Normalization of restricted T-cell–receptor (TCR) repertoire is critical following T-cell–depleted (TCD) stem cell transplantation. We present a prospective study analyzing respective contributions of naive and memory T-cell subsets within the CD4+ and CD8+ compartments to the evolution of overall TCR-repertoire complexity following transplantation of CD34-selected peripheral blood progenitor cells from unrelated donors. During the first year after transplantation, sorted CD4/45RA, CD4/45R0, CD8/45RA, and CD8/45R0 subsets were analyzed at 3-month intervals for TCR-repertoire complexity by CDR3 size spectratyping. Skew in TCR-repertoire was observed only in early memory-type T cells. CD4+ and CD8+ subsets differed in clonal distribution of CDR3 sizes, with rapid Gaussian normalization of bands in CD4/45R0+ T cells. Naive T cells displayed normal repertoire complexity and contributed significantly to skew correction. Our data provide direct evidence for an important role of de novo maturation of naive T cells in normalization of an initially restricted TCR-repertoire following transplantation of CD34-selected, TCD-depleted peripheral blood progenitors from unrelated donors.
Introduction
Reconstitution of a functional T-cell compartment after allogeneic stem cell transplantation (allo-SCT) requires not only normalization of peripheral T-cell numbers but also restoration of a diverse T-cell–receptor (TCR) repertoire.1 Several studies in recent years have demonstrated only minor abnormalities in TCR repertoire diversity following transplantation of unmanipulated bone marrow from HLA-identical related donors,2,3 whereas transplantation from unrelated or HLA-mismatched related donors, particularly after T-cell depletion (TCD), results in severe skewing of the TCR repertoire during the first posttransplantation year.2-5 It has been suggested that in these transplantations, reconstitution of a normal TCR repertoire relies on de novo generation of naive T cells and requires residual thymic function of the host.1,6 7 However, the relative contribution of naive and memory T-cell subsets to overall TCR repertoire diversity after allo-SCT has not been investigated thus far.
In this study, we prospectively analyzed TCR repertoire diversity of CD4+ and CD8+ naive and memory T cells at 3-month intervals in 10 children during the first year after allo-SCT from unrelated donors. The results demonstrate a skewing of the repertoire during the first 6 months after transplantation limited to the memory pool of CD4+ and CD8+ T-cell subsets. In contrast, naive CD4+ and CD8+ T cells exhibit an ongoing diverse TCR repertoire from their earliest detection. Memory-type CD4+ cells tend to normalize their patterns of clonal distribution more rapidly than memory-type CD8+ cells, in which a pattern of clonal dominance can be observed beyond the first year after transplantation. Our results provide direct evidence of corrections in gaps and skewing in the initial post–allo-SCT–restricted TCR repertoire by naive CD4+ and CD8+ T cells and emphasize an important role of de novo maturation of T cells through the thymus for complete reconstitution of the T-cell compartment.
Study design
Ten children underwent transplantation with TCD CD34+-selected peripheral blood stem cells from unrelated donors (Table 1). The study was approved by the University of Tuebingen's institutional review board, and informed consent was obtained from the parents. Graft-versus-host disease (GVHD) prophylaxis was achieved by TCD of the graft using the CliniMACS system (Miltenyi, Bergisch-Gladbach, Germany) without posttransplantation immunosuppression. Engraftment as determined by absolute neutrophil count (ANC) greater than 500/μL over 3 consecutive days was achieved after a median of 13.5 days (range, 9-24 days).
Patient characteristics
| UPN . | Age, y . | Disease . | Conditioning . | HLA-mismatch . | CD34+/kg bw, × 106 . | CD3+/kg bw, × 103 . | Outcome . |
|---|---|---|---|---|---|---|---|
| 415 | 12.1 | PCV | Bu(16)/Cyc(200)/ATG(30) | 0 | 3.4 | 3.6 | Alive |
| 454 | 13.7 | CML | FTBI(12)/Cy(120)/TT(10)/ATG(30) | B, DRB1 minor | 9 | 3 | GVHD, HHV6 reactivation, death day + 328 |
| 464 | 16.3 | AML/HLH | Bu(8)/Cyc(120)/Fludara(150)/ATG(30) | 0 | 8 | 3 | Alive |
| 449 | 1.5 | ALL | Bu(20)/VP16(40)/Cy(120)/ATG(30) | 0 | 9.6 | 1 | Relapse day 132, immunotherapy* |
| 432 | 3.8 | AML | Bu(16)/Cyc(120)/Mel(140)/ATG(30) | DR | 21.4 | 4.4 | Alive |
| 391 | 10.2 | MDS/AML | Bu(16)/Cyc(10)/Mel(140)/ATG(30) | DR | 7.3 | 8 | Aspergillosis, death day + 234 |
| 453 | 10 | ALL | TBI(12)/VP16(40)/TT(10)/ATG(30) | 0 | 10 | 2.4 | Relapse, death day + 307 |
| 442 | 9.8 | ALL | TBI(12)/VP16(40)/TT(10)/ATG(30) | C | 7.4 | 8.9 | Alive |
| 466 | 4.3 | BDA | Busulfex(12)/Cyc(200)/ATG(30) | 0 | 6.3 | 5.7 | Alive |
| 437 | 13.5 | ALL | TBI(12)/VP16(40)/TT(10)/ATG(30) | 0 | 8 | 12.5 | Alive |
| Median | 10.1 | — | — | — | 8 | 3 | — |
| (range) | (1.5-16.3) | (3.4-21.4) | (1-12.5) |
| UPN . | Age, y . | Disease . | Conditioning . | HLA-mismatch . | CD34+/kg bw, × 106 . | CD3+/kg bw, × 103 . | Outcome . |
|---|---|---|---|---|---|---|---|
| 415 | 12.1 | PCV | Bu(16)/Cyc(200)/ATG(30) | 0 | 3.4 | 3.6 | Alive |
| 454 | 13.7 | CML | FTBI(12)/Cy(120)/TT(10)/ATG(30) | B, DRB1 minor | 9 | 3 | GVHD, HHV6 reactivation, death day + 328 |
| 464 | 16.3 | AML/HLH | Bu(8)/Cyc(120)/Fludara(150)/ATG(30) | 0 | 8 | 3 | Alive |
| 449 | 1.5 | ALL | Bu(20)/VP16(40)/Cy(120)/ATG(30) | 0 | 9.6 | 1 | Relapse day 132, immunotherapy* |
| 432 | 3.8 | AML | Bu(16)/Cyc(120)/Mel(140)/ATG(30) | DR | 21.4 | 4.4 | Alive |
| 391 | 10.2 | MDS/AML | Bu(16)/Cyc(10)/Mel(140)/ATG(30) | DR | 7.3 | 8 | Aspergillosis, death day + 234 |
| 453 | 10 | ALL | TBI(12)/VP16(40)/TT(10)/ATG(30) | 0 | 10 | 2.4 | Relapse, death day + 307 |
| 442 | 9.8 | ALL | TBI(12)/VP16(40)/TT(10)/ATG(30) | C | 7.4 | 8.9 | Alive |
| 466 | 4.3 | BDA | Busulfex(12)/Cyc(200)/ATG(30) | 0 | 6.3 | 5.7 | Alive |
| 437 | 13.5 | ALL | TBI(12)/VP16(40)/TT(10)/ATG(30) | 0 | 8 | 12.5 | Alive |
| Median | 10.1 | — | — | — | 8 | 3 | — |
| (range) | (1.5-16.3) | (3.4-21.4) | (1-12.5) |
ALL indicates acute lymphoid leukemia; AML, acute myeloid leukemia; ATG(30), α-thymocyte globulin (rabbit) (3 × 10 mg/kg bw); BDA, Blackfan-Diamond anemia; Busulfex(12), Busulfex (3 × 4 mg/kg bw); Bu(16), busulfan (4 × 4 mg/kg bw); bw, body weight; CML, chronic myeloid leukemia; Cyc(120), cyclophosphamide (2 × 60 mg/kg bw); Fludara(150), fludarabine (5 × 30 mg/m2per day); FTBI (12), fractionated total body irradiation (12 Gy); HHV6, human herpes virus 6; HLA, human leukocyte antigen; HLH, secondary hemophagocytic lymphohistiocytosis; MDS, myelodysplastic syndrome; Mel(140), Melphalan (140 mg/m2 per day); PCV, polycythemia vera; TT(10), thiotepa (10 mg/kg bw); VP16(40), etoposide (40 mg/kg bw).
After receiving donor lymphocyte infusions, this patient was excluded from further analysis.
Peripheral blood mononuclear cells (PBMCs) were collected at 3-month intervals using standard procedures. In the early posttransplantation phase, when memory T cells dominated the peripheral T-cell pool, PBMCs were MACS-sorted into CD4+ and CD8+ cells following the manufacturer's protocol, and purity was confirmed by FACS analysis. When more than 10% of the CD4+/CD45RA+ naive-type T cells were detectable in the peripheral blood, PBMCs were sorted into naive (CD45RA+) and memory (CD45RO+) CD4+and CD8+ cells using the CD4+ Multisort Kit (Miltenyi). TCR repertoire diversity in the subsets was assessed by CDR3 size spectratyping of Vβ1-24, as described previously.8 All cDNAs were controlled using a Cβ-specific primer pair to ensure appropriate quality and quantity before they were subjected to spectratype analysis. Because a normal spectratype has been shown to consist of 5 to 8 bands per family with a Gaussian size distribution of TCR fragments9 10 scoring and size distribution were used to assess the normalization of TCR repertoire. In addition, TCR-repertoire complexity of T-cell subsets was determined in healthy controls (n = 4).
Results and discussion
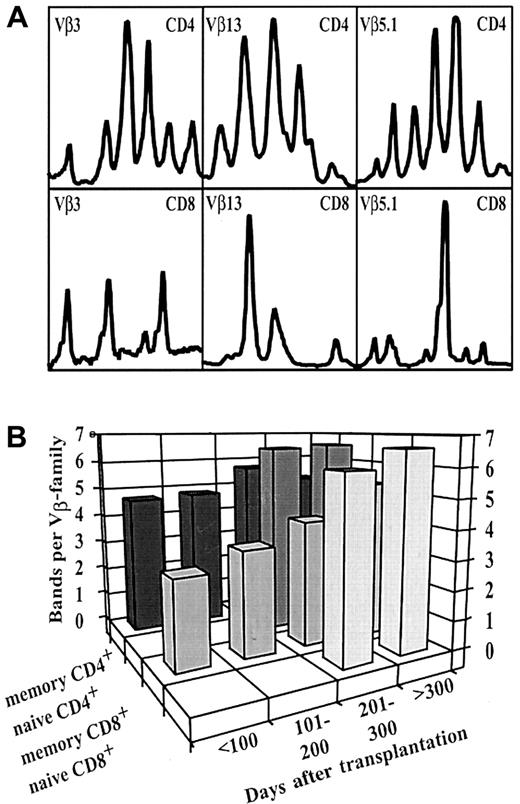
Previous longitudinal CDR3 size spectratype analyses in healthy persons have confirmed the TCR repertoire to be stable over time with a Gaussian distribution of TCR fragments and a median of 6 to 8 bands in each Vβ-family.9,11 Furthermore, complexity and clonal distribution were reported to be equivalent in CD4+ and CD8+ T cells in healthy persons, with minor skews in certain CD8+ Vβ families.12 However, in the early posttransplantation phase, before day 200, characterized by an expansion of only mature memory-type T cells, we observed a markedly restricted TCR repertoire, with different clonotypes identified in CD4+ and CD8+ T cells (Figure1A). Restricted overall TCR diversity has also been reported by other investigators after the transplantation of autologous or allogeneic TCD grafts3,4,10 and can be explained by the low number of cotransplanted T cells in TCD grafts. The identification of different clonotypes in CD4+ and CD8+ subsets demonstrates that these populations are efficiently separated using the MACS technique (median purity, 80.6%). It should be noted that the skew observed in our patients was not caused by limited numbers of T cells available for analysis. In titration experiments we had previously determined the lower threshold of our assay to be in the range of 104 T cells.5 All samples in this study contained T-cell numbers above this limit (median, 6.8 × 105 CD3+ T cells; range, 0.1-149 × 105 CD3+ T cells).
Distinct CDR3 size distributions in CD4+ and CD8+ naive and memory T-cell subsets.
(A) Different CDR3 size fragments identified in CD4+ (top lane) and CD8+ (bottom lane) T-cell subsets. Representative example of Vβ3, Vβ5.1, and Vβ13 families in the sorted subfractions of a patient with acute lymphoid leukemia (UPN 442) on day 147 after transplantation. (B) Increase in the median number of bands per Vβ family in naive and memory CD4+ and CD8+ T-cell subsets after transplantation. Before day 200, no naive-type T cells were available for analysis. In parallel with the appearance of naive-type T cells in the periphery, the mean number of bands per Vβ family of the CD4+ and CD8+memory compartment significantly increased between the study time points, days 101 to 200 and days 201 to 300 (P < .001, Mann-Whitney U test).
Distinct CDR3 size distributions in CD4+ and CD8+ naive and memory T-cell subsets.
(A) Different CDR3 size fragments identified in CD4+ (top lane) and CD8+ (bottom lane) T-cell subsets. Representative example of Vβ3, Vβ5.1, and Vβ13 families in the sorted subfractions of a patient with acute lymphoid leukemia (UPN 442) on day 147 after transplantation. (B) Increase in the median number of bands per Vβ family in naive and memory CD4+ and CD8+ T-cell subsets after transplantation. Before day 200, no naive-type T cells were available for analysis. In parallel with the appearance of naive-type T cells in the periphery, the mean number of bands per Vβ family of the CD4+ and CD8+memory compartment significantly increased between the study time points, days 101 to 200 and days 201 to 300 (P < .001, Mann-Whitney U test).
In the first 200 days after transplantation, peripheral T cells almost exclusively expressed a memory phenotype (CD45RO+ for CD4+, CD27+ for CD8+) (data not shown). It is generally assumed that the antigenic milieu of the host stimulates expansion of selected donor-derived memory T-cell clones that were cotransplanted with the graft,13though several groups reported the persistence of recipient-derived clones in these subsets.1 10 Two to 5 patients in our cohort showed mixed donor-recipient chimerism during follow-up, with a median percentage of 2.5% (Table 2). Therefore, it seems possible that in these patients, expanded recipient clones might have contributed in part to TCR repertoire diversity.
Chimerism and TCR Vβ repertoire reconstitution
| Day . | Chimerism* . | No. bands per Vβ family, mean ± SD . | ||||
|---|---|---|---|---|---|---|
| Mixed . | Complete donor . | CD4+ CD45RA+ . | CD4+CD45RO+ . | CD8+CD45RA+ . | CD8+ CD45RO+ . | |
| 0-100 | 2/10† | 8 of 10 | — | 4.7 ± 2.3 | — | 2.9 ± 1.7 |
| 101-200 | 5/10† | 5 of 10 | — | 4.8 ± 2.5 | — | 3.4 ± 2 |
| 201-300 | 4/9† | 5 of 9 | 6.5 ± 2.4 | 5.7 ± 2.3 | 6 ± 2 | 4.3 ± 2.2 |
| > 300 | 3/6† | 3 of 6 | 6.6 ± 2.2 | 5.2 ± 2.4 | 6.6 ± 1.8 | 5.3 ± 2.4‡ |
| Control | — | — | 6.5 ± 1.3 | 6.5 ± 1.4 | 5.5 ± 1.6 | 4.6 ± 1.7‡ |
| Day . | Chimerism* . | No. bands per Vβ family, mean ± SD . | ||||
|---|---|---|---|---|---|---|
| Mixed . | Complete donor . | CD4+ CD45RA+ . | CD4+CD45RO+ . | CD8+CD45RA+ . | CD8+ CD45RO+ . | |
| 0-100 | 2/10† | 8 of 10 | — | 4.7 ± 2.3 | — | 2.9 ± 1.7 |
| 101-200 | 5/10† | 5 of 10 | — | 4.8 ± 2.5 | — | 3.4 ± 2 |
| 201-300 | 4/9† | 5 of 9 | 6.5 ± 2.4 | 5.7 ± 2.3 | 6 ± 2 | 4.3 ± 2.2 |
| > 300 | 3/6† | 3 of 6 | 6.6 ± 2.2 | 5.2 ± 2.4 | 6.6 ± 1.8 | 5.3 ± 2.4‡ |
| Control | — | — | 6.5 ± 1.3 | 6.5 ± 1.4 | 5.5 ± 1.6 | 4.6 ± 1.7‡ |
Determined by PCR of variable number tandem repeats in whole blood samples as previously described.22
Median percentage of recipient cells in whole blood was 2.5%.
Skewing within the CD8+CD45RO+ compartment could be observed in transplant recipients beyond day 300 and in healthy controls.
Between day 100 and day 200, repertoire complexity of the memory T-cell pool only marginally improved (Figure 1B; Table 2), suggesting that TCR repertoire complexity after TCD transplantation does not normalize without the addition of new clones either by adoptive transfer or by de novo maturation. However, we were able to detect differences between the CD4+ and CD8+ T-cell compartments. CD4+ memory-type T cells showed a faster normalization toward a Gaussian distribution of TCR fragments within the Vβ families (Figure 1A), even before detection of naive T cells in the peripheral blood. This suggests that homeostatic proliferation14,15 of early naive T cells with subsequent acquisition of a memory phenotype might be operative in this setting. The generation of functional CD4+ memory T cells after encounter with endogenous antigen only has been described recently in TCR-transgenic mice.14 Although this phenomenon has also been reported in CD8+ naive T cells,15 we found it to be more relevant in the CD4+ compartment.
Clonal dominance of certain Vβ families in memory CD8+ T cells of transplant recipients persisted well beyond the first posttransplantation year and could be observed in healthy controls as well (data not shown). This pattern has been described in both populations12,16 17 and seems to be a characteristic feature of this T-cell subset.
Newly appearing naive-type T cells emerging after day 200 showed a TCR repertoire comparable to that of healthy controls with a Gaussian distribution of TCR fragments (Figure 1B; Table 2). In parallel with the appearance of naive-type T cells in the periphery, repertoire complexity of the CD4+ and CD8+memory pool significantly improved (P < .001, Mann-Whitney U test; Figure 1B), probably because of the transition of new naive T cells into a memory phenotype. None of the 10 children underwent donor lymphocyte infusion (DLI) during the study period; therefore, the transfer of mature T-cell clones by DLI can be excluded. Because the generation of naive T cells after allo-SCT seems to require residual thymic function, at least in CD4+T cells,18 19 this underscores the importance of thymic involvement for the reconstitution of a complex TCR repertoire after TCD stem cell transplantation.
The results of our study demonstrate that restricted TCR repertoire diversity after allo-SCT of TCD grafts from unrelated donors is limited to the early-appearing memory T-cell pool. Naive-type T cells, which appear several months after transplantation, display a normal TCR repertoire complexity and help to correct the initial skewing. Additional studies will be aimed at enhancing the generation of naive-type T cells, either by the administration of specific cytokines such as interleukin-720 21 or by protection of the thymus from chemotherapy- and radiotherapy-induced toxicity.
We thank the staff of the BMT unit and outpatient department and the technical staff of the stem cell processing unit at the University Children's Hospital, Tuebingen for their excellent and dedicated patient care. We also thank Shangara Lal for critically reviewing the manuscript.
Prepublished online as Blood First Edition Paper, May 13, 2002; DOI 10.1182/blood-2001-11-0005.
Supported by a grant from the fortuene-Program of the University of Tuebingen (no. 587/1999) and in part by the Deutsche Forschungsgemeinschaft Program Project Grant SFB 510-C4 (P.G.S. and D.N.) M.E. is a postdoctoral fellow of the Else Uebelmesser Foundation for Cancer Research (1.3-0415.221.18-03/97).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paul G. Schlegel, Pediatric Stem Cell Transplant Program, Program Project Grant IZKF, Hoppe-Seyler-Strasse 1, Tuebingen, D-72076, Germany; e-mail: p-g.schlegel@med.uni-tuebingen.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal