Human herpesvirus 8 (HHV-8)/Kaposi sarcoma–associated herpesvirus (KSHV) is linked to a number of malignancies thought to be driven by cytokines, including interleukin-6 (IL-6). Rta, a transcriptional activator encoded by HHV-8/KSHV, activates the viral lytic cycle leading to the expression of several viral genes implicated in viral pathogenesis. However, the effect of HHV-8/KSHV Rta on cellular genes has not been reported. We present evidence that the human IL-6(hIL-6) gene is up-regulated by Rta. Rta potently activated (up to 164-fold) the hIL-6 promoter in a dose-dependent manner in a transient transfection reporter system. Rta also induced expression of the endogenous hIL-6 gene, as shown by enzyme-linked immunosorbent assays. Activation of the hIL-6gene by HHV-8/KSHV supports the role of hIL-6 in the development of these malignancies.
Introduction
Human interleukin-6 (hIL-6) is a multifunctional cytokine, and dysregulation of hIL-6 is implicated in the pathogenesis of several malignancies such as Kaposi sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman disease (MCD). hIL-6 serves as an autocrine growth factor for cultured AIDS-KS cells and may induce endothelial cell proliferation in KS through a paracrine pathway.1,2 Supernatants from PEL-derived cell lines and PEL effusions contain large quantities of hIL-6.3,4Anti–hIL-6 neutralizing antibodies delayed PEL tumor progression in SCID mice.5 Overproduction of IL-6 also reproduced some manifestations of MCD in a mouse model.6 Furthermore, anti–hIL-6 or anti–hIL-6 receptor antibodies exerted a therapeutic effect on MCD patients.7 8 Taken together, these data strongly support the involvement of hIL-6 in the pathogenesis of these malignancies.
Another common feature of KS, PEL, and MCD is their association with human herpesvirus 8 (HHV-8)/Kaposi sarcoma–associated herpesvirus (KSHV).9-11 HHV-8/KSHV encodes a potent transcriptional activator, Rta, which is necessary and sufficient for initiating viral lytic replication.12,13 Among the lytic genes expressed are homologues of cytokines and chemokines, including viral IL-6 (vIL-6) and viral macrophage inflammatory proteins.14,15In particular, vIL-6 has been detected in tumor lesions and sera from KS, PEL, and MCD patients and is thought to play an important role in viral pathogenesis.16-18 In addition to pirating cellular genes, it is likely that HHV-8/KSHV has developed strategies to enhance its replication by modulating the regulation of cellular factors. We are investigating the effect of Rta on cellular genes and report here that hIL-6 expression is up-regulated by Rta.
Study design
Plasmid construction
The 1.2-kb hIL-6 promoter region was amplified from total cellular DNA using primers F (5′-GGAAGATCTCTCCTGCAAGAGACACCATCCTGA-3′) and R (5′-CGGGAATTCAGGGCAGAATGAGCCTCAGAGACAT3-3′); the underlined nucleotides represent BglII and EcoRI sites, respectively. The PCR fragment was cloned into pSEAP2-basic (Clontech, Palo Alto, CA) to produce phIL6-1200/SEAP.
Reporter assays
Transfections were performed in 12-well plates using a standard calcium phosphate method for the human embryonic kidney cell line 293T or LipofectAmine PLUS (Invitrogen, Carlsbad, CA) for the immortalized bone marrow stromal cell line R1T.19 At 48 hours after transfection, supernatants and cells were harvested. Supernatants were assayed for secreted alkaline phosphatase (SEAP) activities, using the Great EscAPe SEAP Chemiluminescence Detection Kit (Clontech). Cells were lysed in 1× passive lysis buffer and assayed forRenilla luciferase activities using the Luciferase Reporter Assay System (Promega, Madison, WI).
Enzyme-linked immunosorbent assays
pcDNA3/Rta12 or pcDNA3 was transfected into 293T or R1T cells in 6-well plates using LipofectAmine PLUS. pcDNA3/Rta contained a 3.1-kb genomic sequence encoding Rta, whose expression was driven by the cytomegalovirus immediate-early promoter/enhancer in the vector. Supernatants from transfected cells were collected at 24, 48, and 72 hours after transfection and were assayed for hIL-6 protein levels using an hIL-6 enzyme-linked immunosorbent assay (ELISA) kit (Biosource International).
Results and discussion
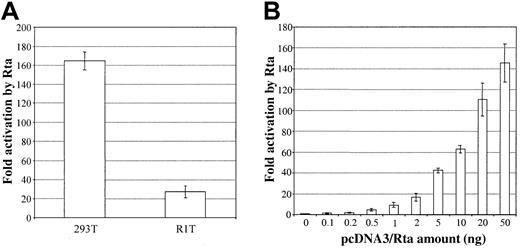
To investigate the role Rta may play in regulatinghIL-6 gene expression, we first examined whether Rta can activate the hIL-6 promoter in a reporter system. A 1200-bp promoter region upstream of the first hIL-6 exon was cloned into the pSEAP2-basic vector to produce phIL6-1200/SEAP. This reporter plasmid was cotransfected into 293T cells with either pcDNA3/Rta (an Rta expression plasmid) or vector alone. To control for transfection efficiency and other experimental variations, pRL-CMV, which constitutively expresses the Renilla luciferase, was included in each transfection. As shown in Figure1A, phIL6-1200/SEAP was potently activated (164-fold) by Rta. To confirm that activation of the hIL-6 promoter was mediated by the Rta protein, we examined the dose dependence of Rta activation. A fixed amount of the reporter plasmid phIL6-1200/SEAP was cotransfected with increasing amounts of pcDNA3/Rta into 293T cells. As the amount of pcDNA3/Rta in each transfection increased, so did the normalized SEAP activity (Figure 1B), indicating that activation of the hIL-6 promoter by Rta is specific.
HHV-8/KSHV Rta activates the hIL-6 promoter in a reporter system.
(A) Activation of the hIL-6 promoter by Rta in 2 different cell lines. Reporter plasmid phIL6-1200/SEAP (20 ng), pRL-CMV (2 ng), filler DNA (720 ng; plasmid DNA that lacks a mammalian promoter/enhancer), and either pcDNA3/Rta or pcDNA3 (50 ng) were transfected into 293T or R1T cells. Supernatants and cells were harvested at 48 hours after transfection and were assayed for SEAP and Renillaluciferase activities, respectively. SEAP activities from the hIL6 promoter were normalized to the corresponding Renillaluciferase activities. Fold activation by Rta was calculated by comparing the normalized SEAP activity stimulated by Rta to that by pcDNA3. (B) Dose-dependent activation of the hIL-6 promoter by Rta in 293T cells. Cells were transfected with 20 ng phIL6-1200/SEAP, 2 ng pRL-CMV, 720 ng filler DNA, and an increasing amount of pcDNA3/Rta (0-50 ng) and a correspondingly decreasing amount of pcDNA3 (50-0 ng) so that the total amount of pcDNA3 vector backbone remained the same. Reporter activities were assayed at 48 hours after transfection; fold activation by different amounts of pcDNA3/Rta was calculated by comparing the normalized SEAP activities to that stimulated by 0 ng pcDNA3/Rta and 50 ng pcDNA3.
HHV-8/KSHV Rta activates the hIL-6 promoter in a reporter system.
(A) Activation of the hIL-6 promoter by Rta in 2 different cell lines. Reporter plasmid phIL6-1200/SEAP (20 ng), pRL-CMV (2 ng), filler DNA (720 ng; plasmid DNA that lacks a mammalian promoter/enhancer), and either pcDNA3/Rta or pcDNA3 (50 ng) were transfected into 293T or R1T cells. Supernatants and cells were harvested at 48 hours after transfection and were assayed for SEAP and Renillaluciferase activities, respectively. SEAP activities from the hIL6 promoter were normalized to the corresponding Renillaluciferase activities. Fold activation by Rta was calculated by comparing the normalized SEAP activity stimulated by Rta to that by pcDNA3. (B) Dose-dependent activation of the hIL-6 promoter by Rta in 293T cells. Cells were transfected with 20 ng phIL6-1200/SEAP, 2 ng pRL-CMV, 720 ng filler DNA, and an increasing amount of pcDNA3/Rta (0-50 ng) and a correspondingly decreasing amount of pcDNA3 (50-0 ng) so that the total amount of pcDNA3 vector backbone remained the same. Reporter activities were assayed at 48 hours after transfection; fold activation by different amounts of pcDNA3/Rta was calculated by comparing the normalized SEAP activities to that stimulated by 0 ng pcDNA3/Rta and 50 ng pcDNA3.
These results from the reporter system indicate that Rta activates the hIL-6 promoter in the absence of chromatin structure. We next examined whether Rta also activates the endogenous hIL-6 gene. pcDNA3/Rta or pcDNA3 was transfected into 293T cells, and supernatants were harvested at different time points after transfection. The hIL-6 protein levels in these samples were then assayed by ELISA. Consistent with the lack of endogenous hIL-6 expression in 293T cells, the hIL-6 protein levels were low (less than 7.8 pg/mL, the detection limit of the kit) in pcDNA3-transfected cells (Figure2A). However, the expression of Rta in 293T cells stimulated hIL-6 expression and resulted in progressively higher amounts of hIL-6 protein accumulating in the supernatant at 48 and 72 hours after transfection (54.0 and 84.5 pg/mL, respectively).
HHV-8/KSHV Rta activates the endogenous
hIL-6 gene. pcDNA3/Rta or pcDNA3 (1 μg) was transfected into 293T (A) or R1T (B) cells. Supernatants were harvested 24, 48, and 72 hours later, diluted where appropriate, and assayed for hIL-6 protein levels by ELISA. Average hIL-6 protein concentrations in the supernatants are indicated by horizontal bars, with the numbers (pg/mL) shown. The detection range of the ELISA kit is 7.8 to 500 pg/mL. Dotted line in panel A indicates the lowest detection limit of the kit. When the hIL-6 level in one or more experiments was lower than 7.8 pg/mL, the average for that time point was not calculated. A logarithmic scale is used in panel B.
HHV-8/KSHV Rta activates the endogenous
hIL-6 gene. pcDNA3/Rta or pcDNA3 (1 μg) was transfected into 293T (A) or R1T (B) cells. Supernatants were harvested 24, 48, and 72 hours later, diluted where appropriate, and assayed for hIL-6 protein levels by ELISA. Average hIL-6 protein concentrations in the supernatants are indicated by horizontal bars, with the numbers (pg/mL) shown. The detection range of the ELISA kit is 7.8 to 500 pg/mL. Dotted line in panel A indicates the lowest detection limit of the kit. When the hIL-6 level in one or more experiments was lower than 7.8 pg/mL, the average for that time point was not calculated. A logarithmic scale is used in panel B.
To further establish the ability of Rta to activate the hIL-6 promoter and to induce hIL-6 protein expression, we performed similar experiments in R1T cells. R1T cells manifest a significant level of basal hIL-6 expression19 and thus complement the use of 293T cells. The reporter plasmid phIL6-1200/SEAP was activated 27-fold by Rta in transient transfection reporter assays in R1T cells (Figure1A). The fold activation in R1T cells was lower than that in 293T cells because of the higher basal level of the reporter plasmid. Moreover, transfection of pcDNA3/Rta stimulated the expression of endogenous hIL-6 in R1T cells, when compared to transfection of pcDNA3, and resulted in hIL-6 levels of 1209, 5762, and 21 447 pg/mL at 24, 48, and 72 hours after transfection, respectively (Figure 2B).
Up-regulation of the hIL-6 gene has emerged as a common theme among herpesvirus infections, and multiple mechanisms may be involved.20-22 In the case of HHV-8/KSHV, latently infected B-cell lines (eg, BC-1 and KS-1) express hIL-6 at high levels.3,4 This is attributed in part to the responsiveness of the hIL-6 promoter to an HHV-8/KSHV-latent gene product, the latency-associated nuclear antigen.19 Because HHV-8/KSHV exists predominantly in a latent state in KS and PEL lesions, the induction of hIL-6 expression by the latency-associated nuclear antigen may play a critical role in the development of these malignancies. Here we have demonstrated that HHV-8/KSHV also stimulates hIL-6 expression through its lytic transcriptional activator, Rta. We hypothesize that activation of hIL-6 by Rta plays an important role in lytic infections. This is especially relevant in patients with HHV-8/KSHV-associated MCD. Our results are consistent with the high plasma hIL-6 levels observed in MCD patients and with the fact that most HHV-8/KSHV–infected cells in MCD lesions express the viral lytic gene expression program driven by Rta.16 17
Interestingly, in a separate study, we demonstrated that Rta also strongly activates the HHV-8/KSHV vIL-6 gene.23Like hIL-6, vIL-6 promotes the growth of IL-6–dependent B cells and activates signal transduction pathways. However, vIL-6 may stimulate a broader spectrum of target cells because it requires only the ubiquitously expressed gp130 receptor, whereas hIL-6 requires both gp130 and IL-6Rα for signal transduction.14,15,24 On the other hand, the amount of vIL-6 required to stimulate the growth of IL-6–dependent B cells was greater than that of hIL-6, and the binding affinity of vIL-6 for soluble gp130 was determined to be 1000-fold lower than that of hIL-6/soluble IL-6R complex for gp130.25 Therefore, hIL-6 and vIL-6 may both be important in HHV-8/KSHV replication and pathogenesis, but they may play overlapping yet different roles.
We thank Mike Johnson and Jiabin An for excellent technical assistance, Dr Tonia Symensma for critical reading of the manuscript, and members of the Sun and Martinez-Maza laboratories for discussion.
Prepublished online as Blood First Edition Paper, May 13, 2002; DOI 10.1182/blood-2002-01-0015.
Supported by National Institutes of Health grants CA91791, CA83525, DE14153, and CA57152, the Jonsson Cancer Center Foundation, the Stop Cancer Foundation, and the Concern Foundation. H.D. is a Lymphoma Research Foundation Fellow.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ren Sun, Department of Molecular and Medical Pharmacology, University of California at Los Angeles, Los Angeles, CA 90095-1735; e-mail: rsun@mednet.ucla.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal