Abstract
Development of mammalian B-lineage cells is characterized by progression through a series of checkpoints defined primarily by rearrangement and expression of immunoglobulin genes. Progression through these checkpoints is also influenced by stromal cells in the microenvironment of the primary tissues wherein B-cell development occurs, ie, fetal liver and bone marrow and adult bone marrow. This review focuses on the developmental biology of human bone marrow B-lineage cells, including perturbations that contribute to the origin and evolution of B-lineage acute lymphoblastic leukemia and primary immunodeficiency diseases characterized by agammaglobulinemia. Recently described in vitro and in vivo models that support development and expansion of human B-lineage cells through multiple checkpoints provide new tools for identifying the bone marrow stromal cell–derived molecules necessary for survival and proliferation. Mutations in genes encoding subunits of the pre-B cell receptor and molecules involved in pre-B cell receptor signaling culminate in X-linked and non–X-linked agammaglobulinemia. A cardinal feature of these immunodeficiencies is an apparent apoptotic sensitivity of B-lineage cells at the pro-B to pre-B transition. On the other end of the spectrum is the apoptotic resistance that accompanies the development of B-lineage acute lymphoblastic leukemia, potentially a reflection of genetic abnormalities that subvert normal apoptotic programs. The triad of laboratory models that mimic the bone marrow microenvironment, immunodeficiency diseases with specific defects in B-cell development, and B-lineage acute lymphoblastic leukemia can now be integrated to deepen our understanding of human B-cell development.
Development of mature blood cells from lymphohematopoietic progenitors is a complex process governed by sequential changes in gene expression and external cues emanating from lymphohematopoietic microenvironments, such as fetal liver and bone marrow (BM). The last decade has witnessed dramatic progress in elucidating the molecular mechanisms that govern blood cell development. Mice with alterations in gene content (transgenics, knockouts, knockins) have been extraordinarily useful in elucidating the role of transcription factors, cytokines, and cytokine receptors in blood cell development. This review focuses on the developmental biology of human BM B-lineage cells and on perturbations in development that can contribute to the progression of B-lineage acute lymphoblastic leukemia (ALL) and immunodeficiency diseases characterized by agammaglobulinemia. My objective is to provide an update on key issues in human B-cell development and, where appropriate, compare and contrast B-cell development in mouse and human. The discussion of B-lineage ALL and immunodeficiency diseases will consider the developmental biology of these diseases as they constitute a deviation from normal programs.
The terminology used in this review is largely consistent with the terminology used by other laboratories studying human B-cell development. Pro-B cells are those B-lineage cells that express cell-surface CD19 but do not express cytoplasmic or cell-surface μ heavy chains (HCs). Pre-B cells express cell-surface CD19 and cytoplasmic μHCs, and variably express cell-surface μHCs associated with surrogate light chains (ψLCs)—ie, the pre-B cell receptor (pre-BCR). Immature B cells express cell-surface CD19 and cell-surface μHCs associated with κ or λLCs—ie, the B-cell receptor (BCR). B-cell precursors include all B-lineage cells prior to immature B cells expressing the BCR.
Sites of B-cell development
Human B-lineage cells are present in multiple tissue sites in early fetal development. However, from midgestation through the eighth decade of life, the BM is the exclusive site of B lymphopoiesis. Pre-B cells are present in 7- to 8-week gestational age fetal liver1and 10-week gestational age fetal omentum.2 A thorough analysis of 18- to 20-week fetal tissues revealed that B-cell development is multifocal; CD19+/surface μHC− B-cell precursors and CD19+/surface μHC+ immature B cells are present in BM, liver, lung, kidneys, and spleen.3 The frequency of B-cell precursors as a percentage of the total lymphoid cell pool is much higher in fetal BM compared with adult BM.3,4 Adult BM also differs from fetal BM by the presence of recirculating CD19+/surface μ, δ HC+mature B cells in the former.3 Similar levels of recombinase-activating gene (RAG)–1, RAG-2, and terminal deoxynucleotidyl transferase (TdT) are detectable by reverse transcriptase–polymerase chain reaction (RT-PCR) in pro-B cells from 18-week fetal BM and 62-year adult BM, underscoring the functional integrity of BM B-cell development throughout life.3 It is noteworthy that recent studies of T-cell development indicate that T-cell receptor (TCR) gene rearrangements actively occur in thymocytes from individuals in their sixth decade of life.5 Thus, ongoing development of B and T lymphocytes throughout life complements the existence of memory B and T lymphocytes in maintaining a functional immune system.
Developmental stages of B-lineage cells
Analysis of gene expression in developing lymphoid cells can be accomplished by multiparameter flow cytometry, immunohistochemistry/fluorescence microscopy, and RT-PCR. Immunologic phenotyping of B-lineage ALL using monoclonal antibodies (mAbs) and flow cytometry has been conducted in many laboratories, and it is not my intent to summarize the many published reports. The reader is referred elsewhere for an in-depth review.6
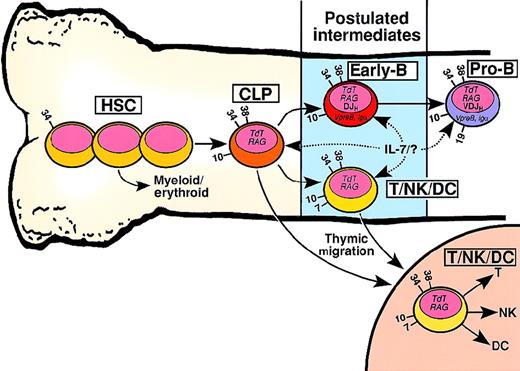
Although lymphoid progenitors are the descendants of hematopoietic stem cells (HSCs) with the capacity to develop into all lymphohematopoietic lineages, the earliest populations of lineage-restricted lymphoid progenitors are poorly characterized. Figure1 shows potential relationships between so-called common lymphoid progenitors (CLPs) and progenitors with increased fitness to form specific lymphoid lineages. The CLP is defined as a progenitor with the capacity to develop into T, B, or natural killer (NK) cells, but little or no capacity to develop into nonlymphoid lineages, such as myeloid/erythroid cells. A cell with the developmental potential of a CLP has been included in the blood cell developmental schemes of hematology textbooks for many years. However, data supporting its existence have only recently been published.7-10 Galy and colleagues used fluorescence-activated cell sorting (FACS) to purify CD34+/CD10+/CD45RA+ BM progenitors that do not express T, B, or NK lineage surface markers (ie, CD2, CD4, CD8, CD16, CD19, CD20, and CD56). A battery of in vitro assays and the severe combined immunodeficiency (SCID)–hu (human) mouse were employed to demonstrate that CD34+/CD10+/CD45RA+ progenitors are capable of developing into B, T, NK, and lymphoid dendritic cell (DC) lineages, but not myeloid/erythroid lineages.7 However, this study did not clarify whether T- and B-lineage cells are derived from a single progenitor. A subsequent study by Ryan and colleagues showed that CD34+/CD19− lymphoid progenitors expressing the interleukin 7 receptor (IL-7R) (CD127) gave rise to CD19+ cells in a colony-forming assay.8The CD34+/IL-7R+ lymphoid progenitors were uniformly TdT+, and the majority expressed CD10. RT-PCR analysis indicated the expression of RAG-1, immunoglobulin (Ig)β (CD79b), and the paired domain transcription factor PAX-5.8 Ryan and colleagues did not assay for T, NK, or DC potential, but the CD34+/IL-7R+/CD19− population contained granulo-monocytic colony–forming units at a frequency 10-fold to 100-fold lower than IL-7R−/CD19− cells.8 A recent report indicated that CD34+ BM cells expressing the CXCR4 chemokine receptor for stromal cell–derived factor-1 (discussed in more detail below) could develop into B- and T-lineage cells, but not myeloid/erythroid cells.9 It seems likely that the 3 reports7-9 described a human lymphoid progenitor with similar developmental potential. A population of IL-7R+adult murine BM cells are also developmentally restricted to become T, B, and NK cells10 and may be the murine counterpart of the lymphoid progenitors isolated from human BM.7-9
Developmental relationship between hematopoietic stem cells (HSCs), common lymphoid progenitors (CLPs), and putative early-B or T/NK/dendritic cell (DC) progenitors.
HSCs include all primitive CD34+/lineage−hematopoietic developmental stages prior to the CLP, shown schematically as 3 cells. Arrows with solid lines indicate developmental flow culminating in increased lineage restriction. Dashed arrows indicate possible cellular targets of IL-7 signaling or an unknown (?) ligand. Numbers on the cell surface indicate CD antigens useful in distinguishing the developmental compartments. Although not shown in this figure, the 3 reports that described the cell surface phenotype of CD19− lymphoid progenitors revealed considerable heterogeneity.7,8,13 For example, CD7 and CD33 were detected on a minority of the lymphoid progenitors in each study.7,8 13 There is no known surface marker that distinguishes the CLP from the early-B cell. It is also likely that IL-7R expression and signaling vary both within and between the lymphoid progenitor compartments.
Developmental relationship between hematopoietic stem cells (HSCs), common lymphoid progenitors (CLPs), and putative early-B or T/NK/dendritic cell (DC) progenitors.
HSCs include all primitive CD34+/lineage−hematopoietic developmental stages prior to the CLP, shown schematically as 3 cells. Arrows with solid lines indicate developmental flow culminating in increased lineage restriction. Dashed arrows indicate possible cellular targets of IL-7 signaling or an unknown (?) ligand. Numbers on the cell surface indicate CD antigens useful in distinguishing the developmental compartments. Although not shown in this figure, the 3 reports that described the cell surface phenotype of CD19− lymphoid progenitors revealed considerable heterogeneity.7,8,13 For example, CD7 and CD33 were detected on a minority of the lymphoid progenitors in each study.7,8 13 There is no known surface marker that distinguishes the CLP from the early-B cell. It is also likely that IL-7R expression and signaling vary both within and between the lymphoid progenitor compartments.
The model in Figure 1 proposes that CLP can differentiate into 1 of 2 lymphoid progenitor intermediates: early-B cells or T/NK/DC tri-lineage cells. Early-B cells are characterized by the initiation of DJH rearrangements and the expression of B-lineage specific proteins such as VpreB and Igα (CD79a). Support for the existence of an early-B cell comes from reports showing that DJHrearrangements,11,12 cytoplasmic Igα protein,13 and VpreB protein14 are present in CD19− lymphoid progenitors. The CLP could also differentiate into a T/NK/DC tri-lineage progenitor possibly defined by the acquisition of CD7. Either the CLP or the T/NK/DC progenitor could migrate to the thymus and undergo subsequent development.15It is unknown whether specific signals transduced by BM stromal cell–derived molecules can promote CLP differentiation into an early-B cell or a T/NK/DC progenitor.
Murine CLPs and possibly human CLPs are particularly sensitive to IL-7 stimulation. Signaling through the IL-7R is essential for the development of murine CLPs, although not by virtue of inducing self-renewal.16 A role for IL-7 signaling in the development of human CD19+ B-lineage cells from CD10+/TdT+/CD19− lymphoid progenitors has been demonstrated in vitro,8 but whether IL-7 exerts an effect on CLPs and/or early-B cells is unknown. The relationship between signaling pathways activated following IL-7 stimulation of CLPs and the developmental fate of CLPs are unknown. IL-7 activation of phosphatidylinositol (PI)–3 kinase/protein kinase B activation is essential for survival/proliferation of human T-cell precursors, whereas IL-7 activation of STAT5 favors T-cell differentiation.17 It will be interesting to determine whether PI-3 kinase, STAT5, or other IL-7 signaling pathways activated in human CLPs can lead to different developmental fates. The CLP→early-B cell step cannotrequire IL-7 (see below), and other cytokines or BM stromal cell–derived compensatory signals must be important.
Figure 2 shows the stages of human B-cell development and counterpart stages in murine B-cell development. Several classification schemes have been developed for the mouse,18 but Figure 2 shows the A through E fractions originally described by Hardy and colleagues.19 Figure 2proposes the existence of an early-B cell (as discussed above) that does not express the cell surface protein CD19. Early-B cells have initiated DJH rearrangements and express cytoplasmic Igα (and to some degree Igβ) as well as VpreB proteins. I would emphasize that this is a tentative definition of an early-B cell, since no cell surface markers that could distinguish early-B cells from CLP have been reported. This early-B cell may be similar to the CD19− B-lineage fractions A1 and/or A2 recently described by Hardy and colleagues,20 or the lin−/c-kitloand lin−/c-kit− progenitors described by Payne and colleagues.21 Human pro-B cells are a well-characterized population expressing CD10, CD34, and CD19.22 The vast majority of pro-B cells express TdT,22-24 and V-to-DJH rearrangements are easily detected.11,12 However, single-cell PCR analysis indicated that a minority of CD19+/CD34+ pro-B cells have both HC alleles in germ-line configuration.24Thus, assigning the early-B cell population a DJHrearrangement status and the pro-B cell population a VDJHrearrangement status is probably an oversimplification. A difference of opinion exists as to whether CD19+/CD34+ pro-B cells express cytoplasmic μHC. Two groups have concluded that pro-B cells express neither cytoplasmic nor low cell-surface μHC.24,25 In contrast, using FACS-purified CD19+/CD34+ pro-B cells, we can reproducibly detect cytoplasmic μHC in 5% to 10% of pro-B cells.26This result is consistent with the existence of readily detectable VDJH rearrangements in pro-B cells.11,12 The human pro-B cell may correspond to Hardy fraction B ± fraction C, based on analysis of VDJH rearrangements.24 27
Stages in human B-cell development.
Six stages beginning with the CLPs and culminating with immature B cells are shown as one model of B-cell development in human BM. The letters in parentheses represent an approximation of the counterpart stages in murine B-cell development, using the nomenclature of Hardy and colleagues.19 20 Numbers on the cell surface indicate CD antigens frequently used to define the individual stages in human B-cell development. Patterns of gene expression inside the cells have been determined by RT-PCR and/or flow cytometry. Dashed arrows indicate possible cellular targets for positive (+) and negative (−) growth regulators/chemotactic factors produced by BM stromal cells. Cells in a particular developmental stage are not necessarily uniform in the expression of a specific receptor. For example, only 10% to 20% of the pre-BI plus the pre-BII cells express the μ-ψLC pre-BCR.
Stages in human B-cell development.
Six stages beginning with the CLPs and culminating with immature B cells are shown as one model of B-cell development in human BM. The letters in parentheses represent an approximation of the counterpart stages in murine B-cell development, using the nomenclature of Hardy and colleagues.19 20 Numbers on the cell surface indicate CD antigens frequently used to define the individual stages in human B-cell development. Patterns of gene expression inside the cells have been determined by RT-PCR and/or flow cytometry. Dashed arrows indicate possible cellular targets for positive (+) and negative (−) growth regulators/chemotactic factors produced by BM stromal cells. Cells in a particular developmental stage are not necessarily uniform in the expression of a specific receptor. For example, only 10% to 20% of the pre-BI plus the pre-BII cells express the μ-ψLC pre-BCR.
A functional VDJH rearrangement is essential for normal pro-B cell differentiation into the pre-BI compartment (Figure 2). Pro-B cells that fail to make a functional VDJHrearrangement undergo apoptosis and are probably phagocytized by BM macrophages. Pro-B cell differentiation into pre-BI cells is characterized by loss of CD34 and TdT, and acquisition of cytoplasmic μHC in more than 95% of the cells.22-24 Similarly to the mouse,18 human pre-B cells can be generally subdivided into large proliferating cells (designated pre-BI in Figure 2) and small postmitotic cells (designated pre-BII in Figure 2) on the basis of cell-cycle analysis.24 The human pre-BI cells would partially overlap with Hardy fraction C.19,20 Pre-BII cells are actively undergoing κLC rearrangements.24 In general, κ rearrangement precedes λ rearrangement, and pre-BII cells that fail to make a functional κ rearrangement can proceed to rearrange the λLC locus. Interestingly, a very small percentage (approximately 1%) of immature B cells in human BM and peripheral blood express κ and λLC on individual cells.28-30 This dual LC expression may reflect immature B cells undergoing receptor editing.
The pre-BCR and related structures
Mammalian B-lineage cells must traverse several critical checkpoints on the road to becoming functional antigen-specific B cells. The cell surface molecular complex appearing at a critical initial checkpoint is the pre-BCR. The pre-BCR is a complex of proteins consisting of μHC, ψLC, and the Igα/Igβ signal transducing heterodimer.31 The mammalian ψLC consists of 2 proteins generally referred to as VpreB and λ5. The genes encoding these 2 proteins were originally discovered in the mouse (Melchers et al32 and references therein) and their organization differs between mouse and human (Minegishi et al33 and references therein) VpreB and λ5 proteins are noncovalently associated on the surface of B-cell precursors and together form a λLC-like structure. In turn, λ5 is covalently coupled to the CH1 domain of μHC via a carboxy-terminal cysteine. Readers are referred to an earlier review for details on the original identification and characterization of the VpreB and λ5 genes and their encoded proteins.32
Analysis of the structure, expression, and function of the human pre-BCR has been facilitated by the development of mAbs against recombinant ψLC proteins.34-39 The initial panel of mAbs made against the human ψLC was used to characterize cytosolic and cell-surface μHC/ψLC complexes and surface expression of ψLC.34,40 A major conclusion in the original study34 was that surface μΗC/ψLC expression was restricted to the pre-B cell compartment (ie, CD19+/CD34− cells in Figure 2); this suggests a critical role for the pre-BCR at a relatively late stage of B-cell development. Subsequent studies using other mAbs yielded conflicting results.24,35,36 A major difference was the identification of normal and leukemic pro-B cells (ie, CD19+/CD34+/μΗC−) that stained with anti-VpreB mAb.24,35 36 The low levels of cell-surface ψLC, differences in the subtype of the mAb (ie, IgM versus IgG1), and differences in epitope recognition by various anti-VpreB mAbs were among the explanations offered for the conflicting results.
A series of papers describing new antihuman VpreB mAbs37-39has provided some resolution to past discrepancies. The recent vintage of anti-VpreB mAbs includes mostly IgG1 subclass mAbs, thereby eliminating potential problems that can confound the use of IgM reagents. The anti-VpreB mAb produced by Wang and colleagues binds to the surface of pre-B cell lines, but only binds to pro-B cell lines following permeabilization.37 As expected, normal CD19+ human B-lineage cells coexpressed low levels of cell surface μHC and VpreB, and approximately 20% of the CD19+/VpreB+ cells were weakly CD34+.37 Interestingly, cytoplasmic VpreB+ is expressed in CD34+/CD19− lymphoid progenitors at a stage prior to V-to-DJH rearrangement,37possibly the early-B cells proposed in Figure 2. Tsuganezawa and colleagues generated mAbs that recognized human VpreB, human λ5, or an epitope expressed only on the assembled pre-BCR.38 Their anti-VpreB mAb binds to the surface of pre-B ALL cell lines but not pro-B ALL cell lines.38 However, cytoplasmic VpreB or λ5 were detected in the majority of pro-B ALL cell lines and freshly isolated pro-B ALL.38 In contrast, the anti-VpreB mAb produced by Gauthier and colleagues39 binds to the surface of some μ− pro-B ALL cell lines.25 One of these pro-B ALL cell lines (designated JEA2) was shown to express cell surface VpreB in association with poorly characterized molecules of approximately 105 to 130 kd. Interestingly, cross-linking VpreB on the μ− JEA2 pro-B ALL cell line led to an increase in Ca++ flux, suggesting that VpreB was one component of a putative signaling receptor on the surface of at least some pro-B ALL cells.25 These authors also detected cell-surface VpreB on CD19+/CD34+ normal pro-B cells and used this data to argue for the existence of 2 distinct populations: CD19+/CD34+/μHC−/VpreB+and CD19+/CD34−/μΗC+/VpreB+. However, there is no biochemical evidence that VpreB is associated with a protein (or proteins) other than μHC on the surface of normal human B-cell precursors. We have used one of the anti-VpreB mAbs (VpreB8) made by Wang and colleagues37 to analyze VpreB expression in fetal BM B-lineage cells. Our results indicate that VpreB is expressed on the surface of 5% to 10% of the B-cell precursors (ie, pro-B plus pre-BI plus pre-BII in Figure 2). Furthermore, within the VpreB+ population, approximately 90% of the cells are CD19+/CD34−, and approximately 10% are CD19+/CD34+. Results from our laboratory show that CD34+/VpreB+ cells also express cell surface μHC (J. A. R. Pribyl, N. Shah, F. E. Bertrand, T. W. LeBien, 1999, unpublished data). Thus, we believe that most, if not all, CD19+/CD34+/VpreB+ normal B-lineage cells express the conventional pre-BCR. Since the vast majority of surface VpreB+ cells are CD34−, I show the expression of the pre-BCR on pre-BI and pre-BII cells only (Figure2). The VpreB+ cells that weakly express CD34 could be developmentally more similar to pre-BI cells than pro-B cells, but this has not been tested. The vast majority of B-lineage ALLs express cytoplasmic or surface VpreB,25 38 probably reflecting the general phenotypic similarity between normal and leukemic B-cell precursors.
The importance of the ψLC to normal B-cell development was first elucidated in a classic study demonstrating that mice with a targeted disruption in the λ5 locus exhibit a block at the pro-B to pre-B transition.41 The block in B-cell development probably occurs because cells at this transition fail to receive a positive selection signal through the pre-BCR. However, the block is not absolute since the number of B cells in secondary lymphoid tissues gradually increases over time, probably owing to the emergence of B cells that rearranged κLC genes prior to μHC genes.42,43 The importance of the ψLC in human B-cell development has been underscored by the discovery of an agammaglobulinemia patient with mutations in both λ5 alleles33 (see below). Immunologic analysis of this single patient indicated a disruption in B-cell development more severe than what occurs in λ5-deficient mice. Since only a single patient with a mutation in the λ5 locus has been described to date, it is unclear whether the gradual recovery of B cells observed in λ5-deficient mice would occur in humans.
How does the pre-BCR perform its critical role at the pro-B→pre-B transition? Despite heuristic appeal, the notion that the pre-BCR functions as a receptor for a specific ligand in the BM or fetal liver microenvironment has not been supported by experimental evidence. What has become clear is that only about one half the μHC proteins encoded by functional VDJHrearrangements are capable of pairing with ψLC in the mouse.44-46 Circumstantial evidence suggests that a similar type of preferential pairing of μHC and ψLC also occurs in human pre-B cells.47,48 This pairing is essential for pre-BCR assembly and expression on the cell surface. Formation of the pre-BCR heralds a sequence of events, including (1) suppression of RAG-1/RAG-2 expression to ensure allelic exclusion at the μHC locus, (2) a rapid burst of proliferation in cells expressing the pre-BCR, and (3) reexpression of RAG-1/RAG-2 and initiation of LC gene rearrangement. Are there separable roles for ψLC and μHC in pre-BCR function? This is controversial. Shaffer and Schlissel reported that transgenic mice expressing a truncated murine μHC (incapable of pairing with ψLC) could still transduce signals leading to changes in surface markers, transcription, and retargeting of the recombinase ensemble in B-lineage cells.49 The authors concluded that the ψLC functions as a chaperone to facilitate pre-BCR assembly and expression, but plays no direct role in signal transduction. They also argued that the capacity of truncated μHC to mediate B-cell differentiation in the absence of ψLC ruled against the pre-BCR/ligand model. However, truncated μHC may undergo enhanced aggregation compared with full-length μHC, thereby leading to increased constitutive signaling.50
Pre-BCR cross-linking in vitro initiates signaling events, including Ca++ flux and protein tyrosine kinase activation.51,52 How could this occur in vivo in the absence of an external cross-linking ligand? Elegant recent studies have provided new insight into the complexity of pre-BCR subunit protein-protein interactions involving μHC, VpreB, and λ5.39,53 These studies suggest mechanisms to explain pre-BCR assembly, VH repertoire selection, and cell signaling. We might assume that signaling through the pre-BCR minimally requires the dimerization/aggregation of at least 2 distinct pre-BCR molecular complexes on the cell surface. With the realization that the ψLC is a non–transmembrane-spanning polypeptide complex disulfide-linked to the μHC and exhibiting VpreB-VHinteractions, it seems possible (as proposed by Melchers54) that the ψLC itself could assume a ligand function for the μHC.
Other protein complexes with potentially similar functions to the pre-BCR have been described. Murine pro-B cell lines express the ψLC associated with several proteins ranging from 65 to 200 kd, including a predominant protein of 130 kd.55 These pro-B cell lines do not express μHC.55 The protein or proteins associated with ψLC in these cells have been occasionally referred to as the surrogate HC. A recent study using a human μ− pro-B leukemic cell line (JEA2) showed that VpreB is noncovalently associated with a p105 and possibly several other proteins on the cell surface.25 However, there is no evidence that VpreB associates with p105 (or any other protein other than μHC) on normal human B-cell precursors. The identity of the murine and human ψLC-associated surface proteins is unknown. A novel complex that has been referred to as the “calnexin pre-BCR”56 was recently described by Nagata and colleagues.57 The calnexin pre-BCR consists of Igα/Igβ noncovalently associated with the molecular chaperone calnexin, and was detected on the surface of pro-B cell lines and early B-lineage cells from RAG-2–deficient mice.57 Cross-linking Igβ on these cells induced the tryosine phosphorylation of syk, ERK, and PI-3 kinase in vitro, and pro-B to pre-B cell differentiation in vivo. A potential μ-independent role for Igβ was discovered when mice with a targeted disruption of the Igβ gene were shown to exhibit a block in B-cell development prior to V-to-DJH rearrangement.58 These results57 58 suggest a role for Igβ+/μHC− molecular complexes in the earliest stages of murine B-cell development.
The IL-7 story
An unresolved issue in studies of human B-cell development is the identity of the molecule(s) essential for the growth of normal B-cell precursors. Much has been written of IL-7, and some historical perspective is warranted. Following the initial cloning and characterization of IL-7 from murine BM stromal cells more than 10 years ago,59 IL-7 was shown to be crucial for the proliferation and development of murine B-cell precursors. IL-7 has been cast as a survival, proliferation, or differentiation factor depending upon the experimental system being employed.60,61Mechanistic insight has been gleaned from studying the effect of single amino acid substitutions on IL-7R α chain function. Corcoran and colleagues showed that a Y-to-F mutation at amino acid residue 449 abrogated the capacity of B-cell precursors to undergo IL-7–induced proliferation through a PI-3 kinase–dependent pathway.62Interestingly, functional studies of the IL-7R α chain harboring this mutation uncovered a novel signaling pathway (PI-3 kinase independent?) that triggered IgH rearrangements and subsequent B-cell differentiation.62 Recent studies from the same group indicated that IL-7R signaling can alter recombinase accessibility of 5′ VH genes.63 The criticality of IL-7 for normal murine B-cell development has been elucidated in gene-targeted mice. Targeted disruptions in the genes encoding IL-7,64 the IL-7R α chain,65 the γc subunit of the receptors for IL-2, 4, 7, 9, and 15,66,67 and the Jak3 tyrosine kinase68,69 all lead to severe impairment in B-cell development. Thymocyte and T-cell development are also impaired, reflecting the multiple actions these 5 cytokines exert on lymphopoiesis. IL-7 is, however, not the only cytokine implicated in the regulation of murine B-cell development. Kincade and colleagues have suggested that at least 16 distinct stromal cell products can exert positive effects on murine B-cell development.61 One recent addition to the list is thymic stromal lymphopoietin (TSLP). TSLP was originally isolated from a murine thymic stromal cell line.70 TSLP reportedly has the capability to replace IL-7 in supporting murine B-cell development in vitro71 and promote the development of surface IgM+ immature B cells from surface IgM−precursors.72 Interestingly, the TSLP receptor complex consists of the IL-7R α chain and a second subunit distinct from the γc. Furthermore, TSLP induced the activation of STAT5 but not any of the known Jak kinases.72Whether IL-7 and TSLP work in a hierarchical or cooperative manner in regulating murine B-cell development is unknown. Human TSLP has been cloned (S. Lyman, PhD, Immunex, written communication, May 1999), but there are no published reports on its bioactivity on human B-cell precursors.
The role of IL-7 in human B-cell development is strikingly different from its role in murine B-cell development. In fact, there is a widespread misconception that IL-7 stimulates the proliferation of human B-cell precursors. Part of the difficulty in determining IL-7 effects can be attributed to differences in the assay systems employed and the biological endpoints measured. Initial studies by us73 and others74,75 demonstrated that recombinant human IL-7 could exert weak proliferative effects on normal human B-cell precursors in vitro. However, it was difficult to exclude the possibility that IL-7 was simply enhancing survival. A stronger effect of IL-7 was observed when normal B-cell precursors were cultured on human BM stromal cells.26,73,75 76 Under these conditions, CD19+ B-lineage cell numbers increased by 10-fold to 20-fold over 2 to 3 weeks in vitro, but underwent rapid cell death thereafter.
Biological insight into IL-7 function in human B-cell development has been gleaned from congenital immunodeficiency patients. Patients with X-linked severe combined immunodeficiency (XSCID) have mutations in the γc subunit of the receptors for IL-2, IL-4, IL-7, IL-9, and IL-15. XSCID patients have severe defects in development of T and NK cells, but have normal or even elevated numbers of peripheral blood B cells.77 Furthermore, immunodeficiency patients with autosomal recessive mutations in the Jak3 tyrosine kinase exhibit a developmental phenotype indistinguishable from XSCID, including normal numbers of peripheral blood B cells.78,79 Likewise, 2 patients with mutations in the IL-7R α chain that led to defective expression also had normal numbers of peripheral blood B cells.80 These collective experiments of nature clearly indicate that IL-7 signaling is not essential for at least the numerically normal development of human B lymphocytes. My laboratory used a human BM stromal cell culture system to show that HSCs could develop into B-lineage cells independently of IL-7 stimulation.81 Our results are in accord with B-cell development occurring in patients with XSCID. However, extensive proliferation of the pro-B cell compartment does not occur,81 and it is presumptuous to assume that this in vitro model completely mimics B-cell development occurring in patients with mutations in the IL-7 pathway.
Although not essential for human B-cell development, IL-7 does transduce signals that lead to specific changes in gene expression. Proliferation of CD19+/CD34+ pro-B cells on human BM stromal cells is enhanced by inclusion of exogenous IL-7.26 IL-7 stimulation induces a specific increase in cell-surface CD19 on human pro-B cells82,83 and a decrease in RAG-1, RAG-2, and TdT messenger RNA (mRNA) levels.83There may be physiologic relevance to these results. For example, IL-7 expression in situ has been detected by RT-PCR analysis of human BM biopsies.84 The identity of the human BM cell producing IL-7 in vivo is unknown, although small amounts (less than 2 pg/mL) of IL-7 can be detected in supernatants from vascular cell adhesion molecule-1 (VCAM-1) (CD106)+ BM stromal cells in vitro.76,81,85 Similarly, purified VCAM-1+murine BM reticular cells express cytoplasmic IL-7 protein.86
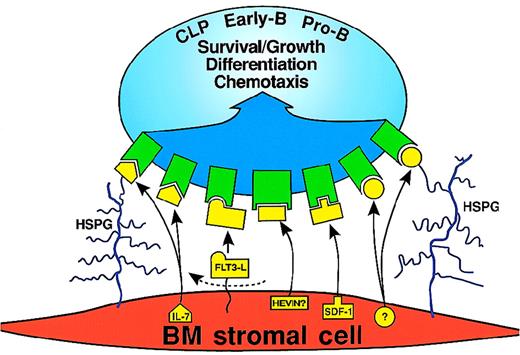
The complete identity of the human BM stromal cell–derived molecule (or molecules) that transduce signals essential for human B-cell development (including the counterpart of the murine IL-7 “signal”) is unknown. Part of the difficulty in identifying an IL-7 alternative is that IL-7 could act on at least 3 distinct stages of lymphoid cell development: CLPs, early-B cells, and pro-B cells (Figure 2). The cell-cycle disposition and self-renewal capacity of these compartments have not been determined. However, we do know that human CD19− progenitors8 and pro-B cells26 are IL-7 responsive. Figure3 shows several cytokines that could regulate human B-cell development. The cytokine-responsive target cells would include CLPs, early-B cells, and pro-B cells. The BM stromal cell molecules that regulate development could be secreted or cleaved from the stromal cell surface. The secreted or cleaved products could in turn be bound to stromal cell proteoglycans such as heparan sulfate proteoglycans (HSPGs). Several candidates come to mind. Namikawa and colleagues reported that a combination of IL-7, IL-3, and Flt3 ligand is superior to IL-7 alone in supporting human pro-B cell growth on murine BM stromal cells.87 My laboratory has confirmed their observation using human BM stromal cells (J.A.R. Pribyl and T.W. LeBien, unpublished observations, February 1999). Flt3 ligand is produced by BM stromal cells88,89 (also in our unpublished observations, February 1999) and could potentially stimulate similar cellular compartments and transduce similar signals to IL-7. Oritani and Kincade developed a cloning strategy to identify murine BM stromal cell gene products that bind to murine pre-B cells.90 One of the molecules identified was a secreted extracellular matrix glycoprotein designated SC1/ECM2.90 Soluble and immobilized SC1/ECM2 enhance the growth of IL-7–dependent murine pre-B cells,90,91 but the mechanism of enhancement is unknown. Interestingly, the carboxy terminus of SC1/ECM2 has high amino acid sequence homology to osteonectin/SPARC,92 which reportedly can bind cytokines, such as platelet-derived growth factor.93 A human homologue of SC1/ECM2 (designated hevin) has been cloned from high endothelial venules,94 but it is not known whether hevin has any effect on the growth of human B-cell precursors. HSPGs play a critical role in “presenting” cytokines to survival/growth factor receptors on lymphohematopoietic cells. HSPGs expressed on murine B-lineage and BM stromal cells can bind IL-7 and enhance the growth of IL-7–dependent murine pre-B cell lines.95 Furthermore, heparan sulfate is important for cytokine-mediated expansion of human long-term-culture–initiating cells.96 Therefore, a currently unknown molecule produced by BM stromal cells could bind to HSPGs and mediate survival/growth of human B-cell precursors (Figure3).
BM stromal cell–derived molecules that could transduce survival/growth, differentiation, or chemotactic signals to CLP, early-B, or pro-B cells.
HSPG indicates heparan sulfate proteoglycan. The dashed arrow indicates that the membrane-bound form of Flt3 ligand is cleaved at the stromal cell surface. The question mark indicates the unknown growth factor that could bind to HSPG. IL-7 and the unknown growth factor are shown binding directly to their cognate receptors, or binding HSPG followed by binding to their cognate receptors.
BM stromal cell–derived molecules that could transduce survival/growth, differentiation, or chemotactic signals to CLP, early-B, or pro-B cells.
HSPG indicates heparan sulfate proteoglycan. The dashed arrow indicates that the membrane-bound form of Flt3 ligand is cleaved at the stromal cell surface. The question mark indicates the unknown growth factor that could bind to HSPG. IL-7 and the unknown growth factor are shown binding directly to their cognate receptors, or binding HSPG followed by binding to their cognate receptors.
A BM stromal cell product shown in Figure 3 that potentially provides a unique function in B-cell development is the chemokine stromal cell–derived factor-1 (SDF-1). Mice with targeted disruptions in the genes encoding SDF-1 and its receptor CXCR4 exhibit perinatal mortality owing to perturbations in organ vascularization and lymphohematopoiesis.97-99 B lymphopoiesis and myelopoiesis are severely impaired,97-99 and a recent study concluded that a functional CXCR4 receptor is essential for retention of B-cell precursors in the BM microenvironment.100 CXCR4 has a complex pattern of expression on CD34+ lymphohematopoietic cells and CD19+ B-lineage cells.9,100-102 CXCR4 is expressed at all stages of B-cell development, but CD19+/CD34−/LC− pre-B cells and mature B cells express higher levels than CD19+/CD34+ pro-B cells.102,103Interestingly, SDF-1–mediated signaling pathways leading to calcium mobilization and chemotaxis are more rigorously activated in less mature B-lineage cells expressing lower levels of cell surface CXCR4.102,103 Thus, BM SDF-1 may trigger signaling pathways that regulate chemotaxis of B-cell precursors to “preferred sites” of proliferation within the stromal cell milieu. It is noteworthy that SDF-1 is expressed in human fetal liver biliary ductal plate epithelial cells, in apposition to lymphoid cells expressing VpreB.104
Models of human B-cell development
Establishment and characterization of in vitro culture systems that at least partially mimic the in vivo BM microenvironment have been extremely important for advancing our understanding of human B-cell development. Progress in this area has been facilitated by the use of BM stromal cells as a supportive microenvironment. Adherence of B-cell precursors to BM stromal cells is essential for normal murine and human B-cell development (reviewed in Kincade et al,61 Jarvis et al,105 and Ryan et al106). Binding of very late antigen–4 (VLA-4) (CD49d/CD29) expressed on human B-cell precursors to VCAM-1 on human BM stromal cells is the primary molecular interaction that facilitates adhesion of these 2 cell types.107-109Cytokines can regulate levels of BM stromal cell surface VCAM-1, thereby influencing the capacity of these cells to support B-cell precursor adhesion.108 There is substantial evidence that cross-linking VLA-4 with VCAM-1 or the CS-1 domain of fibronectin can trigger a protein tyrosine kinase cascade in B-lineage cells. However, there is no evidence that VLA-4 triggers a reciprocal activation of VCAM-1 culminating in a signal transduced in BM stromal cells. Lymphoid cell contact with human BM stromal cells can transduce signals leading to protein tyrosine kinase activation110 and tyrosine phosphorylation of focal adhesion kinase, paxillin, and ERK2.111 This signaling pathway is independent of VCAM-1.110 To what degree these bi-directional (B-cell precursor ↔ BM stromal cell) signaling events might influence B-cell developmental fates in vivo is unknown.
Prompted by our success in establishing in vitro human BM stromal cell culture conditions that support the adhesion108 and short-term growth26,73 of B-cell precursors, we asked whether a more expanded model of B-cell development could be established. In an effort to foster physiologic relevance, we FACS-purified fetal BM CD34++/CD19− HSC and plated them onto third-passage, nontransformed human fetal BM stromal cells.81 CD34++/CD19−HSCs underwent commitment and differentiation into the B-lineage over a 3-week period.81 A hierarchy of developmental changes consonant with B-cell development in vivo occurs in this in vitro model, including (1) loss of CD34 expression, (2) a continuum of increase in cell surface CD19, (3) emergence of cytoplasmic μHC+ pre-B cells, including some expressing the cell-surface pre-BCR, and (4) emergence of immature B cells expressing μ/κ or μ/λ BCR. As discussed above, several lines of evidence rule out a mandatory role for IL-7 in this culture system.81 The fact that CD34++/CD19− HSC can differentiate all the way to immature B cells demonstrates that human fetal BM stromal cells can provide the developmental cues necessary to traverse the major checkpoints defined by rearrangement of HC and LC genes. This human BM stromal cell culture does not support a dramatic numerical expansion of any specific compartment of B-lineage cells, probably attributable to the exclusion of fetal bovine serum and exogenous cytokines. It is also conceivable that the BM stromal cells in this culture (which are exclusively adventitial reticular/fibroblastlike cells by third passage) do not represent the totality of BM stromal cell components essential for optimal proliferation in vivo.
At the time we were developing our human BM stromal cell culture, Rawlings and his colleagues were developing an in vitro culture using the murine S17 stromal cell line.112 They originally showed that enriched CD34+ cord blood cells would develop into CD19+ B-lineage cells after 3 to 4 weeks.112The CD19+ B-lineage cells were at a very early stage of B-cell development since bulk culture analysis by Southern blotting indicated no rearrangements at the IgH locus.112 These CD19+ B-lineage cells could be expanded following transfer to fresh S17 stromal cells, but did not proliferate following stimulation with IL-7 or stem cell factor (SCF). A follow-up report showed that inclusion of Flt3 ligand enhanced the development of CD19+ B-lineage cells by twofold to threefold.113 We have recently shown that inclusion of Flt3 ligand at the initiation of our human BM stromal cell culture also enhances the development of CD19+ B-lineage cells (J.A.R. Pribyl and T.W. LeBien, unpublished observations, February 1999), providing additional support for a role of Flt3 ligand in human B-cell development (Figure3).
Given the differences in the tempo and degree of B-cell development in the 2 models, we conducted a side-by-side comparison of murine S17 stromal cells and human fetal BM stromal cells.114 When human fetal BM CD34+ HSCs were cultured on human fetal BM stromal cells or human skin fibroblasts, robust differentiation to the immature B-cell stage occurred within 3 weeks.114 In contrast, CD19+ B-lineage cells emerging on S17 stromal cells within the same time frame had twofold to fourfold higher levels of cell-surface CD19, but no cells expressing the BCR.114Human and murine S17 stromal cells therefore differ in their capacity to support human B-cell differentiation under the conditions in which we compared them. The identity of the soluble or membrane-bound stromal cell molecules important in both cultures is unknown. When CD34+ cord blood HSCs are cultured on S17 stromal cells for 6 to 8 weeks, small numbers of cytoplasmic and surface μHC+ cells can be detected.115 Furthermore, transferring these 6- to 8-week cultures onto CD40 ligand (CD154+) fibroblasts supplemented with IL-4 and IL-10 results in terminal human B-cell differentiation to Ig-secreting cells.115 Murine stromal cell lines other than S17 also support the development of CD19+ human B-lineage cells from CD34+ cord blood HSCs.116-119 Two of these studies showed that a combination of SCF and granulocyte colony–stimulating factor would enhance the outgrowth of B-cell precursors.117 118
The nonobese diabetic-severe combined immunodeficient (NOD-SCID) mouse120 has become a popular tool for studying engraftment and development of human HSCs in vivo (for review, see Greiner et al121). The CD34++/CD38− HSC that engrafts in NOD-SCID mice has been designated the SCID-repopulating cell.122 Development of CD19+B-lineage cells from human CD34+ BM or cord blood HSCs transplanted into NOD-SCID mice has been reported by several groups.122-126 The degree of human B-cell differentiation was variable in these studies, although spleen and peripheral blood B cells expressing surface μHC and κ or λLC were detected in 2 studies.124 125 These results indicate that xenogeneic factors produced in NOD-SCID mice can promote and support multiple stages of human B-cell development. The murine BM appears to be the primary site of engraftment by human CD34+ HSCs. It follows that commitment into the human B-lineage and traversal through the pre-BCR and BCR checkpoints is likely to occur in murine BM, although this has not been directly demonstrated.
How can these in vitro and in vivo models of human B-cell development be further refined? None of the in vitro BM stromal cell cultures described thus far fully recapitulate the complex microenvironment in which B cells develop. For example, the adventitial reticular (fibroblastlike) adherent cell in the human BM stromal cell culture is only 1 component of the BM microenvironment. No one has examined the capacity of other BM microenvironmental cells (eg, osteoblasts, BM microvascular endothelial cells, macrophages) to support or modulate B-cell development. One technical problem is the difficulty in purifying and establishing long-term cultures of human BM stromal cell components. SV40 large t antigen127 or human papilloma virus E6/E7 genes128 are capable of immortalizing human BM stromal cells. These stromal cell “lines” have been used to study hematopoiesis (eg, Li et al129), but no reports have described their capacity to support human B-cell development. Another possible strategy for long-term maintenance of BM stromal cells would be overexpression of the catalytic subunit of telomerase, which has been shown to exceed the life span of human fibroblasts by up to 20 doublings.130Development of stable long-term BM stromal cell cultures would facilitate the isolation of potentially novel genes that encode survival/growth factors regulating human B-cell development using, for example, the cloning/screening strategy of Oritani and Kincade.90 A more detailed analysis of the NOD-SCID mouse might focus on whether fetal liver or BM stromal cells are comparable to human BM stromal cells in supporting human B-cell development. It is conceivable that a highly conserved murine cytokine is as effective as its human homologue, in which case murine stromal cell complementary DNA (cDNA) libraries could be screened for binding to human B-lineage cells.90
B-lineage immunodeficiencies
Dramatic progress has recently been made in identifying the genetic defects in many congenital human immunodeficiency diseases.131 These diseases are largely classified on the basis of which cellular component or function of the immune response is defective.132 By the grace of good hindsight, it is not surprising that immunodeficiency diseases that primarily affect B-cell development or B-cell function involve genes encoding protein components of the pre-BCR, BCR, or signaling pathways activated following cross-linking these receptors.133 The degree to which B-cell development or function is altered in these patients shows many similarities and some differences compared with the phenotype observed in gene-targeted mice.
X-linked agammaglobulinemia (XLA)
XLA is the prototype immunodeficiency disease that specifically affects the B-lineage.131-133 The Bruton's tyrosine kinase (BTK) gene encodes a cytosolic 659–amino acid protein that is mutated in the vast majority of boys diagnosed with XLA.134,135BTK mutations are found in 80% to 90% of patients following a presumptive diagnosis of XLA based on early-onset hypogammaglobulinemia and few or no detectable peripheral blood B cells.136 More than 300 mutations have been identified in the BTK gene,137 and mutations have been mapped to all 6 of the functional domains.138 The majority of XLA patients have profound hypogammaglobulinemia afflicting all immunoglobulin classes and fewer than 1% of normal numbers of peripheral blood B cells. A single study of 8 patients indicated that maturation arrest occurred at the pro-B/pre-B interface, ie, between CD19+/TdT+/cytoplasmic μHC−and CD19+/TdT−/cytoplasmic μHC+ populations.139
The xid mouse, a murine model of X-linked immunodeficiency disease, harbors a mutation in the Btk gene.140 Mice with targeted disruptions of Btk have a defect in B-cell development that is identical to the xid mouse.141-143 As discussed in detail elsewhere,131,133,140-143 xid and Btkgene–targeted mice have a much milder form of B-cell immunodeficiency than XLA patients, characterized by reduced levels of only 2 immunoglobulin subclasses (IgM and IgG3) and B cell numbers reduced by only 30% to 50%. Comparison of B-cell developmental defects in mice and humans led many investigators to conclude that loss of BTK function has more severe consequences in humans. This could be explained by compensatory/redundant kinases operating in murine B-cell development or by a difference in the role of BTK in murine and human pre-BCR and/or BCR signaling pathways. A more intriguing possibility is the potential contribution of modifying genetic factors/modifier alleles (ie, their gene products) in facilitating the traversal of pro-B to pre-B cells in XLA patients.133 138 This may explain the variability in immunologic symptoms present in family members with identical genetic backgrounds. Despite considerable effort, no correlation has been determined between XLA genotype and the severity of clinical symptoms in XLA patients. Whether human XLA has a more severe defect in B-cell development than the murine models is still a matter of some controversy, but the unique function of BTK in B-cell development in both species is undeniable.
The role of BTK in signal transduction pathways has been extensively studied, and Rawlings has recently reviewed this subject.138 BTK is expressed throughout the B-lineage, but expression decreases in terminally differentiated plasma cells.144,145 BTK activation following BCR cross-linking has been studied in detail.138 Briefly, BCR cross-linking activates PI-3 kinase, which generates limiting amounts of membrane-associated PI-3,4,5-trisphosphate. The latter recruits cytosolic BTK to the membrane by interacting with the BTK SH3 domain.146 BTK activation then proceeds through 2 steps: transphosphorylation of Y551 within the BTK kinase domain (most likely by the src family kinase Lyn), followed by autophosphorylation of Y223 in the BTK SH3 domain.147Membrane-associated BTK then binds to an unidentified tyrosine-phosphorylated ligand,138 which facilitates co-localization of BTK with phospholipase Cγ (PLC-γ), activation of PLC-γ, and culmination in a sustained calcium signal involving extracellular calcium influx.148 Very recent studies suggest that the “unidentified tyrosine-phosphorylated ligand” could be the B-cell linker protein (BLNK).149 The outcome of this complex pathway leading to sustained calcium signaling is enhanced proliferation and changes in transcription. I would emphasize that this model of BTK function has been developed with the use of BCR cross-linking as an activation stimulus. Evidence that BTK functions through the same pathway following pre-BCR activation is lacking. It is possible that once the pre-BCR becomes activated (eg, through ligand-independent tonic signals as discussed above), BTK occupies a critical point in the pre-BCR signaling pathway whose function is nonredundant. Given the complexity of BTK protein domain organization, it is remarkable that so many distinct BTKmutations culminate in a relatively similar block in B-cell development.
Non–X-linked agammaglobulinemia
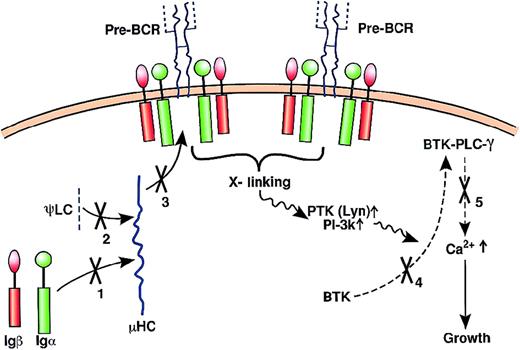
As discussed above, 10% to 20% of B-lineage immunodeficiency patients do not harbor BTK mutations. Mary Ellen Conley and her colleagues have systematically screened BM DNA samples from patients lacking BTK mutations in an effort to identify other mutated genes that could underlie these immunodeficiency diseases. By this approach, they have identified patients with mutations in the μHC,150 the λ5/14.1 component of the ψLC,33 and Igα.151 Seven patients from 3 families harbored mutations that disrupted the μHC.150These included 75-kb to 100-kb homozygous deletions of the D and J regions plus the μ constant region, and a homozygous base-pair substitution that removed an alternate splice site used to generate the membrane form of the μHC. Analysis of peripheral blood from 4 of 7 patients revealed no detectable B cells, and analysis of the BM from 1 of the 4 evaluable patients indicated maturation arrest at the pro-B to pre-B interface.150 In a second report, a 5-year-old boy with severe hypogammaglobulinemia was found to have fewer than 1% of the normal number of peripheral blood B cells.33 A detailed analysis of the mutated λ5 alleles and their encoded proteins suggested that the mutated λ5 protein underwent improper folding and was subsequently degraded.33 Analysis of this patient's BM suggested a block at the pro-B to pre-B transition. In the most recent report, a 2-year-old girl with agammaglobulinemia was found to have a deletion of exon 3 in the gene encoding Igα.151 This exon encodes the transmembrane domain of Igα leading to the prediction that the Igα transcript made in this patient would encode a truncated protein incapable of assembling with the pre-BCR. Analysis of this patient further revealed the complete absence of peripheral blood B cells and a block at the pro-B to pre-B transition. A non-XLA patient with a block at the pro-B cell stage and a decrease in Igα, Igβ, and VH-Cu transcripts may represent yet another distinct mutation in genes essential for B-cell development.152 It is remarkable that mutations in BTK and genes encoding components of the pre-BCR can lead to a relatively similar block in B-cell development at the pro-B to pre-B transition. Figure4 suggests why this may occur. Expression of the pre-BCR requires assembly of the μHC, ψLC, and Igα/Igβ subunits. Any mutation that leads to an absence of one subunit will block full assembly of the pre-BCR. The physical absence of an intact pre-BCR will result in a failure of the pre-BI compartment (Figure 2) to expand. Independently of how the pre-BCR signals, the presence of the Igα/Igβ heterodimer predicts that pre-BCR and BCR signaling pathways will be highly conserved.31 138 Thus, in XLA patients, pre-BCR cross-linking would be normal at least to the point where BTK is translocated to the membrane, but BTK-dependent events leading to a sustained increase in Ca++ flux would be greatly decreased or absent (Figure 4).
Components of the pre-BCR and pre-BCR signaling pathways disrupted in B-lineage immunodeficiencies.
The left side shows the assembly of the structural components of the pre-BCR: μHC, ψLC, and the Igα/Igβ heterodimer. For simplicity, only the formation of a Fab is shown. As discussed in the text, the mechanism of pre-BCR cross-linking is unknown. By whatever mechanism, pre-BCR cross-linking activates protein tyrosine kinases (PTKs) such as Lyn, followed by a complex series of events (see Benschop et al31 and Rawlings138 for detailed reviews) culminating in initial activation of PLC-γ. Concomitant activation of PI-3 kinase leads to production of PI (3,4,5) P3, which recruits BTK to the membrane where it is phosphorylated by Lyn. BTK then phosphorylates PLC-γ, leading to sustained Ca++ flux and enhancement of growth. The assembly (solid arrows) or signaling pathways (dashed arrows) disrupted in antibody-deficiency diseases are shown by an X and a number (1 indicates mutation in Igα that would impair pre-BCR assembly; 2 indicates mutation in ψLC that would impair pre-BCR assembly; 3 indicates mutation in μHC that would impair pre-BCR assembly; 4 indicates BTK mutation in pleckstrin homology domain that would compromise binding to PI (3,4,5) P3; and 5 indicates BTKmutation in catalytic site that would compromise tyrosine phosphorylation of PCL-γ).
Components of the pre-BCR and pre-BCR signaling pathways disrupted in B-lineage immunodeficiencies.
The left side shows the assembly of the structural components of the pre-BCR: μHC, ψLC, and the Igα/Igβ heterodimer. For simplicity, only the formation of a Fab is shown. As discussed in the text, the mechanism of pre-BCR cross-linking is unknown. By whatever mechanism, pre-BCR cross-linking activates protein tyrosine kinases (PTKs) such as Lyn, followed by a complex series of events (see Benschop et al31 and Rawlings138 for detailed reviews) culminating in initial activation of PLC-γ. Concomitant activation of PI-3 kinase leads to production of PI (3,4,5) P3, which recruits BTK to the membrane where it is phosphorylated by Lyn. BTK then phosphorylates PLC-γ, leading to sustained Ca++ flux and enhancement of growth. The assembly (solid arrows) or signaling pathways (dashed arrows) disrupted in antibody-deficiency diseases are shown by an X and a number (1 indicates mutation in Igα that would impair pre-BCR assembly; 2 indicates mutation in ψLC that would impair pre-BCR assembly; 3 indicates mutation in μHC that would impair pre-BCR assembly; 4 indicates BTK mutation in pleckstrin homology domain that would compromise binding to PI (3,4,5) P3; and 5 indicates BTKmutation in catalytic site that would compromise tyrosine phosphorylation of PCL-γ).
B-lineage ALL
In approximately 75% of pediatric patients with newly diagnosed ALL, the disease is classified as B-lineage in origin on the basis of immunoglobulin gene rearrangements and expression of cell surface markers.153-155 The karyotypic and molecular genetic abnormalities in B-lineage ALL have been extensively characterized,153-155 and chromosomal translocations giving rise to distinct fusion genes including TEL-AML1, MLL-AF4 (or MLL rearrangements with other genes), and E2A-PBXare present in more than 30% of newly diagnosed pediatric B-lineage ALL.153 However, the totality of molecular genetic abnormalities in B-lineage ALL is much greater than these landmark translocations. Despite this impressive progress, there is still a deficiency in our understanding of how these many genetic abnormalities ultimately subvert normal B-cell precursor developmental programs. Related questions are how these genetic abnormalities tip the survival scale to apoptotic resistance and whether external cues (ie, cytokines) play any role in regulating the survival/growth of B-lineage ALL in vivo.
The universal common denominator of pediatric B-lineage ALL is a BM origin of the disease. However, as discussed by Greaves,156infant and pediatric ALLs are biologically and clinically distinct diseases. For purposes of this discussion, we will consider both of them as “B-lineage,” even though infant ALL with MLL-AF4 translocations have characteristics of bi-phenotypic B-lineage/myeloid cells.156 Manifestation of molecular genetic abnormalities shifts the B-cell precursor developmental program from (1) a process governed by functional immunoglobulin gene rearrangements and appropriate homeostatic response to positive and negative growth regulators to (2) a transformed clone more resistant to apoptosis and (generally) incapable of undergoing differentiation. The apoptotic death of normal B-cell precursors probably resembles death by neglect,157 ie, an apoptotic fate that follows decreased availability or absence of a continuous survival signal. Neglect might reflect primarily the fate of a cell that cannot express the pre-BCR (eg, a cell with 2 nonfunctional μHC rearrangements) and hence does not receive a tonic (survival) signal that follows pre-BCR expression. Normal murine and human B-cell precursors express antiapoptotic bcl-2 family members such as bcl-2 and bcl-x,27,158-160 but are nonetheless very sensitive to apoptotic stimuli.158,159Many laboratories have studied B-lineage ALL for expression of bcl-2 family members in an attempt to determine whether expression can be correlated with the clinical or biological characteristics of the disease.161-166 The results of these studies are quite variable, and no simple conclusion can be drawn regarding bcl-2 family member expression and clinical outcome. Subcellular distribution (particularly in mitochondrial membranes) and homodimerization/heterodimerization characteristics of bcl-2 family members are crucial in determining apoptotic sensitivity in many eukaryotic cells.167-169 It is therefore interesting that a recent study suggested that mitochondrial levels of bcl-2 may portend the sensitivity of leukemic cells to apoptosis.164
A fascinating relationship between a molecular genetic abnormality and apoptotic resistance in B-lineage ALL is the E2A-HLF translocation. TheE2A-HLF fusion gene occurs as a consequence of the t(17;19)(q23;p13) in some cases of pro-B ALL.170,171 The E2A-HLF fusion protein contains the transactivation domains of the transcription factor E2A tethered to the basic leucine zipper DNA–binding domain of the transcription factor HLF. Inaba and colleagues demonstrated that a dominant-negative form of E2A-HLF induced apoptosis in a human pro-B ALL cell line harboring the E2A-HLF translocation, and transfection of a murine IL-3–dependent pro-B cell line with E2A-HLF reversed apoptosis that normally occurred following IL-3 withdrawal.172 These data strongly suggest that E2A-HLF functions by blocking an early step in an apoptotic pathway.172 Reasoning that IL-3 mediates cell survival by activation of 1 or more transcription factors whose activity can be substituted by E2A-HLF, the same group went on to show that nuclear factor regulated by IL-3 (NFIL3) is a target gene of E2A-HLF.173 Enforced expression of NFIL3 promoted the IL-3–independent survival of pro-B cells.173 Two recent studies used representational difference analysis to identify additional genes regulated by E2A-HLF.174,175 One study identified Annexin VIII and a novel cDNA designated SRPUL, but neither protein prevented apoptosis in murine pro-B cells deprived of IL-3.174 In the second study, E2A-HLF was shown to up-regulate a zinc-finger transcription factor designated SLUG.175 Importantly, SLUG was nearly as effective as bcl-2 or bcl-x in preventing apoptosis in IL-3–deprived pro-B cells.175 Murine models of E2A-HLF mediated oncogenesis have been developed.176,177 In both studies, E2A-HLF transgenic mice exhibited thymic hypoplasia and subsequent development of thymic lymphomas. Although a block in splenic B-cell maturation was noted in one of the studies,177 leukemias involving B-lineage progenitors were rare.
A second fusion gene containing E2A that is grudgingly giving up its function is E2A-PBX1. The E2A-PBX1 fusion gene was originally identified in pre-B ALL blasts harboring the t(1;19)(q23;p13) cytogenetic abnormality by two groups.178,179 The E2A-PBX1 fusion protein contains the N-terminal domain of E2A fused to the homeodomain of PBX1. This genetic abnormality is specific for pre-B ALL expressing cytoplasmic μHC and is present in approximately 25% of newly diagnosed pre-B ALL (reviewed in Hunger180).Transgenic mice expressing the E2A-PBX1fusion gene develop thymic lymphomas and myeloid leukemias, but not B-lineage malignancies.181,182 Surprisingly, BM B-lineage cells are reduced to 20% of normal values in E2A-PBX1 transgenic mice, suggesting that the fusion protein increases the sensitivity of these cells to apoptosis.181 Representational difference analysis was used to isolate a novel WNT gene, designated WNT-16, as an activating target of E2A-PBX1.184 WNT-16 is a member of the vertebrate WNT family, which includes more than 20 genes encoding cysteine-rich secreted proteins that mediate cell-cell interactions.183 WNT-16 mRNA is expressed in E2A-PBX1+ pre-B ALL but not a variety of E2A-PBX1− B-lineage malignancies.184Furthermore, Frizzled genes that encode receptors for WNT family members are expressed in B-lineage ALL, including those expressing E2A-PBX1. These data implicate WNT-16 as one component in a survival/growth pathway that is operative in pre-B ALL harboring theE2A-PBX1 fusion gene.184
The earliest stages of clonal expansion in B-lineage ALL (ie, the subclinical phase of the disease wherein the progeny of a single clone begin to expand) may be characterized by a dependency on BM stromal cells for survival and growth. This would be a stage in the natural history of the disease in which the BM microenvironment is completely intact and lymphohematopoiesis is unperturbed. How long this BM stromal cell dependency might be retained is unknown. Acquisition of sequential genetic changes may portend the emergence of a dominant subclone with a decreased, or complete absence of, a requirement for BM stromal cell–derived survival/growth factors. By the time a patient is diagnosed with B-lineage ALL and the marrow is filled with leukemic blasts, a physical displacement of normal lymphohematopoiesis and BM architecture will have occurred. Indeed, physical disruption/displacement of the BM stromal cell microenvironment is more frequently seen in ALL than in AML or CML.185 It is likely that a dominant B-lineage ALL subclone would be BM stromal cell–independent at this stage.
In addition to the subversion of apoptotic programs by genetic changes in B-lineage ALL, are there external cues that could regulate survival/growth? Several laboratories have examined the effect of recombinant cytokines on the growth of B-lineage ALL using short-term in vitro assays. Sporadic responsiveness to IL-3, IL-7, and flt3-ligand was observed.186-195 However, no single cytokine has been demonstrated to exert a consistent proliferative effect on a significant percentage of cases. Furthermore, the response to these cytokines (ie, the degree of proliferation) is generally weak. A potentially more rational approach to identifying growth factors is to assume that BM stromal cells produce the collective array of survival/growth factors essential for clonal expansion of B-lineage ALL. This assumes that adhesive interactions exist to bring the leukemic clone into apposition with BM stromal cell surfaces and the surrounding extracellular matrix. Similarly to their normal counterparts,107-109 B-lineage ALL cells generally adhere to BM stromal cells through VLA-4/VCAM-1 interactions.109,196-198 Using a fluorescent bead adhesion assay that facilitated flow cytometric analysis of integrin expression/function, Geijtenbeek and colleagues reported that leukemic cells from 17 of 20 B-lineage ALL BM specimens exhibited defects in expression or activation of LFA-1 and VLA-4.199 The biological significance of their results is uncertain. On the one hand, weaker or reduced adhesion of leukemic cells to BM stromal cells could lead to more rapid egress into the peripheral blood. On the other hand, interaction of leukemic cells with BM stromal cells generally inhibits apoptosis (see below), indicating that adherence could play an important role in the survival/growth of B-lineage ALL.
Adherence of B-lineage ALL cells to BM stromal cells could be followed by a more complex, energy-dependent interaction, characterized by migration of the leukemic cells underneath BM stromal cells. Interestingly, migration (at least in vitro) is VCAM-1–independent.196,198 The biological significance of in vitro migration is unclear, but may reflect a chemotactic response by the leukemic cells to BM stromal cells. The CXCR4 chemokine receptor and its SDF-1 ligand may be involved in leukemic cell migration since at least some B-lineage ALLs undergo chemotaxis in response to SDF-1.102,103 SDF-1 may also promote the survival of B-lineage ALL.200
Campana's laboratory has extensively examined the capacity of nontransformed human BM stromal cells to inhibit the apoptotic fate of freshly isolated B-lineage ALL. They initially showed that allogeneic BM stromal cells support survival or inhibit apoptosis of the majority of B-lineage ALLs tested, although survival of a minority of B-lineage ALL was unaffected.201 They went on to demonstrate that direct contact with BM stromal cells was necessary for optimal survival of normal B-cell precursors and some (but not all) B-lineage ALLs.202 Heterogeneity in BM stromal cell contact requirements for B-lineage ALL survival/growth was also reported by other investigators.203,204 The survival of B-lineage ALL on allogeneic BM stromal cells also correlates with prognosis. The probability of 4-year event-free survival was greater among patients whose leukemic cells exhibited reduced survival on BM stromal cells, compared with patients whose leukemic cells exhibited elevated survival on BM stromal cells.205 A very recent report from Campana's group provided strong evidence that hyperdiploidy (51 to 65 chromosomes) in B-lineage ALL showed a significant correlation with reduced capacity of the leukemic cells to survive on BM stromal cells.206 This is a strong endorsement for the utility of this biological assay in predicting clinical outcome and likely reflecting the in vivo apoptotic sensitivity of this subcategory of B-lineage ALL.
Many B-lineage ALL cell lines have been established, but the vast majority (if not all) require only supplementation of tissue culture medium with fetal bovine serum for optimal growth.207 My own laboratory has established a panel of human B-lineage ALL cell lines that retain a dependency on human BM stromal cells for long-term survival and growth. Using the same human BM stromal cell culture employed for studies of normal B-cell precursors,26,81 we established a cell line designated BLIN-2 (B-lineage 2).208BLIN-2 cells express the pre-BCR, have a dic(9;20) chromosomal abnormality and a bi-allelic deletion of the p16INK4a and p14ARF genes. BLIN-2 has an absolute dependence on human BM stromal cells for survival and growth, and direct contact is necessary for optimal growth. Removal of BLIN-2 from BM stromal cells results in membrane blebbing and apoptotic body formation in 72 hours. Using a variety of assays to characterize apoptotic fate, we have recently shown that BLIN-2 cell death has caspase-dependent and caspase-independent features.209 Although the identity of the BM stromal cell molecules that are essential for growth of BLIN-2 are unknown, heparan sulfate proteoglycans may play at least a partial role.210We have also produced 2 additional cell lines, designated BLIN-3 and BLIN-4, that have overlapping but unique growth factor requirements compared with BLIN-2.210 BLIN-3 requires human BM stromal cells supplemented with exogenous IL-7 for optimal growth, survives but does not proliferate in the presence of BM stromal cells alone, and undergoes apoptosis in the absence of BM stromal cells.210BLIN-4 grows on BM stromal cells and undergoes apoptosis in their absence. However, growth of BLIN-4 can be supported by a cooperative stimulus of exogenous IL-7 plus flt3-ligand in the absence of BM stromal cells.210 The BLIN cell lines represent a composite of growth-factor requirements that may mirror the physiologic dependency of normal and leukemic B-cell precursors on the BM microenvironment.
Conclusion
The general blueprint for mammalian B-cell development has been determined, and the investigative fine-tuning has begun. A number of questions regarding human B-cell development remain unanswered. For example, how does a B-lineage cell develop from a multilineage progenitor (eg, a CLP in Figure 1), and how is B-lineage commitment defined in molecular terms? Transcription factors are obviously the key. One of the great accomplishments in hematology during the nineties was the isolation and characterization of transcription factors that regulate the development of murine lymphohematopoietic lineages (for recent reviews, see Glimcher et al211 and Engel et al212). A stunning recent discovery directly implicated the paired box transcription factor PAX5 in murine B-lineage commitment.213,214 The major message from these 2 studies is that PAX5-deficient murine pro-B cells (ie, B-lineage cells that have undergone DJH but not VDJH rearrangements) harbor the capacity to differentiate into a constellation of other lineages—including macrophages, osteoclasts, dendritic cells, granulocytes, NK cells, and thymocytes.213,214 This surprising result was used to propose that PAX5 plays an essential role in fostering B-lineage commitment by suppressing the expression of genes that (directly or indirectly) promote development of non-B lineage cells. It is reasonable to assume that human B-lineage commitment and development are governed by similar transcription factors, but is there experimental evidence? Answers may be forthcoming. Jaleco and colleagues have very recently described a strategy that represents the first success in elucidating the role of transcription factors in human B-cell development. They constructed a retroviral vector encoding green fluorescent protein (GFP) and the dominant negative helix loop helix protein Id3.215 Human fetal liver HSCs were then infected with this vector and plated on murine or human stromal cells, and GFP expression was used to trace the effect of overexpression of Id3 on B-cell development. The results indicated that Id3 overexpression blocked B-cell development at a stage prior to expression of the IL-7 receptor.
Another issue that requires resolution is the identity of the molecule (or molecules) produced in the BM microenvironment that are essential for the survival/proliferation of human B-cell precursors. I propose that stromal cell–derived molecules potentially bound to HSPGs (Figure3) are reasonable candidates. Time will tell if these molecules turn out to be previously cloned cytokines/chemokines. These putative cytokines/chemokines could also play an important role in the survival/proliferation of at least some B-lineage ALL. The intracellular signaling pathways that affect survival/proliferation in normal and leukemic B-lineage cells are not completely understood. Very recent reports reveal a critical role for the linker protein BLNK in human216 and murine217 218 B-cell development, although the absence of BLNK function may result in a more severe phenotype in humans than mice. Thus, additional efforts will lead to the discovery of new components, or novel functions for known components, in signaling pathways essential for the proliferation and differentiation of B-cell precursors. The identity of the essential survival/proliferation factor is linked to a related question: what is the mechanism of cell death that ensues in a B-cell precursor that does not receive a survival/proliferation signal (eg, in a pre-BI cell that fails to express the pre-BCR)? Which caspase pathways are involved? Are these pathways subverted in B-lineage ALL and accentuated in XLA?
Finally, “genome prospecting,”219 using DNA microarray technology with all its analytical power and bio-informatic challenges, has burst onto the scene. Golub and colleagues used DNA microarrays to evaluate gene expression in human acute leukemias, included B-lineage ALL.220 Their results indicate that microarray-based quantitation of gene expression (1) confirms well-known leukemia classifications, (2) provides a new tool for diagnosis, and (3) generates a staggering amount of new information of unknown significance (ie, quantitative expression of approximately 6800 human genes). Once this technology is applied to normal B-cell precursors, we will witness the beginning of a complete fingerprint of comparative gene expression. This database will provide an investigative substrate for the next millenium, taking us deeper into regulation of cell fate/function in normal and abnormal human B-cell development.
Acknowledgments
Mary Ellen Conley (St. Jude Children's Research Hospital) and Les Silberstein (Harvard Medical School) kindly provided preprints of their work. I thank Ted Bertrand for helpful comments on the manuscript and Sandi Sherman for word-processing support.
Supported by grants R01 CA31685 and R01 CA76055 from the National Cancer Institute, National Institutes of Health, Bethesda, MD.
Reprints:Tucker W. LeBien, University of Minnesota Cancer Center, 420 Delaware St SE, Box 806 Mayo, University of Minnesota, Minneapolis, MN 55455; e-mail: lebie001@tc.umn.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal