to prevent resurfacing of the receptor and stained with anti-CXCR4 antibody (clone 12G5; R & D Systems) as described above.
Western blotting
Early- and late-lineage B cells were stimulated with medium alone, 1000 ng/mL CCL4, 100 ng/mL CXCL12 or prestimulated first with 1000 ng/mL CCL4, and then after 5 minutes, stimulated with 100 ng/mL CXCL12. Stimulations were done for 2 minutes at 37°C. The reactions were stopped by adding ice-cold PBS. Protein samples were resolved on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA). Membranes were blocked in 5% BSA/Tris-buffered saline (TBS)-Tween 20 buffer and sequentially incubated with 0.5 μg/mL antiphospho-ERK1/2 or antiphospho-p38 antibodies (New England Biolabs) at 4°C for 3 hours, followed by incubation with horseradish peroxidase (HRP)–conjugated goat antirabbit antibody for 1 hour and subsequently developed using an enhanced chemoluminescence (ECL) kit (Amersham Pharmacia Biotech, Uppsala, Sweden). Membranes were then stripped with 1 times stripping buffer (Chemicon, Temecula, CA) and reblotted with anti-ERK1/2 or anti-p38 antibodies to verify the amount of proteins on the gels. The intensity of phosphoprotein was determined by densitometry using ImageQuant Version 1.1 (Molecular Dynamics, Sunnyvale, CA).
Enzyme-linked immunosorbent assay for detection of chemokines
Secretion of MIP, CCL3, CCL4, regulated on activation, normal T cell–expressed and –secreted protein CCL5, monocyte chemoattractant protein (MCP-1; CCL2), and Eotaxin (CCL11) was detected by Quantikine Human Immunoassay (R & D Systems), according to the manufacturer's protocol. Briefly, FACS-sorted early- (pro/pre combined) and late- (immature/mature combined) lineage B cells were cultured for 24 hours in serum-free medium (StemSpan H2000) at the concentration of 106 cells/1 mL medium. Conditioned media derived from cells were analyzed by the quantitative sandwich enzyme immunoassay.
Results
Expression of CCR5 on different subsets of primary B cells from bone marrow
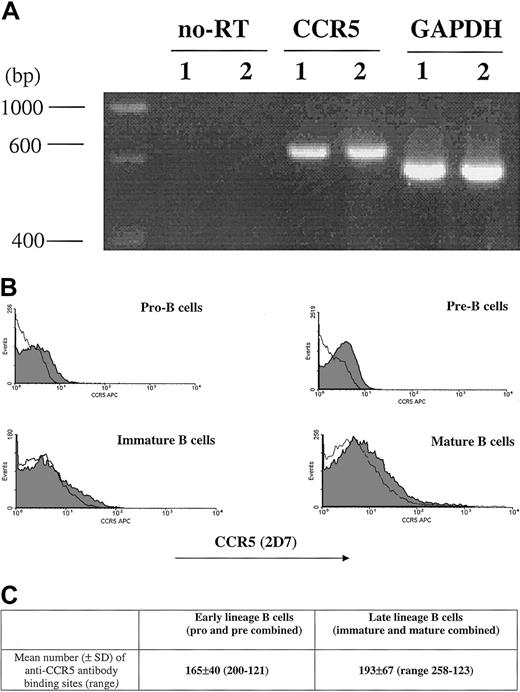
The percentage of CCR5+ cells measured by 2D7 antibody staining is shown (Figure 1). CCR5 expression was next evaluated by RT-PCR as well as by determining the number of anti-CCR5 binding sites per cell (Figure 1). Due to limitations in cell number, the sum of anti-CCR5 binding sites was calculated on a cell population designated as early lineage or late-lineage B cells. This early lineage B-cell population included both pro-B and pre-B cell subsets (CD19+, κ−/λ−) and late lineage B-cell population included both immature and mature B cells (CD19+, κ+/λ+).
CCR5 expression.
CCR5 expression during B-cell development in bone marrow as measured by RT-PCR (A), FACS (B), and QFACS (C). (A) CCR5 detection by RT-PCR of mRNA extracted from bone marrow B cells; lane 1, early-lineage B cells (pro-B and pre-B combined); lane 2, late-lineage B cells (immature B and mature B combined). no-RT indicates amplification of the PCR product not preceded by reverse transcription, to confirm that amplification from gDNA has not occurred. The results are representative of 3 experiments. (B) Surface expression of CCR5 on B-cell populations from bone marrow is shown. The shaded part of the histogram represents cells stained with anti-CCR5 antibody; the black line represents staining with the isotype control. The results are representative of 4 experiments. (C) The values represent the mean number of anti-CCR5 ABSs per cell for early lineage and late lineage bone marrow B cells. The results are representative of 3 experiments.
CCR5 expression.
CCR5 expression during B-cell development in bone marrow as measured by RT-PCR (A), FACS (B), and QFACS (C). (A) CCR5 detection by RT-PCR of mRNA extracted from bone marrow B cells; lane 1, early-lineage B cells (pro-B and pre-B combined); lane 2, late-lineage B cells (immature B and mature B combined). no-RT indicates amplification of the PCR product not preceded by reverse transcription, to confirm that amplification from gDNA has not occurred. The results are representative of 3 experiments. (B) Surface expression of CCR5 on B-cell populations from bone marrow is shown. The shaded part of the histogram represents cells stained with anti-CCR5 antibody; the black line represents staining with the isotype control. The results are representative of 4 experiments. (C) The values represent the mean number of anti-CCR5 ABSs per cell for early lineage and late lineage bone marrow B cells. The results are representative of 3 experiments.
The number of anti-CCR5 binding sites per cell was comparable for all stages of B-cell development in bone marrow (mean ± SD, n = 4): 165 ± 40 for early lineage (pro/pre) B cells, and 193 ± 67 for late lineage (immature/mature) B cells.
Both by flow cytometry as well as QFACS, the level of CCR5 expression on progenitor B cells is lower than previously reported for CXCR4.15
CCL4-induced responses of developmental B-cell populations
Chemokine receptors may still be biologically functional, even though they are expressed at relatively low levels on the cell surface.18 Thus, we assessed calcium mobilization responses of B-lineage cell subsets sorted from bone marrow after stimulation with 1000 ng/mL CCL4 (Figure2A).
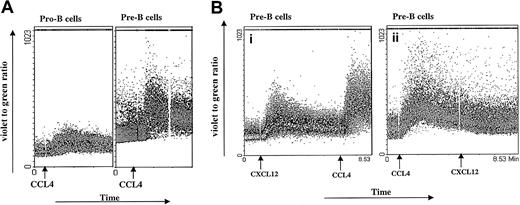
Calcium mobilization.
(A) CCL4-induced calcium mobilization of B-cell subsets sorted from bone marrow. Sorted B cells were loaded with Indo-1amand stimulated at the indicated time points (arrows) with the 1000 ng/mL CCL4. The results are representative of 3 experiments using primary B cells from bone marrow. (B) Inhibition of CXCL12-induced calcium mobilization by CCL4. Pre-B cells were sequentially stimulated with 100 ng/mL CXCL12 first and then restimulated with 1000 ng/mL CCL4 (i) or first with CCL4 and then restimulated with CXCL12 (ii). The results are representative of 2 independent experiments.
Calcium mobilization.
(A) CCL4-induced calcium mobilization of B-cell subsets sorted from bone marrow. Sorted B cells were loaded with Indo-1amand stimulated at the indicated time points (arrows) with the 1000 ng/mL CCL4. The results are representative of 3 experiments using primary B cells from bone marrow. (B) Inhibition of CXCL12-induced calcium mobilization by CCL4. Pre-B cells were sequentially stimulated with 100 ng/mL CXCL12 first and then restimulated with 1000 ng/mL CCL4 (i) or first with CCL4 and then restimulated with CXCL12 (ii). The results are representative of 2 independent experiments.
Sixty percent of pro-B cells showed an agonist-dependent calcium response to CCL4, appearing 30 seconds after stimulation and returning to base level after 2 to 3 minutes. Most of the pre-B cells (94% cells) showed increased calcium mobilization responses after addition of CCL4, with kinetics similar to those observed for pro-B cells. In contrast, mature B cells showed a low calcium mobilization response to CCL4 (only 2%-7% of stimulated cells responded; data not shown).
Different B-cell subsets were also stimulated sequentially, first with CXCL12 and subsequently with CCL4. As a control, another aliquot of bone marrow B cells sorted from the same donor was stimulated first with CCL4 and subsequently restimulated with CXCL12 (Figure 2B). Because CXCL12 and CCL4 are believed to bind specifically to CXCR4 and CCR5 receptors, respectively, we did not expect any cross-influence as measured by the calcium response. As indicated in Figure 2B, pre-B cells exhibited a robust calcium response after stimulation with CXCL12, as well as after restimulation with CCL4. In contrast, when pre-B cells were first stimulated with CCL4, the subsequent response to CXCL12 did not cause an increase in intracellular calcium concentration.
It is possible that the lack of calcium response to a second, subsequent ligand may be caused by depletion of the intracellular calcium stores. However, our measurements of Indo-1am cell loading after 2 consecutive stimulations are consistent with adequate calcium reserves. Further, when CCL4 follows CXCL12 stimulation, a vigorous sustained calcium response is seen, which indicates adequacy of calcium stores.
Chemotactic responses of different B-cell subsets toward CCL4
In view of the robust calcium responses of early B cells to CCL4 stimulation, we examined whether this chemokine could also elicit the migration of bone marrow B cells.
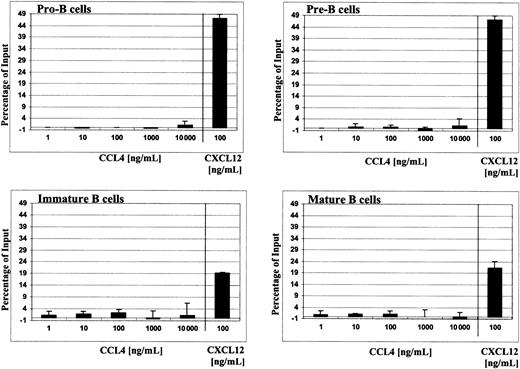
To avoid the influence of cell stress factors (ie, cell sorting), total bone marrow cells were loaded into Transwell and the migrated cells were then stained with appropriate markers to define B-cell subsets, as described in “Materials and methods.” CCL4 did not cause chemotaxis of pro-B, pre-B, immature, or mature B cells. The migration was tested along a wide range of chemokine concentrations (1-10 000 ng/mL; 0.125 nM to 1.25 μM) and sufficient time was allowed for the cells to migrate (2-4 hours). The number of cells, which migrated in the presence of CCL4, however, was not significantly different than spontaneous migration in the presence of medium alone (negative control; Figure 3).
Migration of different B-cell subsets from bone marrow to human CCL4.
Chemotaxis of B cells from total bone marrow in response to serial dilutions of CCL4 (1-10 000 ng/mL) is shown. Results are expressed as the percentage (mean ± SD, n = 4) of the input cells that specifically migrated to CCL4. For comparison, chemotaxis responses of B cells to 100 ng/mL CXCL12 are shown.
Migration of different B-cell subsets from bone marrow to human CCL4.
Chemotaxis of B cells from total bone marrow in response to serial dilutions of CCL4 (1-10 000 ng/mL) is shown. Results are expressed as the percentage (mean ± SD, n = 4) of the input cells that specifically migrated to CCL4. For comparison, chemotaxis responses of B cells to 100 ng/mL CXCL12 are shown.
For comparison, the known migration responses of bone marrow B cells to CXCL1214-16 are shown thus indicating their potential of chemotaxis. To confirm the biologic activity of CCL4 used in our assays—to initiate the migration of the lymphocytes—we determined that non–B-cell populations in bone marrow (ie, CD19−cells) did, indeed, exhibit chemotactic responses to CCL4 (data not shown).
Inhibition of CXCL12-induced chemotaxis of bone marrow B cells mediated by CCR5 binding chemokines
Our finding that stimulation with CCL4 inhibited CXCL12-induced calcium responses prompted our examination of whether this phenomenon could be extended to a more complex biologic event, such as cell migration requiring the activation of multiple downstream signaling pathways. Thus, we examined if exposure of bone marrow B cells to 1000 ng/mL CCL4 could inhibit their migration toward CXCL12. The experiments were performed with CCL4 present only in the upper well of the Transwell system or with the chemokine present in both upper and lower wells of the chemotaxis chamber.
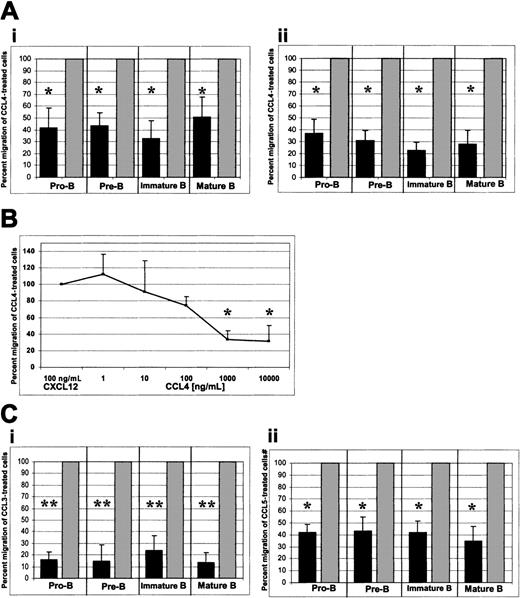
When CCL4 was added only to the upper well of the Transwell system, a considerable inhibition of migration toward CXCL12 was observed. This inhibition is expressed as the percent migration of CCL4-treated cells relative to the migration of untreated cells to CXCL12 (Figure4Ai). Where the migration of untreated cells to CXCL12 was considered to be 100%, the percent migration (mean ± SD, n = 6) of CCL4-treated pro-B, pre-B, immature, and mature bone marrow B cells was 42% ± 16%, 43% ± 10%, 33% ± 14%, and 51% ± 16%, respectively. In all cases the number of migrated cells was sufficiently high for reliable statistical analysis (see below).
CCR5-mediated inhibition of CXCL12-induced chemotaxis by bone marrow B cells.
(A) CCL4-mediated inhibition of CXCL12-induced chemotaxis. (i) CCL4 present in the upper well only; (ii) CCL4 present in the upper and lower wells of the chemotaxis chamber. (B) Titration of the CCL4 inhibitory effect. Panel B shows a dose-response curve of CCL4-mediated inhibition of CXCL12-induced chemotaxis. Pro-B cell data are shown. Similar results were obtained for the other bone marrow B-cell subsets (pre-B, immature B, mature B cells). (C) CCL3- and CCL5-mediated inhibition of CXCL12-induced chemotaxis. (i) Inhibition of the CXCL12-induced chemotaxis by CCL3. (ii) Inhibition of the CXCL12-induced chemotaxis by CCL5. Dark bars represent the percent migration of CCL3-, CCL4-, or CCL5-treated cells relative to the migration of cells pretreated with medium alone (gray bars). The data shown in panel A are representative of 14 individual experiments, each done in triplicate. The data shown in panels B and C are representative of 4 individual experiments, each done in triplicate. The changes in chemotaxis toward CXCL12 after exposure to CCL3, CCL4, and CCL5 were statistically highly significant (*P < .005, **P < .0005, paired t test 2-sided, whereP represents a statistical difference of CCL-exposed cells compared with the control of medium-treated cells) and migration of untreated cells toward CXCL12 was considered to be 100%.
CCR5-mediated inhibition of CXCL12-induced chemotaxis by bone marrow B cells.
(A) CCL4-mediated inhibition of CXCL12-induced chemotaxis. (i) CCL4 present in the upper well only; (ii) CCL4 present in the upper and lower wells of the chemotaxis chamber. (B) Titration of the CCL4 inhibitory effect. Panel B shows a dose-response curve of CCL4-mediated inhibition of CXCL12-induced chemotaxis. Pro-B cell data are shown. Similar results were obtained for the other bone marrow B-cell subsets (pre-B, immature B, mature B cells). (C) CCL3- and CCL5-mediated inhibition of CXCL12-induced chemotaxis. (i) Inhibition of the CXCL12-induced chemotaxis by CCL3. (ii) Inhibition of the CXCL12-induced chemotaxis by CCL5. Dark bars represent the percent migration of CCL3-, CCL4-, or CCL5-treated cells relative to the migration of cells pretreated with medium alone (gray bars). The data shown in panel A are representative of 14 individual experiments, each done in triplicate. The data shown in panels B and C are representative of 4 individual experiments, each done in triplicate. The changes in chemotaxis toward CXCL12 after exposure to CCL3, CCL4, and CCL5 were statistically highly significant (*P < .005, **P < .0005, paired t test 2-sided, whereP represents a statistical difference of CCL-exposed cells compared with the control of medium-treated cells) and migration of untreated cells toward CXCL12 was considered to be 100%.
Some chemokines have the ability to cause nonspecific movement of stimulated cells (chemokinesis) or cause movement away from the chemokinetic agent (repulsion).19 These phenomena may have biased the observed CCL4-mediated inhibition of chemotaxis to CXCL12. To test for this possibility, we added CCL4 at the same concentration to both upper and lower wells to ensure that there was no dynamic gradient of CCL4 present between the wells. In these experiments, CCL4-mediated inhibition of chemotaxis to CXCL12 was even greater. Relative to the migration of untreated cells to CXCL12, the migration (mean ± SD, n = 8) of CCL4-treated pro-B, pre-B, immature, and mature B cells to CXCL12 was 37% ± 11, 31% ± 8%, 23% ± 6%, and 28% ± 11%, respectively (Figure 4Aii).
Statistical analysis indicated that the decrease in chemotaxis toward CXCL12 after exposure to CCL4 was statistically highly significant for all B-cell subsets examined (P < .005, pairedt test, 2-sided).
CCL4-mediated inhibition of the CXCL12 chemotaxis was concentration dependent. A maximum inhibitory effect of CCL4 was observed at a concentration of 1000 ng/mL (P < .005) and was not further increased by raising the dose of CCL4 to 10 000 ng/mL (P < .005; Figure 4B).
CCL4-mediated inhibition of CXCL12-induced chemotaxis was observed only when chemotaxis was run for 15 minutes. When the duration of chemotaxis was extended to 30 minutes, the desensitization/inhibition effect of CCL4 diminished (data not shown). We postulate that the decrease of the inhibitory effect is due to homologous desensitization of the CCL4-binding chemokine receptor.20
We also examined whether other CCR5-binding chemokines (eg, CCL3 and CCL5) would inhibit CXCL12-induced chemotaxis of bone marrow B cells. Both CCL3 and CCL5 mediated significant inhibition of the CXCL12-induced chemotaxis. Relative to the migration of untreated cells to CXCL12, the migration (mean ± SD, n = 4) of CCL3-treated pro-B, pre-B, immature, and mature B cells to CXCL12 was 15% ± 6%, 14% ± 13%, 23% ± 12%, and 13% ± 8%, respectively (Figure 4Ci;P < .0005) and the migration (mean ± SD, n = 4) of CCL5-treated pro-B, pre-B, immature, and mature B cells to CXCL12 was 41% ± 7%, 43% ± 12%, 42% ± 9%, and 35% ± 12%, respectively (Figure 4Cii; P < .005).
In conclusion, CCL3 appeared to cause an even greater inhibition of CXCL12-induced chemotaxis than CCL4, whereas CCL5 was a less potent inhibitor than CCL4.
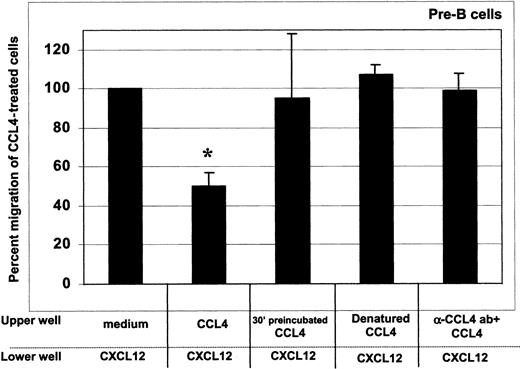
As shown in Figure 5, CCL4 inhibition of the CXCL12 chemotaxis was dependent on the presence of intact, cell surface bound chemokine, as both denaturation of CCL4 and blocking of its binding to the cell surface with neutralizing antibodies abrogated the desensitization of CXCL12-induced chemotaxis. The inhibition was further blocked when the cells were preincubated with CCL4 for 30 minutes before the assay (Figure 5).
CCL4-mediated inhibition of CXCL12-induced chemotaxis of bone marrow B cells: specificity controls for CCL4.
To assess whether the inhibition of chemotaxis to CXCL12 was specific for CCL4, we used the following controls: (1) cells preincubated at 37°C with 1000 ng/mL CCL4 for 30 minutes, then added into upper well of the chemotaxis chamber (third bar); (2) 1000 ng/mL heat-denatured CCL4 (fourth bar); and (3) 1000 ng/mL CCL4 preincubated for 30 minutes with CCL4-neutralizing antibody (fifth bar). The degree of inhibition is expressed as the percent migration of CCL4-treated cells toward CXCL12 (second bar), compared with the migration of cells pretreated with medium alone (first bar). Results acquired for the pre–B-cell subset are presented. Similar results were obtained for the other bone marrow B-cell subsets (pro-B, immature B, mature B cells). The means (± SD) of 2 experiments, each done in triplicate, are presented. Migration toward CXCL12 of untreated cells was considered 100%. *indicates the percent migration of CCL4-treated cells is statistically significant (P < .005, pairedt test 2-sided) compared with the migration of untreated (medium only) cells toward CXCL12. In contrast, the percent migration of cells treated with CCL4 controls was not statistically different.
CCL4-mediated inhibition of CXCL12-induced chemotaxis of bone marrow B cells: specificity controls for CCL4.
To assess whether the inhibition of chemotaxis to CXCL12 was specific for CCL4, we used the following controls: (1) cells preincubated at 37°C with 1000 ng/mL CCL4 for 30 minutes, then added into upper well of the chemotaxis chamber (third bar); (2) 1000 ng/mL heat-denatured CCL4 (fourth bar); and (3) 1000 ng/mL CCL4 preincubated for 30 minutes with CCL4-neutralizing antibody (fifth bar). The degree of inhibition is expressed as the percent migration of CCL4-treated cells toward CXCL12 (second bar), compared with the migration of cells pretreated with medium alone (first bar). Results acquired for the pre–B-cell subset are presented. Similar results were obtained for the other bone marrow B-cell subsets (pro-B, immature B, mature B cells). The means (± SD) of 2 experiments, each done in triplicate, are presented. Migration toward CXCL12 of untreated cells was considered 100%. *indicates the percent migration of CCL4-treated cells is statistically significant (P < .005, pairedt test 2-sided) compared with the migration of untreated (medium only) cells toward CXCL12. In contrast, the percent migration of cells treated with CCL4 controls was not statistically different.
CCR5 receptor specificity of CCL4-mediated inhibition of CXCL12-induced chemotaxis of bone marrow B cells
In the next experiments, we examined if the inhibitory effect of CCL4 was mediated by interaction with its specific receptor CCR5 or alternatively by another yet uncloned receptor present on the surface of B cells.
To test this hypothesis, we used a CCR5 neutralizing antibody (clone 2D7) at a concentration that caused complete specific inhibition of CCL4-induced migration of natural killer (NK) T cells.21As specificity controls, along with 2D7, separate aliquots of the cells were preincubated with the same amount of either nonneutralizing anti-CCR5 antibody (clone 45529) or with isotype control antibody (IgG2a).
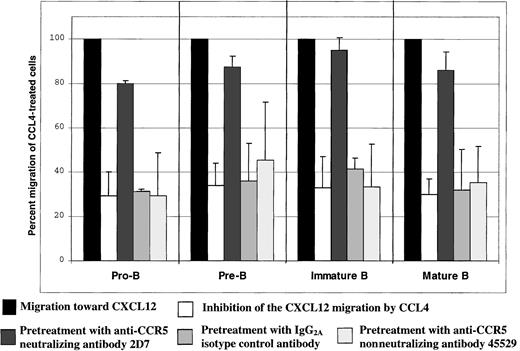
Preincubation of B cells with the CCR5 neutralizing antibody 2D7 before the assay almost completely abrogated CCL4-mediated inhibition of CXCL12-induced chemotaxis by all B-cell subsets (P < .05; Figure 6). The neutralizing effect was specific for the CCR5 receptor, because preincubation of the cells before the assay with the same amount of either isotype control antibody or nonneutralizing anti-CCR5 antibody did not have any effect on the CCL4-mediated inhibition of CXCL12-induced chemotaxis (Figure6).
CCL4-mediated inhibition of CXCL12-induced chemotaxis of bone marrow B cells: specificity controls for CCR5.
The degree of inhibition is expressed as the percent migration of CCL4-treated cells toward CXCL12 (second bar for each B-cell subset), compared with the migration of cells pretreated with medium alone (first bar for each B-cell subset). The third bar for each B-cell subset indicates migration of the cells pretreated with 2D7-neutralizing anti-CCR5 antibody; the fourth bar indicates migration of the cells pretreated with IgG2a isotype control antibody; and the fifth bar indicates migration of the cells pretreated with the nonneutralizing anti-CCR5 antibody (clone 45529). Migration toward CXCL12 of untreated cells was considered 100%.
CCL4-mediated inhibition of CXCL12-induced chemotaxis of bone marrow B cells: specificity controls for CCR5.
The degree of inhibition is expressed as the percent migration of CCL4-treated cells toward CXCL12 (second bar for each B-cell subset), compared with the migration of cells pretreated with medium alone (first bar for each B-cell subset). The third bar for each B-cell subset indicates migration of the cells pretreated with 2D7-neutralizing anti-CCR5 antibody; the fourth bar indicates migration of the cells pretreated with IgG2a isotype control antibody; and the fifth bar indicates migration of the cells pretreated with the nonneutralizing anti-CCR5 antibody (clone 45529). Migration toward CXCL12 of untreated cells was considered 100%.
CCL4 does not change the level of CXCR4 expression on bone marrow B cells
Previous reports indicated that chemotactic responses of cells toward chemokines could be changed by other cytokines as a result of modulation of chemokine receptor expression on the cell surface.22 23
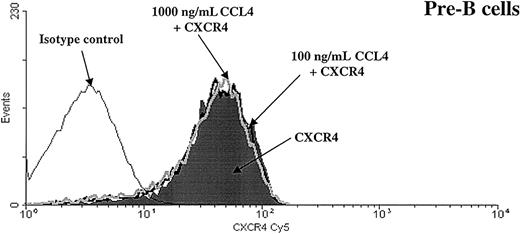
To determine whether CCL4-induced inhibition of CXCL12 chemotaxis was caused by altered cell surface expression of the CXCR4 receptor, we examined surface expression of CXCR4 on B cells after exposure to CCL4. Bone marrow B cells were preincubated with 100 or 1000 ng/mL CCL4 for 30 minutes and CXCR4 expression on these cells was compared with the expression on the cells that were incubated in medium alone.
As shown in Figure 7, we found that CCL4 did not change the level of CXCR4 expression on B cells.
CXCR4 expression is not down-regulated after preincubation with CCL4.
Expression of the CXCR4 chemokine receptor was analyzed on pre-B cells after preincubation for 30 minutes at 37°C with medium alone (shaded part of the histogram) or with CCL4 (100 ng/mL CCL4, black thick line, or 1000 ng/mL CCL4, gray thick line). Black thin line represents the isotype control. Similar results were obtained for the other bone marrow B-cell subsets (pro-B, immature B, mature B cells). The results are representative of 3 experiments using primary human bone marrow B cells.
CXCR4 expression is not down-regulated after preincubation with CCL4.
Expression of the CXCR4 chemokine receptor was analyzed on pre-B cells after preincubation for 30 minutes at 37°C with medium alone (shaded part of the histogram) or with CCL4 (100 ng/mL CCL4, black thick line, or 1000 ng/mL CCL4, gray thick line). Black thin line represents the isotype control. Similar results were obtained for the other bone marrow B-cell subsets (pro-B, immature B, mature B cells). The results are representative of 3 experiments using primary human bone marrow B cells.
Non–CCR5-binding chemokines (eg, CCL19, CCL20, and CXCL13) do not inhibit CXCL12-induced chemotaxis of bone marrow B cells
CCL19, CCL20, and CXCL13 were chosen because their receptors, CCR7, CCR6, or CXCR5, respectively, are expressed on developing bone marrow B cells. Moreover, these chemokines trigger strong biologic responses of bone marrow B cells at a concentration used in our experiments (data not shown; manuscript in preparation).
As shown in Figure 8, none of these chemokines affected CXCL12-induced chemotaxis of bone marrow B cells.
The influence of non–CCR5-binding chemokines on CXCL12-induced chemotaxis of bone marrow B cells.
The effects of CCL19 (A), CCL20 (B), and CXCL13 (C) on CXCL12-induced chemotaxis of bone marrow B cells are shown. Dark bars represent the percent migration of CCL19-, CCL20-, or CXCL13-treated cells relative to the migration of cells pretreated with medium alone (gray bars). The data shown are representative of 4 individual experiments, each done in triplicate. Migration of untreated cells toward CXCL12 was considered to be 100%.
The influence of non–CCR5-binding chemokines on CXCL12-induced chemotaxis of bone marrow B cells.
The effects of CCL19 (A), CCL20 (B), and CXCL13 (C) on CXCL12-induced chemotaxis of bone marrow B cells are shown. Dark bars represent the percent migration of CCL19-, CCL20-, or CXCL13-treated cells relative to the migration of cells pretreated with medium alone (gray bars). The data shown are representative of 4 individual experiments, each done in triplicate. Migration of untreated cells toward CXCL12 was considered to be 100%.
CCL4-mediated inhibition of CXCL12-induced activation of the MAPK pathway
Because CCL4-mediated heterologous desensitization of CXCR4 receptor is independent from CXCR4 receptor internalization (Figure 7), we reasoned that CCL4-mediated signaling through CCR5 was involved. We therefore tested whether exposure of bone marrow B cells to CCL4 would affect a known CCL12/CXCR4 signaling pathway. In our evaluation, we focused on the activation of the MAPK pathway, which includes various downstream kinases, such as ERK1/2 and p38.24
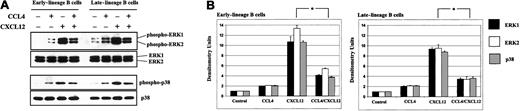
We showed that both CXCL12 and CCL4 can induce phosphorylation of the MAPK pathway (Figure 9). However, activation of the ERK1/2 and p38 was inhibited when bone marrow B cells were stimulated sequentially, first with CCL4 and subsequently after 5 minutes with CXCL12.
CCL4 inhibits CXCL12-induced phosphorylation of the MAPK pathway in bone marrow B cells.
(A) Early- and late-lineage B cells were stimulated with medium alone, CCL4, CXCL12 or prestimulated first with CCL4, and then after 5 minutes, stimulated with CXCL12. The lysates were run on the gel, transferred to membrane, and immunoblotted with antibodies antiphospho-ERK1/2 or antiphospho-p38, followed by reprobing with anti-ERK1/2 or anti-p38 antibodies to verify the amount of proteins on the gels. (B) Densitometric histograms of the Western blot experiments. The fold increases in phosphorylation after stimulation represent mean values ± SD obtained from 2 experiments. *P < .05 paired t test 2-sided, where P represents a statistically significant decrease in ERK1, ERK2, and p38 phosphorylation in cells prestimulated with CCL4.
CCL4 inhibits CXCL12-induced phosphorylation of the MAPK pathway in bone marrow B cells.
(A) Early- and late-lineage B cells were stimulated with medium alone, CCL4, CXCL12 or prestimulated first with CCL4, and then after 5 minutes, stimulated with CXCL12. The lysates were run on the gel, transferred to membrane, and immunoblotted with antibodies antiphospho-ERK1/2 or antiphospho-p38, followed by reprobing with anti-ERK1/2 or anti-p38 antibodies to verify the amount of proteins on the gels. (B) Densitometric histograms of the Western blot experiments. The fold increases in phosphorylation after stimulation represent mean values ± SD obtained from 2 experiments. *P < .05 paired t test 2-sided, where P represents a statistically significant decrease in ERK1, ERK2, and p38 phosphorylation in cells prestimulated with CCL4.
These data presented further proof that CCR5 was functional on developing B cells.
Secretion of CC chemokines by early- and late-lineage bone marrow B cells
CC chemokines are secreted by hematopoietic progenitor cells of several lineages and thus may influence hematopoiesis in an autocrine-paracrine pathway.25 We questioned whether bone marrow B cells also could produce chemokines. Primary bone marrow B cells were cultured for 24 hours in serum-free conditions and the presence of secreted CCL3, CCL4, CCL2, CCL5, and CCL11 chemokine proteins was measured.
Sorted cells (> 98.5% purity) representing early and late stages of B-cell development secreted CCL3, CCL4, and CCL2 (Table1). Early lineage B cells (pro-B and pre-B) secreted (mean ± SD, n = 3) 230 ± 298 pg/mL CCL3, 134 ± 189 pg/mL CCL4, and 70 ± 36 pg/mL CCL2. Late lineage B cells (immature B and mature B) secreted higher amounts of chemokines than early-lineage B cells (mean ± SD, n = 3): 492 ± 354 pg/mL CCL3, 1192 ± 341 pg/mL CCL4, and 156 ± 35 pg/mL CCL2. Primary B cells isolated from bone marrow, however, did not secrete CCL5 or CCL11. The differences in CCL3, CCL4, and CCL2 production between late- and early-lineage B cells were statistically significant (P < .05).
Production of CC chemokines by early- and late-lineage B cells from bone marrow
| Sample . | CCL3 secretion (pg/mL ± SD) . | CCL4 secretion (pg/mL ± SD) . | CCL5 secretion (pg/mL ± SD) . | CCL2 secretion (pg/mL ± SD) . | CCL11 secretion (pg/mL ± SD) . |
|---|---|---|---|---|---|
| Medium | < dt | < dt | < dt | < dt | < dt |
| Early-lineage B cells | 230 ± 298 | 134 ± 189 | < dt | 70 ± 36 | < dt |
| Late-lineage B cells | 492 ± 354 | 1192 ± 341 | < dt | 156 ± 35 | < dt |
| Sample . | CCL3 secretion (pg/mL ± SD) . | CCL4 secretion (pg/mL ± SD) . | CCL5 secretion (pg/mL ± SD) . | CCL2 secretion (pg/mL ± SD) . | CCL11 secretion (pg/mL ± SD) . |
|---|---|---|---|---|---|
| Medium | < dt | < dt | < dt | < dt | < dt |
| Early-lineage B cells | 230 ± 298 | 134 ± 189 | < dt | 70 ± 36 | < dt |
| Late-lineage B cells | 492 ± 354 | 1192 ± 341 | < dt | 156 ± 35 | < dt |
Early- (pro-B and pre-B cells combined) and late- (immature and mature B cells combined) lineage B cells sorted from bone marrow were cultured for 24 hours in serum-free medium at a concentration of 106 cells/mL medium. The detection threshold of the enzyme-linked immunosorbent assays for CCL3, CCL5, and CCL2 was greater than 31 pg/mL, and for CCL4 and CCL11 more than 15 pg/mL. The means ± SD of 3 experiments, each done in duplicate, are presented. < dt indicates below the threshold of detection.
Discussion
In the present study, we show that CCR5-binding chemokines induce biologically significant responses of progenitor B cells in human bone marrow. First, we find that CCL4, similar to CXCL12,15induces strong calcium mobilization responses of pro-B and pre-B cells. Although it is not clear how many chemokine receptor molecules are necessary to mediate calcium mobilization, other studies have shown calcium mobilization in response to CXCL12 by cell populations expressing 9% to 11% of CXCR4+ cells.18 This frequency is similar to the frequency of CCR5+ cells detected in our analyses of bone marrow B cells.
CCL4 did not cause calcium mobilization of either immature or mature B cells, although the level of CCR5 expression on these cell populations was comparable to pro-B and pre-B cells. The mechanism(s) underlying this difference in responsiveness is presently unknown but may be explained by G-protein–coupled receptor-related regulatory mechanisms,26 27 which might be operational at distinct stages of cellular development.
As mentioned above, progenitor B cells show strong calcium mobilization responses to both CXCL12 and CCL4. However, when pre-B cells are first stimulated with CCL4 and then subsequently restimulated with CXCL12, no calcium mobilization is observed in response to CXCL12. It is noteworthy that such cross-desensitization is not observed when cells are first stimulated with CXCL12. Interestingly, a similar pattern of cross-regulation of CXCR4 by CCR2 has been described in 293T cells cotransfected with CCR2 and CXCR4.28
Two types of desensitization are known: homologous and heterologous. Homologous desensitization is specific for a receptor and its ligands.29 Heterologous desensitization refers to a process whereby ligand-induced activation of one chemokine receptor results in the desensitization of different receptors.30The cross-desensitization of CXCR4 by CCL4 is of the heterologous type because CXCL12 is considered to be the exclusive ligand for CXCR4.31 Moreover, we show that this cross-regulation of CXCR4 on B-cell progenitors is dependent on signaling through CCR5 (Figure 6).
Receptor desensitization occurs during short-term (seconds to minutes) exposure of cells to activating ligands. The termination of signaling is mediated by uncoupling of activated receptors from G-proteins. The mechanisms involved in the cross-desensitization of chemokine receptors occur at different levels and include cross-phosphorylation of the receptor as well as cross-regulation at a site distal to the receptor/G-protein coupling involving downstream signaling pathways (eg, phospholipase C).32 In contrast, receptor internalization and subsequent down-regulation is due to loss of receptors from the cell surface and requires longer exposure (30 minutes to hours) of cells to ligands.
In separate experiments we determined that CCL4 does, in fact, bind to B cells (data not shown) but does not cause internalization of CXCR4 (Figure 7). Thus, we speculate that the mechanism of CCL4-mediated cross-desensitization of CXCR4 might be dependent on cross-phosphorylation of CXCR4 or a further downstream signaling event such as phosphorylation of phospholipase Cβ3.33 34 This notion is strengthened by the finding that CCL4-mediated signaling is associated with inhibition of CXCL12-induced MAPK activation (Figure9).
This cross-desensitization has biologic consequences, because we show that inhibition of CXCL12-induced responses by CCR5-binding chemokines could also be measured by inhibition of chemotaxis. Of note is the absence of inhibition when the cells are exposed to CCL4 for 30 minutes before the chemotaxis assay (Figure 5). We speculate that prior exposure to CCL4 in the range of 30 minutes effects down-regulation of the CCR5 receptor resulting primarily from internalization of the receptor.35 36
One of the most important functions of chemokines and their receptors is the regulation of directional migration of blood cells within secondary lymphoid organs and tissues.13,37 In specific tissue compartments, cells are exposed to multiple chemokines presented in the context of very complex dimensional and temporal patterns. Therefore, cells must use chemokine receptors sequentially to move along different chemotactic gradients. The switch from the use of one type of receptor to another could well be determined by the capacity of the first to undergo ligand-induced desensitization.38Such mechanisms are likely to be operational in the retention or correct positioning of progenitor cells in the extravascular compartment of the bone marrow. In this regard, increasing evidence suggests that the CXCR4/CXCL12 axis plays an important role in retaining B-cell precursors within the supportive niches of bone marrow for further maturation. However, progenitor B cells simultaneously receive signals mediated by a whole range of molecules (cytokines, chemokines, adhesion molecules) expressed by various cell types (stroma-, lymphoid-, and myeloid-lineage cells) in the bone marrow microenvironment.6,25 In this regard, bone marrow developing B cells could provide these signals in an autocrine manner, as they produce numerous CC chemokines in vitro (Table 1). Conceivably, some signals have an additive effect, whereas others may have a negative effect on target cells. Short-lasting desensitization of B-cell responses could enable progenitor B cells to temporarily “escape” CXCL12-induced retention in developmentally specific microenvironmental niches. This process would then allow the progenitor B cells to respond to other migratory gradients and to move toward sinusoids and into the circulation. Had the desensitization been of longer duration, it could have eventually led to the premature release of progenitor cells from the bone marrow as occurs following the infusion of CCL3-mimicking compounds,39 which also signal through CCR5.
Based on the results of our studies on bone marrow–derived B cells, we postulate that cross-desensitization of CXCR4 through signaling of CCR5 is relevant to B lymphopoiesis. Through desensitization or modulation of CXCR4 function or both, CCR5-binding chemokines could influence the multistep processes of movement and maturation of progenitor B cells in the extravascular compartment of bone marrow.8 9
The authors are very grateful to Drs John Manis and Mark Shlomchik for their critical review of the manuscript.
Prepublished online as Blood First Edition Paper, May 31, 2002; DOI 10.1182/blood-2002-01-0248.
Supported by National Institutes of Health grant P50HL54516.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Leslie E. Silberstein, Joint Program in Transfusion Medicine, Harvard Medical School, Children's Hospital Boston, Bader 410, 300 Longwood Ave, Boston, MA 02115; e-mail:leslie.silberstein@tch.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal