Abstract
Thalidomide (Thal) is a drug with antiangiogenic, anti-inflammatory, and immunomodulatory properties that was found to inhibit the production of tumor necrosis factor-α (TNF-α) in vitro. We studied single nucleotide polymorphisms at positions −308 and −238 of the TNF-α gene promoter and measured the corresponding TNF-α cytokine levels in 81 patients (pts) with refractory and relapsed multiple myeloma (MM) who were treated with Thal. In myeloma pts carrying the TNF-238A allele (n = 8), we found a correlation with higher pretreatment TNF-α levels in peripheral blood (P = .047). After Thal administration, this TNF-238A group had a prolonged 12-month progression-free and overall survival of 86% and 100% versus 44% and 84% (P = .003 andP = .07) in pts with the TNF-238G allele, respectively. These findings suggest that regulatory polymorphisms of the TNF-α gene can affect TNF-α production and predict the outcome after Thal therapy, particularly in those MM pts who are genetically defined as “high producers” of TNF-α.
Introduction
Thalidomide (Thal) is able to block the production of tumor necrosis factor-α (TNF-α) by human monocytes in a dose-dependent manner.1 It has been speculated that the suppression of TNF-α expression is the major mechanism of action of Thal in a variety of diseases.2,3 In multiple myeloma (MM), TNF-α is involved in the generation of malignant plasma cells, as monoclonal plasma cells were produced when mononuclear cells from myeloma patients (pts) were exposed to TNF-α and interleukin-4 in vitro.4 In addition, TNF-α has stimulatory effects on plasma cell growth by triggering the secretion of interleukin-6 and shows proangiogenic properties in vitro.5 6 However, thus far it is not clear whether the anti–TNF-α effect of Thal contributes to its efficacy in MM.
TNF-α expression is regulated at the transcriptional level, and polymorphisms of the TNF-α gene promoter are involved in genetic variability of TNF-α production.7-9 Single nucleotide polymorphisms have been identified at positions −308 and −238 in the human TNF-α gene promoter,10,11 with the rarer allele containing adenine instead of guanine at each polymorphic site. These polymorphisms are associated with diseases in which TNF-α is pathogenetically involved.12-14
The aim of this study was to investigate the role of polymorphisms of the TNF-α gene promoter at positions −308 and −238 on TNF-α production in MM. In addition, we studied the effect of TNF-α promoter polymorphisms on the outcome after Thal therapy in 81 pts with refractory and relapsed MM.
Study design
Treatment design
From December 1998 to March 2001, 81 pts with refractory and relapsed MM were enrolled in a clinical phase II trial and treated with Thal. The study has been approved by the ethical guidelines of the Joint Committee of Clinical Investigation of the University of Heidelberg. All pts had to sign an informed consent according to the Declaration of Helsinki. Primary end points of the study were progression-free survival (PFS) and overall survival (OS). The design of the study, the pretreatment and monthly follow-up evaluations, as well as the assessment of response have been described previously.15 The laboratory assays for TNF-α were performed by quantitative sandwich enzyme immunoassay (ELISA) (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. All measurements were done in duplicate.
Analysis of TNF-α polymorphisms
The single nucleotide polymorphisms at positions −308 and −238 of the TNF-α gene promoter were studied using a sequence-specific polymerase chain reaction assay.16 For typing of the relevant polymorphic variants of these cytokine genes, we designed 4 primer pairs (Applied Biosystems, Foster City, CA), which detected all 4 possible haplotypes. In the TNF-α reagents we used a nonallelic control primer pair, which amplified a 440 bp fragment of the C-reactive protein gene.
Statistical analysis
The Mann-Whitney was used to compare plasma cytokine levels in the 2 independent groups defined by genetic polymorphisms in the TNF-α locus. Survival probabilities were estimated by the method described by Kaplan and Meier. The statistical analyses were performed using the software packages StatXact (Cytel Software Corp, Cambridge, MA) and S-Plus (Insightful, Seattle, WA).
Results and discussion
To investigate the role of TNF-α polymorphisms at positions −308 and −238 of the gene promoter on TNF-α production and the outcome after Thal therapy, we analyzed 81 MM pts with relapsed and refractory MM. In particular, there were 60 males and 21 females with a median age of 59 years (range 34-86). According to the classification of Salmon and Durie,17 6 pts had stage II and 75 pts stage III MM. All 81 pts received chemotherapy prior to Thal, including a median of 7 (range 3-30) chemotherapy cycles and at least 1 cycle of high-dose chemotherapy with peripheral blood stem cell transplantation in 60 pts.
To determine whether pts with refractory and relapsed MM had increased levels of TNF-α in peripheral blood (PB; n = 81) or bone marrow (BM; n = 57), we determined cytokine levels in plasma and compared the data with a group of healthy volunteers (n = 22), including 13 males and 9 females with a median age of 56 years (range 21-69). In comparison with the control group, MM pts had 1.9- and 2.6-fold elevated median TNF-α levels in PB (5.36 pg/mL vs 2.83 pg/mL;P < .0001) and BM (6.94 pg/mL vs 2.63 pg/mL;P < .000 01). In line with this finding, Lichtenstein and coworkers demonstrated that freshly obtained BM cells from MM pts produced a significantly greater amount of TNF-α than BM cells from control individuals, suggesting a pathophysiological role of TNF-α production in MM.18
The incidence of TNF-α polymorphisms at positions −308 and −238 of the TNF-α gene promoter was determined in all 81 Thal-treated pts as well as in a larger group of 255 MM pts, including 166 males and 89 females with a median age of 56 years (range 28-84). In addition, 200 healthy, local blood donors were analyzed as controls (Table1). We found a significant excess of the TNF-α “high-producer” genotype at position −308 in MM pts. Consistently, Davies et al found double heterozygotes of TNF-α at position −308 and lymphotoxin-α at position +252 twice as often in myeloma pts.19 These findings suggest an association of TNF-α “high-producer” genotypes with plasma cell disorders.
Allele frequencies and genotype/haplotype distributions at positions −308 and −238 of the TNF-α gene promoter in 255 multiple myeloma (MM) patients (pts), including 81 Thal-treated pts with relapsed and refractory MM; in addition, 200 healthy, local blood donors were analyzed as controls
| Characteristic . | All MM pts . | Controls . | P* . | ||
|---|---|---|---|---|---|
| No. . | (%) . | No. . | (%) . | ||
| Codon −308 | |||||
| Allele | |||||
| G | 425 | (83.3) | 341 | (85.3) | .49 |
| A | 85 | (16.7) | 59 | (14.7) | |
| Genotypes | |||||
| GG | 184 | (72.1) | 142 | (71.0) | .006 |
| GA | 57 | (22.3) | 57 | (28.5) | |
| AA | 14 | (5.5) | 1 | (0.5) | |
| Codon −238 | |||||
| Allele | |||||
| G | 491 | (96.3) | 377 | (94.3) | .20 |
| A | 19 | (3.7) | 23 | (5.7) | |
| Genotypes | |||||
| GG | 236 | (92.5) | 177 | (88.5) | .15 |
| GA | 19 | (7.5) | 23 | (11.5) | |
| AA | 0 | (0.0) | 0 | (0.0) | |
| Codons −308 and −238 (1. allele/2. allele) | |||||
| Haplotypes | |||||
| GG/GG | 166 | (65.1) | 122 | (61.0) | .01 |
| AG/GG | 56 | (22.0) | 54 | (27.0) | |
| GA/GG | 18 | (7.1) | 20 | (10.0) | |
| AG/AG | 14 | (5.5) | 1 | (0.5) | |
| AG/GA | 1 | (0.4) | 3 | (1.5) | |
| Characteristic . | All MM pts . | Controls . | P* . | ||
|---|---|---|---|---|---|
| No. . | (%) . | No. . | (%) . | ||
| Codon −308 | |||||
| Allele | |||||
| G | 425 | (83.3) | 341 | (85.3) | .49 |
| A | 85 | (16.7) | 59 | (14.7) | |
| Genotypes | |||||
| GG | 184 | (72.1) | 142 | (71.0) | .006 |
| GA | 57 | (22.3) | 57 | (28.5) | |
| AA | 14 | (5.5) | 1 | (0.5) | |
| Codon −238 | |||||
| Allele | |||||
| G | 491 | (96.3) | 377 | (94.3) | .20 |
| A | 19 | (3.7) | 23 | (5.7) | |
| Genotypes | |||||
| GG | 236 | (92.5) | 177 | (88.5) | .15 |
| GA | 19 | (7.5) | 23 | (11.5) | |
| AA | 0 | (0.0) | 0 | (0.0) | |
| Codons −308 and −238 (1. allele/2. allele) | |||||
| Haplotypes | |||||
| GG/GG | 166 | (65.1) | 122 | (61.0) | .01 |
| AG/GG | 56 | (22.0) | 54 | (27.0) | |
| GA/GG | 18 | (7.1) | 20 | (10.0) | |
| AG/AG | 14 | (5.5) | 1 | (0.5) | |
| AG/GA | 1 | (0.4) | 3 | (1.5) | |
The exact χ2 test was used to assess differences in allele frequencies and genotype/haplotype distributions between 255 MM pts and 200 controls.
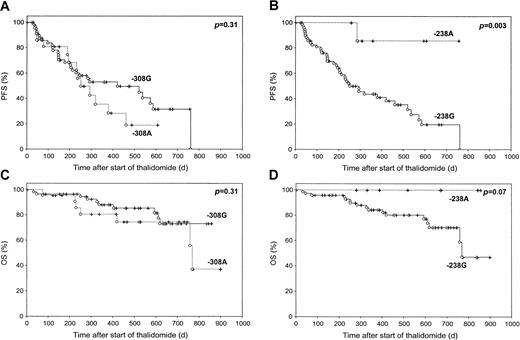
Prior to Thal therapy, we analyzed the correlation of TNF-α polymorphisms at positions −308 and −238 of the gene promoter on TNF-α production. Myeloma pts carrying the TNF-238A allele had statistically significant higher TNF-α levels in PB as compared with TNF-238G allele pts (9.7 pg/mL vs 5.2 pg/mL; P = .047). In myeloma pts carrying the TNF-238A allele (n = 8), we found a prolonged 12-month PFS and OS of 86% and 100% versus 44% and 84% (P = .003 and P = .07) in pts with the TNF-238G allele (n = 73), respectively (Figure1). Of note, TNF-238A allele pts showed a better response rate of 75%, as compared with 38% in TNF-238G allele pts (P = .05, χ2 test), as evidenced by a reduction of monoclonal protein of at least 25%. In addition, the TNF-238A allele group included the only pt who achieved a complete response to Thal therapy. Myeloma pts carrying the TNF-308A allele had neither statistically significant higher TNF-α levels in PB and BM nor a favorable outcome after the initiation of Thal therapy as compared with TNF-308G pts.
Progression-free survival (PFS) and overall survival (OS) of 81 patients with relapsed and refractory multiple myeloma, characterized by their TNF-α–238A/G and TNF-α–308A/G polymorphism status.
PFS was calculated from the start of thalidomide therapy to disease progression or death from any cause. At the start of thalidomide treatment, the drug was administered at a dose of 100 mg daily, following a weekly dose increment of 100 mg daily, for a final dose of 400 mg daily beginning at day 22.
Progression-free survival (PFS) and overall survival (OS) of 81 patients with relapsed and refractory multiple myeloma, characterized by their TNF-α–238A/G and TNF-α–308A/G polymorphism status.
PFS was calculated from the start of thalidomide therapy to disease progression or death from any cause. At the start of thalidomide treatment, the drug was administered at a dose of 100 mg daily, following a weekly dose increment of 100 mg daily, for a final dose of 400 mg daily beginning at day 22.
In comparison with the polymorphic site at position −308, the role of the −238 locus of the TNF-α promoter on TNF-α expression levels has not been examined yet. However, it has been speculated that the location of this polymorphism at the putative repressor site is leading to high levels of TNF-α expression.20 Our data demonstrate for the first time that a single G to A substitution at position −238 of the TNF-α gene promoter is associated with higher pretreatment levels of TNF-α and a more favorable PFS and OS, suggesting that regulatory polymorphisms of the TNF-α gene promoter can affect TNF-α production and predict the outcome after Thal therapy. To investigate whether TNF-α polymorphisms are independent predictors for response to Thal, larger studies are necessary. Because more effective anti–TNF-α drugs than Thal are available, future approaches should consider the testing of these new agents in MM, particularly in those pts with an increased TNF-α expression.
We thank Axel Benner (Central Unit Biostatistics, German Cancer Research Center, Heidelberg, Germany) for statistical analysis, Dr Kai Zwingenberger (Gruenenthal, Aachen, Germany) for kindly providing the study medication, and Renate Stahl for excellent technical assistance.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Anthony D. Ho, Department of Internal Medicine V, University of Heidelberg, Hospitalstr 3, 69115 Heidelberg, Germany; e-mail: anthony_dick.ho@urz.uni-heidelberg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal