Abstract
Fludarabine can exacerbate idiopathic thrombocytopenia (ITP) in chronic lymphocytic leukemia (CLL). We report 3 CLL patients with refractory fludarabine-associated ITP who responded to rituximab. The patients had Rai stages III, III, and IV disease. Before fludarabine treatment, the platelet counts were 141 000/μL, 118 000/μL, and 70 000/μL. ITP developed within week 1 of cycle 3 in 2 patients and within week 2 of cycle 1 in 1 patient. Platelet count nadirs were 4000/μL, 1000/μL, and 2000/μL, respectively, and did not respond to treatment with steroids or intravenous immunoglobulin. Rituximab therapy (375 mg/m2 per week for 4 weeks) was begun on days 18, 23, and 20 of ITP. Patient 1 achieved a platelet count of more than 50 000/μL at day 21 and more than 133 000/μL at day 28, patient 2 achieved a platelet count of more than 50 000/μL at day 4 and more than 150 000/μL at day 10, and patient 3 achieved a platelet count of more than 50 000/μL at day 5 and 72 000/μL at day 28 of rituximab therapy, with platelet response durations of 17+, 6+, and 6 months. These results suggest rituximab can rapidly reverse refractory fludarabine-associated ITP.
Introduction
Patients with chronic lymphocytic leukemia (CLL) are at increased risk for autoimmune complications. Autoimmune hemolytic anemia (AIHA) and idiopathic thrombocytopenia (ITP) are observed in approximately 20% to 35% and 2% of patients, respectively.1,2 Despite the change in the standard initial treatment from alkylating agents to fludarabine.3,4 it is unclear whether the incidence of severe and refractory AIHA has increased.5-8Fludarabine-associated ITP in CLL is uncommon; only 6 cases have been reported.9-13
Rituximab is a chimeric anti-CD20 monoclonal antibody with activity in indolent lymphomas14 but with less efficacy in CLL.15 Several reports have suggested rituximab may also be effective in immune-mediated pure red cell aplasia, AIHA, and ITP.15-18 In the present report, we describe the successful use of rituximab in 3 CLL patients with severe fludarabine-associated ITP.
Study design
Between 1998 and 2001, 3 of 21 patients with B-cell CLL treated at the National Cancer Institute developed severe ITP while receiving treatment on a fludarabine-containing protocol. The protocols were approved by the institutional review board and informed consent was provided according to the Declaration of Helsinki. All patients required treatment for disease progression, and their clinical characteristics and treatment histories are shown in Table1. The diagnosis of B-CLL was confirmed by peripheral blood flow cytometry, with CD19+, dim CD20+, CD22+, CD23+, CD5+, and CD10− small lymphocytes; bone marrow biopsies showed diffuse infiltration by small lymphocytes. Patients 1 and 2 received 25 mg/m2 fludarabine daily for 5 days every 4 weeks, and patient 3 received 25 mg/m2fludarabine daily for 5 days with UCN-01, an investigational staurosporine analog, every 4 weeks. Patient 1, with a remote history of posttraumatic splenectomy, developed a transient decrease in platelets to 89 000/μL on day 5 of cycle 1 and to 37 000/μL on day 24 of cycle 2 with recovery. However, during cycle 3 his platelet count precipitously fell to 4000/μL on day 8. Epistaxis, buccal hematoma, palatal petechiae, and ecchymoses in the scrotal area developed, and he did not respond to multiple platelet transfusions. Patient 2 had a decrease in the platelet count to 67 000/μL by day 9 of cycle 1 and to less than 1000/μL on day 25, accompanied by palatal petechiae and epistaxis refractory to platelet transfusions. Patient 3 had a decrease in platelet count to 10 000/μL on day 12 of cycle 3 with extremity and pharyngeal petechiae, which was refractory to platelet transfusions with a platelet nadir of 4000/μL.
Patient characteristics
| Baseline characteristics . | Patient 1 . | Patient 2 . | Patient 3 . |
|---|---|---|---|
| Age, y/sex | 50/M | 62/F | 59/M |
| Duration of CLL, y | 8 | 4 | 16 |
| Previous treatment | None | CD 40 ligand-transfected autologous lymphocytes 24 months ago | Multiple courses of fludarabine, 1994-2000 Rituximab 1 dose 6 mo ago |
| Baseline CBC | |||
| WBC, μL | 704 000 | 207 000 | 58 800 |
| Hemoglobin, g/dL | 9.1 | 7.0 | 10.1 |
| Platelets, μL | 120 000 | 135 000 | 80 000 |
| Stage (Rai) | III | III | IV |
| Current fludarabine cycles before ITP | 3 | 1 | 3 |
| ITP onset from last fludarabine cycle, d | 5 | 9 | 12 |
| Platelet nadir, μL | 4 000 | 1 000 | 3 000 |
| ITP clinical manifestations | Epistaxis, buccal hematoma, petechiae | Epistaxis, palatal petechiae | Palatal and skin petechiae |
| Serum platelet antibody | GPIb and GPIIb/IIIa | GPIb | Negative |
| ITP treatment | Prednisone | Prednisone | Prednisone |
| IVIG (Gammar) | IVIG (Sandoglobulin) | IVIG (Polygam SD) | |
| Win Rho | Rituximab | Rituximab | |
| Rituximab | |||
| Response to rituximab, μL | Complete response | Complete response | Partial response |
| Platelets 291 000 | Platelets 267 000 | Platelets 72 000 | |
| Duration of response, mo | 17+ | 6+ | 6 |
| Baseline characteristics . | Patient 1 . | Patient 2 . | Patient 3 . |
|---|---|---|---|
| Age, y/sex | 50/M | 62/F | 59/M |
| Duration of CLL, y | 8 | 4 | 16 |
| Previous treatment | None | CD 40 ligand-transfected autologous lymphocytes 24 months ago | Multiple courses of fludarabine, 1994-2000 Rituximab 1 dose 6 mo ago |
| Baseline CBC | |||
| WBC, μL | 704 000 | 207 000 | 58 800 |
| Hemoglobin, g/dL | 9.1 | 7.0 | 10.1 |
| Platelets, μL | 120 000 | 135 000 | 80 000 |
| Stage (Rai) | III | III | IV |
| Current fludarabine cycles before ITP | 3 | 1 | 3 |
| ITP onset from last fludarabine cycle, d | 5 | 9 | 12 |
| Platelet nadir, μL | 4 000 | 1 000 | 3 000 |
| ITP clinical manifestations | Epistaxis, buccal hematoma, petechiae | Epistaxis, palatal petechiae | Palatal and skin petechiae |
| Serum platelet antibody | GPIb and GPIIb/IIIa | GPIb | Negative |
| ITP treatment | Prednisone | Prednisone | Prednisone |
| IVIG (Gammar) | IVIG (Sandoglobulin) | IVIG (Polygam SD) | |
| Win Rho | Rituximab | Rituximab | |
| Rituximab | |||
| Response to rituximab, μL | Complete response | Complete response | Partial response |
| Platelets 291 000 | Platelets 267 000 | Platelets 72 000 | |
| Duration of response, mo | 17+ | 6+ | 6 |
Bone marrow aspirations and biopsy specimens showed adequate megakaryocytes without evidence of microangiopathic hemolysis in all 3 patients. Antiplatelet antibodies were detected in 2 patients (Table1). Standard treatment with 1 mg/kg oral prednisone per day and 1 g/kg per day intravenous immunoglobulin (IVIG) for 2 days (Gammar P, Sandoglobulin, and Polygam SD, respectively) was begun in all 3 patients. Patient 1 also received 250 IU/kg RhoGam I (WinRho) on day 14, and patient 2 was switched to 150 mg/d intravenous methyl prednisone for 5 days. Because no patient had a significant platelet response and all continued with evidence of petechiae or mucosal bleeding, 375 mg/m2 rituximab per week for 4 weeks was begun.
Results and discussion
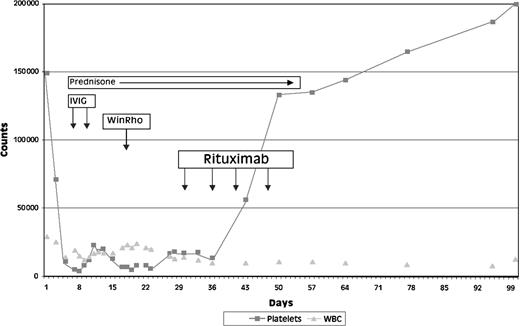
All 3 patients in this series responded to fludarabine but had to discontinue treatment because of severe refractory ITP. Rituximab treatment was begun on days 18, 7, and 6 of platelet nadirs in patients 1, 2 and 3, respectively, because of refractory ITP. In each patient, there was a prompt response to rituximab with a rise in platelet count and a resolution of bleeding as shown for patient 1 in Figure1. In patients 1 and 2, the platelet counts achieved normal levels within 4 weeks and lasted for 17+ and 6+ months. Patient 3 had a slow, incomplete treatment response, possibly related to massive splenomegaly, but did achieve a platelet count of 72 000/μL that persisted for 6 months. This patient eventually died of progressive disease with a WBC count of 504 000/μL and development of disease-related thrombocytopenia.
Clinical course of patient 1.
Fludarabine-induced ITP and response to rituximab.
We reviewed the literature and identified 6 previous cases of fludarabine-associated ITP in CLL (3 ITP and 3 Evans syndrome).9-13 All patients acquired severe thrombocytopenia following fludarabine treatment and variable responses to standard interventions. One patient was refractory to treatment and died of a hemorrhage. In 5 patients, responses were ultimately achieved with cyclosporine in 2 patients, splenectomy in 1 patient, and prednisone/IVIG in 1 patient. Of note, there was also a report of 1 patient with Evans syndrome who responded to rituximab.
Although ITP is relatively uncommon, like AIHA and pure red cell aplasia, there is an overall increased risk in CLL, suggesting that immune dysregulation plays an important role. Of note is a report showing platelet membrane–bound IgG in 8 of 9 patients with advanced CLL, determined by an antiglobulin consumption assay, suggesting an immune mechanism.19 It has also been suggested that the immune suppression from fludarabine may derepress an antibody response against hematopoietic antigens. Such observations form the basis for the hypothesis that an imbalance in CD4-helper and CD8-suppressor cells may produce immune dysregulation and autoimmune complications in CLL.20 Various other theories have also been suggested.21,22 Caligaria-Cappio and Hamblin23 hypothesized that profound suppression of CD4 cells by fludarabine may cause aberrations of the immunoregulatory circuits involving malignant B-cells. CLL and normal B cells can coexpress CD40, which, when stimulated by CD40 ligand on T cells, may up-regulate CD80 and CD86 costimulatory molecules and acquire the ability to present hematopoietic antigens to normal bystander B cells and invoke an immune response.
In the present report, we describe the successful use of rituximab in 3 patients with advanced CLL and refractory, life-threatening, fludarabine-associated ITP. In all patients, the platelet counts showed a rapid and unexpected decline during fludarabine treatment that were not explained by the cytotoxic effects of fludarabine. Bone marrow biopsy findings showed adequate megakaryocytes, confirming fludarabine-associated ITP. Patients were begun on steroids and IVIG, and fludarabine treatment was discontinued. Although the patients received steroids and IVIG for 8, 7, and 6 days, respectively, they continued to have bleeding complications without platelet recovery and were begun on rituximab because of reports of its efficacy in ITP and other autoimmune diseases.16-18,24 25 Two patients experienced a rapid and durable improvement in platelet count that became evident on the first day of therapy. In the third patient, a major improvement in platelet count was achieved but never reached normal values, possibly because of massive splenomegaly.
These rapid increases in platelet count were most likely attributable to rituximab and not to delayed effects of steroids and IVIG. First, all patients showed a significant increase within 24 hours of rituximab therapy; second, the platelet counts continued to rise despite steroid discontinuation. A rapid response to rituximab has been reported by other investigators in refractory ITP, suggesting that depletion of antiplatelet antibodies is not the mechanism of rituximab action.16 18 This observation has led to speculation that rituximab causes opsonization of B cells that inhibit macrophage Fc receptor function and clearance of IgG-coated platelets. This action may lead to the eventual suppression of autoreactive B cells and the sustained remissions observed in some patients.
Successful treatment of refractory ITP with rituximab has also been reported in other settings. Perotta et al17 reported 10 patients with refractory ITP treated with 375 mg/m2rituximab per week for 4 weeks. Of these, 5 patients achieved complete responses and 1 achieved a partial response, lasting from 1 to 14 months. In another report, Stasi et al18 used the same rituximab dose in 25 patients with chronic refractory ITP, and by 4 weeks, 20% of the patients achieved complete or partial responses, and 12% had minor responses. Most responding patients showed rapid platelet count increases within the first week of rituximab treatment.
Our results, in concert with previous reports, suggest that rituximab is effective for the treatment of fludarabine-associated ITP. Indeed, its efficacy in steroid-refractory disease and its low incidence of long-term toxicity suggest it may be preferable to standard treatments of ITP. Of note is that strategies that combine rituximab and fludarabine may not only improve CLL response rates but may decrease the incidence of autoimmune phenomenon.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bruce Cheson, Georgetown University Hospital, Lombardi Cancer Center, 3800 Reservoir Road, NW, Washington, DC 20007.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal