Fcγ receptor–mediated phagocytosis is a complex process involving the activation of protein tyrosine kinases, events that are potentially down-regulated by protein tyrosine phosphatases. We used the J774A.1 macrophage cell line to examine the roles played by the protein tyrosine phosphatase SHP-1 in the negative regulation of Fcγ receptor–mediated phagocytosis. Stimulation with sensitized sheep red blood cells (sRBCs) induced tyrosine phosphorylation of CBL and association of CBL with CRKL. These events were completely or partially abrogated by PP1 or the heterologous expression of dominant-negative SYK, respectively. Heterologous expression of wild-type but not catalytically inactive SHP-1 also completely abrogated the phagocytosis of IgG-sensitized sRBCs. Most notably, overexpressed SHP-1 associates with CBL and this association led to CBL dephosphorylation, loss of the CBL-CRKL interaction, and the suppression of Rac activation. These data represent the first direct evidence that SHP-1 is involved in the regulation of Fcγ receptor–mediated phagocytosis and suggest that activating signals mediated by SRC family kinases SYK, CBL, phosphatidyl inositol-3 (PI-3) kinase, and Rac are directly opposed by inhibitory signals through SHP-1.
Introduction
Fcγ receptor–mediated phagocytosis in macrophages is an important primary mode of defense in the immune system. Fcγ-receptor engagement leads to the activation of nonreceptor tyrosine kinases HCK, LYN, FGR,1,2 and SYK3,4; phosphorylation of adapter proteins; and activation of effector molecules including Rac, Rho, and Rab.5,6 Previous results from our laboratory established that SYK is activated following FcγR stimulation and associates with the FcγRIγ-receptor subunit.3 Hence, the role played by tyrosine kinases in this phenomenon has been examined extensively.7-9 In contrast, there is very limited information on the role of protein phosphatases in the regulation of the FcγR pathway. Herein, we present the first direct evidence that the protein tyrosine phosphatase SHP-1 negatively regulates Fcγ receptor–mediated phagocytosis and controls the phosphorylation state of CBL and the activation of the small guanosine triphosphatase (GTPase), Rac.
Mouse macrophages express 3 types of Fc gamma receptors—FcγRI, FcγRII, and FcγRIII10-13—which can bind to the Fc portion of IgG coating the surface of the foreign invaders. Of these receptors, FcγRI and FcγRIIIA are known to transmit the signals through a tyrosine phosphorylation activation motif (ITAM) contained within the γ subunit, while FcγRIIB has a tyrosine phosphorylation inhibitory motif (ITIM) in its cytoplasmic domain. Multiple isoforms of FcγRIIB are expressed in J774 cells emanating from alternative splice variants, suggesting additional complexity of this receptor in macrophage signaling.12Receptors containing ITIM can act as negative regulators of the signals initiated by receptors containing ITAM.14 FcγRIIB is involved in inhibition of cell activation by FcγR, FcγRIIA, and T-cell receptor upon coaggregation.15 In B cells coligation of B-cell antigen receptor (BCR) and FcγRIIB leads to inhibitory signaling.16 Similarly, ITIM is found in other receptor systems, such as the gp49 family of proteins on mast cells and natural killer (NK) cells, killer cell inhibitory receptor (KIR),17 and inhibitory receptor SHPS-1, abundantly expressed in neurons.18 Upon activation of these receptors the tyrosine residue in the ITIM gets phosphorylated by LYN, a Src family kinase,19 to provide a site of attachment for phosphatases such as SHP-1, SHIP,20 and SHP-2,19 which can start the negative regulatory pathway. Recently, Clynes et al used the FcγRII knockout mouse to demonstrate a role for FcγRIIB in the regulation of Fcγ receptor–mediated phagocytosis.21 These observations strongly suggest a potential role for phosphatases in FcγR signal transduction, which focused our attention on involvement of SHP-1 (also known as SH-PTP1, HCP, and PTP1C) in phagocytic signal transduction.
SHP-1 is a nontransmembrane protein involved in multiple signaling systems. It contains 2 tandem Src homology 2 (SH2) domains. The N-terminal SH2 domain serves both a regulatory and a recruiting function, while the C-terminal SH2 domain functions predominantly as a recruiting domain.22 SHP-1 plays an important role in regulating the macrophage proliferative pathway. Macrophages from motheaten viable (Mev) mice have a frameshift mutation in SHP-123 and display hyperproliferation in response to macrophage colony stimulating factor 1 (CSF-1). Studies from these mice have indicated that CSF-1 receptor and SHP-1 are phosphorylated upon growth factor stimulation and associate with each other. It has been suggested that Grb2, an adapter molecule with an SH2 domain, binds to SHP-1, possibly recruiting phosphotyrosine-containing proteins for dephosphorylation by SHP-1.24 SHP-1–mediated dephosphorylation is also involved in delivery of the Fas apoptosis signal in lymphoid cells.25 SHP-1 is positively involved in epidermal growth factor (EGF) and interferon-γ–induced signal transducer and activator of transcription (STAT) activation in HeLa cells.26 In macrophages, SHP-1 also selectively regulates the tyrosine phosphorylation of Stat1 and Jak1 while leaving Tyk2 and Stat2 unaffected.27 In contrast, Keilhack et al28 have described the binding of SHP-1 to the EGF receptor, leading to dephosphorylation of the receptor and attenuation of receptor signaling. In the motheaten mouse, FcγRIIB signaling is deficient, suggesting a role of SHP-1 in control of ITIM function.29 In contrast, Ono et al 30,31 have shown that inhibitory signaling by FcγRIIB does not require SHP-1 but involves the 5′inositol phosphatase SHIP. Data from Sharlow et al32 have indicated that SHP-1 acts as a negative regulator of erythropoietin-induced differentiation of normal erythroid progenitor cells, preventing their premature commitment to terminal differentiation. Hence it has been suggested that tyrosine phosphatases exert both positive and negative regulation of specific signals and integrate these signals temporally and spatially within the cell.
To investigate the role played by SHP-1 in phagocytic signal transduction, we overexpressed wild-type SHP-1 in J774A.1 cells, using a recombinant vaccinia virus expression system. SHP-1 overexpression led to a complete abrogation of phagocytosis of sensitized sheep red blood cells (sRBCs) by J774A.1 cells. This is the first evidence that SHP-1, a tyrosine phosphatase, regulates Fcγ receptor–mediated phagocytosis. Most notably, SHP-1 associated with the CBL adapter protein, and this association led to loss of CBL phosphorylation and the suppression of Rac. These data support a role for SHP-1 in the control of phagocytosis and suggest that CBL dephosphorylation mediates, at least in part, negative control of FcγR-dependent phagocytosis.
Materials and methods
Antibodies
Anti-CBL (SC170) and anti-CRKL antibodies (SC-319) were obtained from Santa Cruz Biotechnology, (Santa Cruz, CA). Plasmids encoding enhanced green fluorescent protein (EGFP)–tagged SYK kinase and FcγRIIA were prepared by standard subcloning methods in pEGFP and pcDNA3.1, respectively. DNA constructs encoding SHP-1, SHP-2, catalytically dead SHP-1 (C453S), or catalytically dead SHP-2 (C486S) were subcloned into the pcDNA3.1 vector. Anti-SYK antibody was provided by Dr Tamara Hurley (Salk Institute, San Diego, CA), and anti–SHP-1 antibody was generated in our laboratory. For detection of FcγRIIA expression in COS7 cells, we used an allophycocyanin (APC) conjugate anti-CD32 monoclonal antibody (FL18.26).
Cell lines and vaccinia virus expression system
J774A.1, a macrophagelike cell line, was obtained from ATCC (Manassas, VA; catalog no. 67-TIB). The cells were grown in Dulbecco Modified Eagle Medium (DMEM) containing 10% fetal calf serum (FCS). Recombinant vaccinia virus vectors were provided by Dr Bernard Moss (Bethesda, MD). The dominant-negative SYK vaccinia construct (encoding the N terminus of SYK, residues 1-255) was provided by Dr A. Scharenberg, as previously described.33Recombinant vaccinia viruses containing SHP-1 and dominant-negative SYK were prepared as described. Briefly, recombinant vaccinia virus was propagated in 149B cells grown in RPMI containing 10% FCS. A confluent culture of cells was infected with recombinant vaccinia virus at a concentration of 0.5 plaque-forming units (pfu) per cell for 48 hours. The cells were scraped in the same medium, pelleted down, and resuspended in 5 mL of 10 mM Tris-HCl pH 9. The cells were disintegrated by freezing in liquid nitrogen and thawing at 37°C 3 times, after which the volume was made up to 20 mL with 10 mM Tris-HCl pH 9 and the cells were subjected to 40 strokes in a homogenizer. Nuclei and cell debris were separated from the cell lysate by centrifugation at 1000 rpm for 5 minutes. The cell lysate containing the recombinant vaccinia virus was then subjected to sonication for 1 minute. The cell lysate was loaded on a cushion of 36% sucrose solution and centrifuged at 13 000 rpm for 80 minutes at 4°C in an ultracentrifuge (Beckman, Palo Alto, CA) using an SW.28 rotor. Viral pellet obtained at the bottom was suspended in 1 mL of 10 mM Tris-HCl pH 9 and loaded on a sucrose gradient composed of 6.6 mL each of 40%, 36%, 32%, 28%, and 24% sucrose solutions made in 10 mM Tris-HCl pH 9 to be centrifuged at 12 500 rpm for 50 minutes at 4°C in an ultracentrifuge (Beckman) using an SW.28 rotor. A bluish white ring containing purified virus was collected, diluted with 10 mM Tris-HCl pH 9, and recentrifuged at 13 000 rpm for 60 minutes at 4°C in an ultracentrifuge (Beckman) using an SW.28 rotor to pellet the virus down. Purified recombinant vaccinia virus thus obtained was suspended in 10 mM Tris-HCl pH 9 and titered as follows. An aliquot was used for making serial dilutions of the viral suspension. These were used to infect a confluent lawn of 149B cells grown in a 6-well plate for 2 hours at 37°C in 1 mL RPMI containing 10% FCS. The medium was then replaced with 3 mL of fresh RPMI containing 10% FCS. After 24 hours the medium was discarded and the plaques were visualized by staining with crystal violet to determine the titer.
Preparation of recombinant vaccinia virus containing catalytically inactive mutant
A construct (C2mSHP-1S453KT3) containing catalytically inactive SHP-1 mutant was kindly provided by Dr Taolin Yi. The insert was amplified by polymerase chain reaction (PCR), using the following 5′ and 3′ primers: CTCGTCGACAGGATGGTGAGGTGGTTTCAC and AGTCCCGGGAGATCACTTCCTCTTGAGAGAA, respectively. The amplified product was subcloned into PCR2.1 vector with a TA cloning kit (Invitrogen, San Diego, CA), isolated by using SmaI and SalI restriction digest, and ligated to the recombinant vaccinia vector pSC65. The construct C2pSC65 was used to make a recombinant vaccinia virus using packaging cell line CV1 and the wild-type vaccinia virus. Recombinant virus was purified from the wild-type virus by single-plaque purification. It was amplified, purified, and titered as described above.
Phagocytic assays
J774A.1 cells were plated at a density of 2 × 105 cells per well in a 12-well plate (Costar, Corning, NY) overnight. Cells were infected with recombinant vaccinia virus pSC65 or pSC65-SHP-1 at a density of 2 pfu/cell for 4 hours at 37°C in 5% CO2. After 4 hours the medium was changed and the cells were subjected to sRBCs coated with IgG at subagglutinating concentration. The target-to-effector ratio was kept at 100:1. The cells were scraped after 2 hours; cytospins were prepared, fixed, and stained with Wright Giemsa stain (Dade, AG, Switzerland); and the slides were observed under a microscope for rosette formation. The rest of the cells were subjected to water shock to lyse the uningested sRBCs. The cells were suspended in DMEM containing 20% FCS. The cells were spun down on glass slides and fixed and stained with Wright Giemsa stain. A minimum of 150 cells were counted for each slide and the phagocytic index was calculated as follows: phagocytic index (PI) = number of sRBCs internalized by 100 J774 cells counted in 10 random fields of slide. In the case of the inhibitors the cells were subjected to treatment with the inhibitor at different concentrations along with an appropriate dimethyl sulfoxide (DMSO) control for 1 hour in DMEM with 10% FCS before the phagocytosis assay was carried out as described previously.
β-Galactosidase and rosette formation assays
As a control for effects of recombinant vaccinia virus, J774A.1 cells were tested for equal viral load by quantitation of β-galactosidase activity measured with X-gal. The plasmid pSC65, used for cloning of dominant-negative SYK, contains the gene β-galactosidase, which cleaves X-gal to give a color product that can be quantitated colorimetrically. In every experiment, we quantitated recombinant viral load for control (cells infected with empty vector recombinant vaccinia virus) compared with experimental sample (cells infected with vaccinia virus containing dominant-negative SYK) and they were equivalent (data not shown). The capacity of J774A.1 cells to form rosettes via Fcγ receptor was not altered by vaccinia virus. Cells infected with empty vector recombinant vaccinia virus or cells infected with vaccinia virus containing dominant-negative SYK showed 100% rosette formation within 1 minute of addition of sensitized sRBCs, indicating that the surface expression of Fcγ receptors and the extracellular function of the FcγRs as it relates to binding of sRBC targets was not affected by recombinant vaccinia virus alone (data not shown). Rosette formation and phagocytosis did not occur in absence of sensitizing antibody against sRBCs, which establishes the FcγR specificity of this response. Cells equivalent to 1× 105 were suspended in 400 μL of DMEM containing 10% FCS to which 50 μL of 1% X-gal (Sigma, St Louis, MO) was added. After incubation at 37°C the color of the medium turns blue. The supernatant was diluted 1:10 and the optical density was measured at 595 nm in a spectrophotometer (Molecular Devices, Menlo Park, CA).
Overexpression of SHP-1 and dominant-negative SYK in J774A.1 cells
Cells (2 × 105) infected with different viruses were lysed with 50 μL of sample buffer. The lysates were resolved on acrylamide gels and probed for appropriate protein expression with corresponding antibodies as described above.
Heterologous COS7 cell phagocytosis system
COS7 cells were plated at 1 × 105 cells per well on a 6-well plate overnight. Cells were transfected with plasmids using lipofectamine reagent for 4 hours followed by a washing step and further incubation for 24 or 48 hours. The phagocytosis assay was carried out as described above for J774 cells. Phagocytic index = number of sRBCs internalized by 100 COS7 cells randomly sampled. During all transfections total plasmid DNA concentration and composition were equilibrated using empty vector plasmids. All transfected proteins, EGFP-SYK, FcγRIIA, SHP-1, and C2-SHP-1, were quantitated by Western blot analysis or flow cytometry. Thus it can be interpreted that the effect on phagocytic response (PR) is causally linked to SHP-1 transfection. In all experiments performed, all transfected proteins were quantitated in parallel populations of COS7 cells to ensure that the effects observed with transfection of SHP-1 vs SHP-2 were attributable to this variable and not levels of FcγRIIA or SYK kinase. The determination of conversion of guanosine diphosphate (GDP)–Rac to GTP-Rac was as described.34
Immunoprecipitation
J774A.1 cells were infected with recombinant vaccinia virus at a concentration of 2 pfu/mL for 4 hours. The cells were then collected and suspended at a concentration of 2 ×106 cells per mL of DMEM and stimulated with IgG-coated sRBCs at 37°C for 5 minutes. The samples were centrifuged at 500g in a refrigerated centrifuge and the supernatant was aspirated. The cell pellet was used for immunoprecipitation as described earlier.35
Results
Dominant-negative SYK inhibits phagocytosis
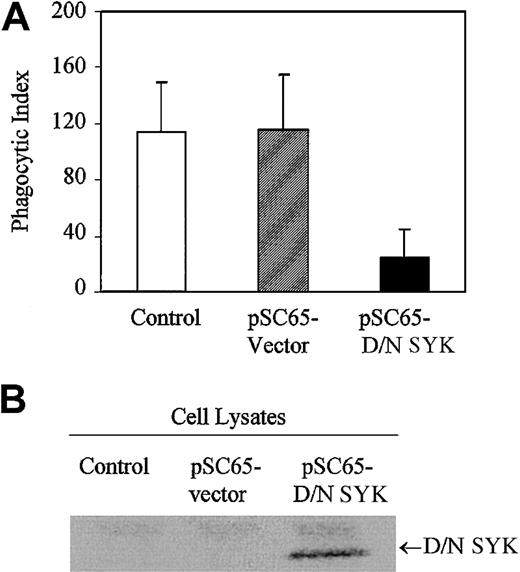
In order to investigate the role played by nonreceptor tyrosine kinase SYK in IgG-mediated phagocytosis, we expressed the dominant-negative form of SYK in J774A.1 cells with recombinant vaccinia virus. The SYK mutant encodes a truncated form of SYK containing only the tandem SH2 domains with no catalytic domain and is expected to dock with the ITAM, thereby preventing the endogenous catalytically active SYK kinase from interacting with the FcγRγ subunit. Expression of dominant-negative SYK in J774A.1 cells strongly inhibits phagocytosis of IgG-coated sRBCs (Figure1A). Normal phagocytosis occurs in J774A.1 cells infected with empty vector recombinant vaccinia virus. In comparison, J774A.1 cells infected with vaccinia virus containing dominant-negative SYK exhibit minimal phagocytosis. Dominant-negative SYK expression is confirmed by Western blot (Figure 1B, lane 3). The data we obtained using dominant-negative SYK are consistent with other data in the literature, including data from SYK knockout mice,8 strongly supporting a role for SYK in propagating signals required for IgG-mediated phagocytosis in this J774 system.
Dominant-negative SYK inhibits phagocytosis.
(A) Phagocytosis of IgG-sensitized sRBCs by noninfected J774A.1 cells (control), cells infected with recombinant vaccinia virus containing empty vector (pSC65-vector), or cells infected with dominant-negative SYK (pSC65-D/N SYK). The cells were infected with vaccinia viruses or empty vector for 4 hours at 37°C with 5% CO2, after which they were subjected to IgG-sensitized sRBCs in fresh medium at a target-to-effector ratio equal to 100:1 for 2 hours at 37°C. Nonengulfed sRBCs were lysed by water shock and the cells were fixed and stained with Wright Giemsa staining before the phagocytic index was counted. Columns indicate phagocytic index of J774A.1 (mean ± SD). (B) Western blot analysis shows expression of dominant-negative SYK in J774A.1 cells infected with different recombinant vaccinia viruses: lane 1, no vector; lane 2, empty vector; lane 3, dominant-negative SYK.
Dominant-negative SYK inhibits phagocytosis.
(A) Phagocytosis of IgG-sensitized sRBCs by noninfected J774A.1 cells (control), cells infected with recombinant vaccinia virus containing empty vector (pSC65-vector), or cells infected with dominant-negative SYK (pSC65-D/N SYK). The cells were infected with vaccinia viruses or empty vector for 4 hours at 37°C with 5% CO2, after which they were subjected to IgG-sensitized sRBCs in fresh medium at a target-to-effector ratio equal to 100:1 for 2 hours at 37°C. Nonengulfed sRBCs were lysed by water shock and the cells were fixed and stained with Wright Giemsa staining before the phagocytic index was counted. Columns indicate phagocytic index of J774A.1 (mean ± SD). (B) Western blot analysis shows expression of dominant-negative SYK in J774A.1 cells infected with different recombinant vaccinia viruses: lane 1, no vector; lane 2, empty vector; lane 3, dominant-negative SYK.
Src and phosphatidyl inositol-3 (PI-3) kinase are required for phagocytosis of IgG coated sRBCs by J774A.1 cells
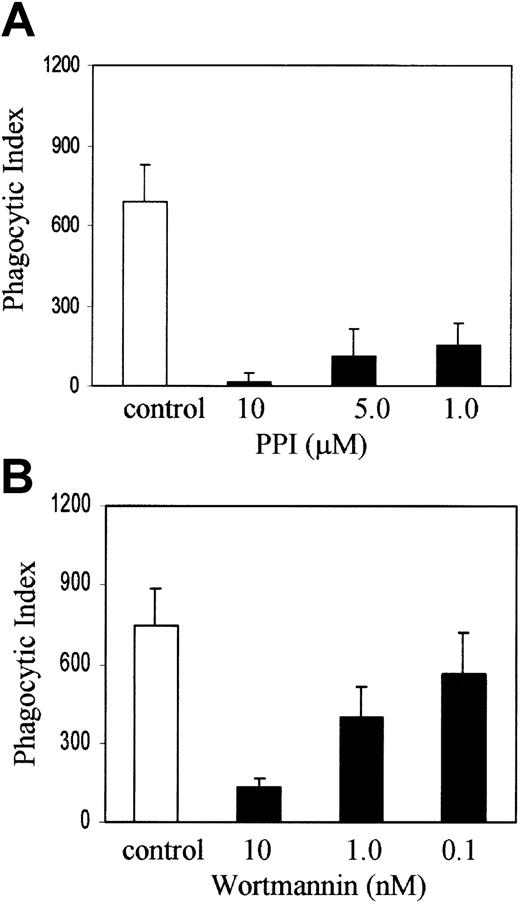
Recent evidence from HCK/LYN/FGR knockout mice suggests that members of the Src family of nonreceptor protein tyrosine kinases are upstream of SYK and PI-3 kinase in myeloid ITAM signaling.8 To examine the role of Src family kinases and PI-3 kinase in FcγR phagocytosis in our system, we treated J774 cells with PP1 (Calbiochem, La Jolla, CA), an inhibitor of Src family tyrosine kinases at 10, 5, and 1 μM concentration, or wortmannin, an inhibitor of PI-3 kinase at a concentration of 10, 1, and 0.1 nM, with appropriate DMSO controls for 1 hour in DMEM with 10% FCS and then added sensitized sRBCs at a target-to-effector ratio equal to 100:1. Figure 2A shows that PP1 abrogates phagocytosis at 10 and 1 μM concentration and the effect is dose dependent. Figure 2B demonstrates that wortmannin blocks phagocytosis significantly at 10 and 1 nM concentrations. These observations are consistent with other reports in the literature, from studies performed in other cell lines, which strongly suggest that Src family kinase activity and PI-3 kinase are required for phagocytosis of IgG-coated sRBCs by J774A.1 cells.
SRC and PI-3 kinases are required for Fcγ receptor–mediated phagocytosis.
J774A.1 cells were treated with (A) PP1 or (B) wortmannin at the indicated concentrations along with an appropriate DMSO control for 1 hour in DMEM with 10% FCS and then IgG-sensitized sRBCs were added at a target-to-effector ratio equal to 100:1. Columns indicate phagocytic index of J774A.1 cells treated with DMSO (control), PP1, or wortmannin (mean ± SD).
SRC and PI-3 kinases are required for Fcγ receptor–mediated phagocytosis.
J774A.1 cells were treated with (A) PP1 or (B) wortmannin at the indicated concentrations along with an appropriate DMSO control for 1 hour in DMEM with 10% FCS and then IgG-sensitized sRBCs were added at a target-to-effector ratio equal to 100:1. Columns indicate phagocytic index of J774A.1 cells treated with DMSO (control), PP1, or wortmannin (mean ± SD).
Dominant-negative SYK and PP1 inhibit Fcγ-induced CBL phosphorylation
Previous reports from our laboratory and others have demonstrated that Fcγ-receptor cross-linking induces the tyrosine phosphorylation of the complex adapter protein CBL.36 37 These observations prompted us to determine whether a phagocytic signaling event would induce the phosphorylation of CBL. To further understand the role of specific kinases in this phosphorylation event, we used dominant-negative SYK and a Src family kinase inhibitor, PP1, to determine a role for these kinases in CBL tyrosine phosphorylation.
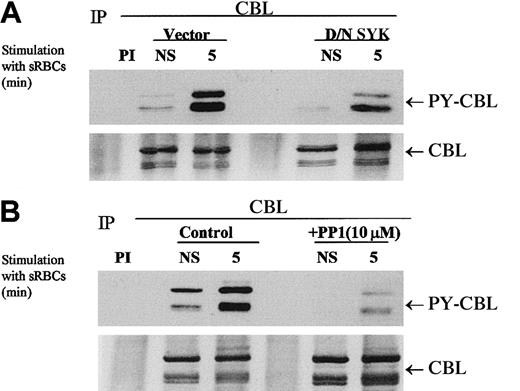
The expression of dominant-negative SYK kinase representing the N-terminal SH2 domains completely abrogates the phagocytic response but has a modest effect on the tyrosine phosphorylation status of CBL in vivo (Figure 3A). We demonstrated that CBL tyrosine phosphorylation is induced under conditions of phagocytosis and that PP1 abrogates the tyrosine phosphorylation of CBL (Figure 3B). This effect is dose dependent (data not shown), as is the effect of PP1 on Fcγ-receptor phagocytosis (Figure 2A). Interestingly, dominant-negative SYK inhibited CBL tyrosine phosphorylation to a lesser extent but completely abrogated the phagocytic response. Both PP1 and D/N SYK suppressed the basal tyrosine phosphorylation state of CBL in vivo. These data suggest that both the Src family kinase catalytic activity and the capacity of SYK to dock with the ITAM receptor are required for the induction of the CBL phosphorylation in response to stimulation with sensitized sRBCs and that these 2 events are required for phagocytosis in vivo. The dominant-negative SYK would not be expected to alter the upstream activity of SRC kinases, hence SRC-mediated phosphorylation of CBL is not altered to the same extent. The data are consistent with the model that SRC and SYK are both involved in FcγR-mediated phagocytosis, likely mediated by the downstream activation of PI-3 kinase (Figure2B), and set the stage for a biochemical analysis of the negative regulation of this ITAM receptor by the intracellular phosphatase SHP-1. Hence, we conclude that the SRC/SYK/CBL signaling axis is a likely target for protein tyrosine phosphatase (PTPase) action as it relates to the negative regulation of phagocytosis.
Effect of dominant-negative SYK and PP1 on tyrosine phosphorylation of CBL in response to stimulation with IgG-sensitized sRBCs.
(A-B) Western blot analysis of CBL immunoprecipitates to assay the phosphorylation of CBL following treatment of IgG-sensitized sRBCs in J774A.1 cells expressed by dominant-negative SYK, treated with Src family kinase inhibitor, PP1 (10 μM). J774A.1 lysates prepared from resting cells or cells stimulated with sensitized sRBCs for 5 minutes were immunoprecipitated with polyclonal anti-CBL antibody and immunoblotted with monoclonal antiphosphotyrosine antibody to determine phosphorylation of CBL or immunoblotted with polyclonal anti-CBL antibody to determine total protein amounts of CBL under the same nitrocellulose membrane.
Effect of dominant-negative SYK and PP1 on tyrosine phosphorylation of CBL in response to stimulation with IgG-sensitized sRBCs.
(A-B) Western blot analysis of CBL immunoprecipitates to assay the phosphorylation of CBL following treatment of IgG-sensitized sRBCs in J774A.1 cells expressed by dominant-negative SYK, treated with Src family kinase inhibitor, PP1 (10 μM). J774A.1 lysates prepared from resting cells or cells stimulated with sensitized sRBCs for 5 minutes were immunoprecipitated with polyclonal anti-CBL antibody and immunoblotted with monoclonal antiphosphotyrosine antibody to determine phosphorylation of CBL or immunoblotted with polyclonal anti-CBL antibody to determine total protein amounts of CBL under the same nitrocellulose membrane.
Overexpression of SHP-1 in J774A.1 cells results in the dephosphorylation of CBL and inhibits phagocytosis
The data presented here as well as in other published work clearly demonstrate a requirement for tyrosine kinases in phagocytosis1-4 7-9 (Figures 1 and 2). These findings therefore also suggest that dephosphorylation of specific sites of tyrosine phosphorylation may negatively regulate this response. To begin to address this question we overexpressed SHP-1 in J774A.1 cells. Phagocytosis of sensitized sRBCs was unaltered in cells infected with empty vector recombinant vaccinia virus or vaccinia expressing a catalytically inactive SHP-1 protein (C2, Figure4). In contrast, overexpression of wild-type SHP-1 in J774 cells markedly inhibited phagocytosis of IgG-coated sRBCs (Figure 4, left lower panel). Figure 4A shows phagocytosis of sensitized sRBCs by J774A.1 infected with empty vector recombinant vaccinia virus, vaccinia virus containing catalytically dead SHP-1 C2 mutant, or vaccinia virus expressing wild-type SHP-1. Quantitation of the phagocytic index in the different groups is shown in Figure 4B and Western blot analysis for expression of SHP-1 and catalytically dead SHP-1 mutant in these cells is shown in Figure 4C. As a control for effects of recombinant vaccinia virus, J774A.1 cells were exposed to an equal multiplicity of infection (MOI) of virus and then quantitated β-galactosidase activity was measured with X-gal. There was no effect of SHP-1 or other protein constructs on the capacity of cells to form rosettes (data not shown), an assay that assesses the capacity of J774 cells to form sRBC-J774 cell conjugates. These findings indicate that SHP-1 negatively regulates intracellular events required for the IgG-mediated phagocytic response in macrophages, and the catalytic activity of SHP-1 is required for this suppression.
Overexpression of SHP-1 in J774A.1 cells inhibits phagocytosis.
(A) Composite photomicrographs of Wright Giemsa–stained J774 cells undergoing phagocytosis of sRBCs. Original magnification, × 100. We show noninfected J774 A.1 cells (control); cells infected with recombinant vaccinia virus containing empty vector (pSC65-vector); J774 cells infected with recombinant vaccinia virus encoding wild-type SHP-1 (pSC65-SHP-1); cells containing catalytically dead SHP-1 mutant (pSC65-C2). The cells were infected with vaccinia viruses or empty vectorfor 4 hours at 37°C with 5% CO2, after which they were subjected to IgG-sensitized sRBCs in fresh medium at a target-to-effector ratio equal to 100:1 for 2 hours at 37°C. (B) Quantitation of phagocytosis of IgG-sensitized sRBCs by J774A.1 cells. Columns indicate phagocytic index of J774A.1 cells (mean ± SD). (C) Western blot analysis shows expression of SHP-1 protein in J774A.1 cells infected with recombinant vaccinia virus: lane 1, no vector; lane 2, empty vector; lane 3, catalytically dead C2-SHP-1 mutant; lane 4, wild-type SHP-1 .
Overexpression of SHP-1 in J774A.1 cells inhibits phagocytosis.
(A) Composite photomicrographs of Wright Giemsa–stained J774 cells undergoing phagocytosis of sRBCs. Original magnification, × 100. We show noninfected J774 A.1 cells (control); cells infected with recombinant vaccinia virus containing empty vector (pSC65-vector); J774 cells infected with recombinant vaccinia virus encoding wild-type SHP-1 (pSC65-SHP-1); cells containing catalytically dead SHP-1 mutant (pSC65-C2). The cells were infected with vaccinia viruses or empty vectorfor 4 hours at 37°C with 5% CO2, after which they were subjected to IgG-sensitized sRBCs in fresh medium at a target-to-effector ratio equal to 100:1 for 2 hours at 37°C. (B) Quantitation of phagocytosis of IgG-sensitized sRBCs by J774A.1 cells. Columns indicate phagocytic index of J774A.1 cells (mean ± SD). (C) Western blot analysis shows expression of SHP-1 protein in J774A.1 cells infected with recombinant vaccinia virus: lane 1, no vector; lane 2, empty vector; lane 3, catalytically dead C2-SHP-1 mutant; lane 4, wild-type SHP-1 .
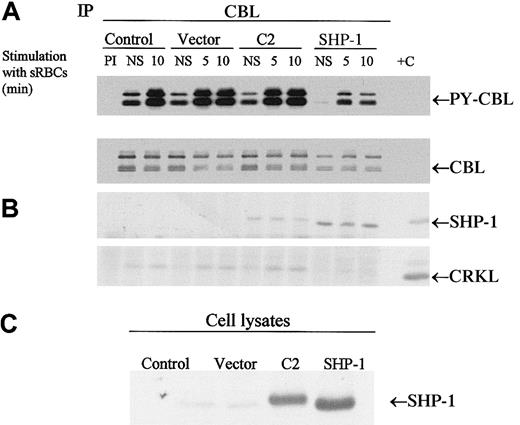
To determine whether the CBL adapter protein is a potential substrate for SHP-1 in vivo, we expressed SHP-1 or the C2 mutant of SHP-1 in J774 cells and then stimulated the cells with sensitized sRBCs followed by immunoprecipitation of CBL. Both the wild-type and mutant SHP-1 coimmunoprecipitated with CBL. Most notably, only the expression of wild-type SHP-1 resulted in the dephosphorylation of CBL in vivo (Figure 5). We next evaluated whether changes in CBL phosphorylation led to alterations in downstream CBL-dependent signaling events, including the formation of the CBL-CRKL signaling complex. The CBL-CRKL interaction has been implicated previously in ITAM and Fcγ receptor signaling.38 In the presence of SHP-1 (but not C2 SHP-1), phosphorylation decreased markedly and the interaction of CBL-CRKL was abrogated (Figure 5). Upon dephosphorylation of CBL, the CBL-CRKL adapter protein interaction is lost. The expression of C2 (catalytically dead SHP-1) is noted to associate with CBL to a lesser extent and not to induce the dephosphorylation of CBL or to disengage the CBL-CRKL interaction in vivo. From these data we conclude that CBL is an in vivo substrate for SHP-1 under conditions of Fcγ-receptor engagement in myeloid cells and that dephosphorylation of CBL alters the generation of phospho-CBL-dependent downstream signaling complexes in vivo.
CBL is a substrate for SHP-1.
(A-B) Western blot analysis of CBL immunoprecipitates to assay the tyrosine phosphorylation of CBL and to determine protein-protein interactions between CBL and SHP-1 or CRKL following treatment of IgG-sensitized sRBCs in J774A.1 cells expressed by wild-type SHP-1 and catalytically dead mutant SHP-1. J774A.1 lysates prepared from resting cells or cells stimulated with sensitized sRBCs for 5 and 10 minutes were immunoprecipitated with polyclonal anti-CBL antibody and immunoblotted with antiphosphotyrosine antibody (PY-CBL blot), anti-CBL antibody (CBL blot), anti–SHP-1 antibody (SHP-1 blot), and anti-CRKL antibody (CRKL blot). (C) Western blot analysis shows expression of SHP-1 proteins in J774A.1 cells infected with recombinant vaccinia virus: lane 1, no vector; lane 2, empty vector; lane 3, catalytically dead SHP-1 mutant; lane 4, wild-type SHP-1 phosphatase.
CBL is a substrate for SHP-1.
(A-B) Western blot analysis of CBL immunoprecipitates to assay the tyrosine phosphorylation of CBL and to determine protein-protein interactions between CBL and SHP-1 or CRKL following treatment of IgG-sensitized sRBCs in J774A.1 cells expressed by wild-type SHP-1 and catalytically dead mutant SHP-1. J774A.1 lysates prepared from resting cells or cells stimulated with sensitized sRBCs for 5 and 10 minutes were immunoprecipitated with polyclonal anti-CBL antibody and immunoblotted with antiphosphotyrosine antibody (PY-CBL blot), anti-CBL antibody (CBL blot), anti–SHP-1 antibody (SHP-1 blot), and anti-CRKL antibody (CRKL blot). (C) Western blot analysis shows expression of SHP-1 proteins in J774A.1 cells infected with recombinant vaccinia virus: lane 1, no vector; lane 2, empty vector; lane 3, catalytically dead SHP-1 mutant; lane 4, wild-type SHP-1 phosphatase.
Specificity of SHP-1 in control of phagocytosis
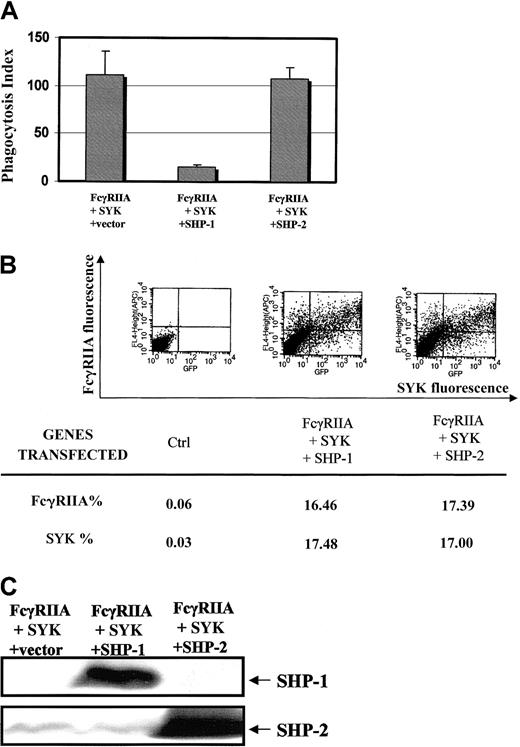
To begin to address the specificity of SHP-1 in these signaling events, we compared the effects of SHP-1 transfection and SHP-2 transfection on FcγRIIA phagocytosis (Figure6A). A heterologous COS7 cell system reconstituted with the FcγRIIA receptor and SYK kinase was used to ask the question, Is the effect of SHP-1 on Fcγ receptor ITAM signaling specific?34 Equivalent levels of FcγRIIA and EGFP-SYK were confirmed in COS7 cell transfectants (Figure 6B) and levels of SHP-1 and SHP-2 expression were quantitated in cells analyzed for phagocytosis and by flow cytometry (Figure 6C). The data clearly suggest that SHP-2 has no significant effect on the FcγRIIA-induced phagocytic response. This result provides evidence that the effect of SHP-1 on ITAM signaling seen in our study is specific for this blood-specific phosphatase.
Specificity for SHP-1 effect on ITAM signaling.
(A) Effect of SHP-1 vs SHP-2 on FcγRIIA-induced phagocytosis using a COS7 cell heterologous system.34 Bars represent SD. COS7 cells were transiently transfected with FcγRIIA and EGFP-tagged SYK kinase in the presence of SHP-1 or SHP-2. Phagocytic index was determined as defined in “Materials and methods.” (B) Flow cytometry is used to document that cotransfection conditions result in equal amounts of EGFP-tagged SYK kinase and FcγRIIA expression in all transfectant populations. (C) Western blot analysis of SHP-1 or SHP-2 expression in COS7 cells evaluated in panels A and B. We performed immunoblot analysis of all COS7 cell transfectants used in the above analysis of phagocytosis.
Specificity for SHP-1 effect on ITAM signaling.
(A) Effect of SHP-1 vs SHP-2 on FcγRIIA-induced phagocytosis using a COS7 cell heterologous system.34 Bars represent SD. COS7 cells were transiently transfected with FcγRIIA and EGFP-tagged SYK kinase in the presence of SHP-1 or SHP-2. Phagocytic index was determined as defined in “Materials and methods.” (B) Flow cytometry is used to document that cotransfection conditions result in equal amounts of EGFP-tagged SYK kinase and FcγRIIA expression in all transfectant populations. (C) Western blot analysis of SHP-1 or SHP-2 expression in COS7 cells evaluated in panels A and B. We performed immunoblot analysis of all COS7 cell transfectants used in the above analysis of phagocytosis.
SHP-1 regulates RAC activation in response to FcγRIIA engagement
It is clear that small GTPases of the Rho family play an important role in the modulation of the actin-cytoskeletal network of proteins required for Fcγ receptor–mediated phagocytosis. The small GTPase Rac must be converted from a GDP-bound state to GTP-Rac in order to activate downstream effectors required for cytoskeletal reorganization and polymerization of actin. Data from a Rac2 knockout model developed in our laboratory39 provide direct evidence that Rac2 is required for macrophage-mediated phagocytosis (D.L.D., unpublished observation, August 2001). Prior reports have also implicated Rac in FcγR-mediated phagocytosis.5 To provide further biochemical evidence for SHP-1 in the regulation of phagocytosis, we determined the effect of SHP-1 on the FcγRIIA-induced conversion of GDP-Rac to GTP-Rac (Figure7). Using the heterologous COS7 cell system, we observed that SHP-1 and not the C2 mutant of SHP-1 blocked FcγRIIA phagocytosis (Figure 4). SHP-1 overexpression suppressed the FcγRIIA-induced conversion of GDP-Rac to GTP-Rac (Figure 7; compare lanes 3 and 6). In the absence of FcγRIIA or SYK, Rac is not activated (Figure 7, lanes 7-9). The transfection of SHP-2 had no effect on phagocytosis (Figure 6A), Rac activation (data not shown), or CBL phosphorylation state (Figure 8). These data are internally consistent with the observed effect of SHP-1 on CBL phosphorylation and the CBL-CRKL interaction and provide confirmatory evidence that SHP-1 regulates this signaling axis required for ITAM signaling.
SHP-1 regulates Rac.
We used a heterologous COS-7 cell system and p21 activated kinase (PAK) binding domain pull-down assay to evaluate ITAM receptor–induced activation of Rac34 in the presence or absence of SHP-1 cotransfection. The transfection condition for each group is shown above the lanes. Lanes 1, 4, and 7 show no stimulation (NS); lanes 2, 5, and 8, sRBC stimulation for 1 minute at 37°C; lanes 3, 6, and 9, stimulation of transfected COS7 cells for 5 minutes with sRBC. Lane 10 shows a positive control for GTP-Rac, a COS7 cell lysate incubated with GTPγS. (A) SHP-1 blocks FcγRIIA-induced conversion of GDP-Rac to its GTP-bound state. Western blot was performed with anti-Rac1 antiserum on glutathione-S transferase PAK binding domain (GST-PBD) fusion protein pull-down to detect levels of GTP-Rac1 in COS7 cell lysates following sRBC stimulation. (B) Anti–SHP-1 Western blot analysis of cell lysates shown in panel A. Lane 1, no transfection; lane 2, transfection with FcγRIIA and EGFP-SYK; lane 3, transfected with empty vector plasmids; lane 4, transfected with FcγRIIA, EGFP-SYK, and SHP-1.
SHP-1 regulates Rac.
We used a heterologous COS-7 cell system and p21 activated kinase (PAK) binding domain pull-down assay to evaluate ITAM receptor–induced activation of Rac34 in the presence or absence of SHP-1 cotransfection. The transfection condition for each group is shown above the lanes. Lanes 1, 4, and 7 show no stimulation (NS); lanes 2, 5, and 8, sRBC stimulation for 1 minute at 37°C; lanes 3, 6, and 9, stimulation of transfected COS7 cells for 5 minutes with sRBC. Lane 10 shows a positive control for GTP-Rac, a COS7 cell lysate incubated with GTPγS. (A) SHP-1 blocks FcγRIIA-induced conversion of GDP-Rac to its GTP-bound state. Western blot was performed with anti-Rac1 antiserum on glutathione-S transferase PAK binding domain (GST-PBD) fusion protein pull-down to detect levels of GTP-Rac1 in COS7 cell lysates following sRBC stimulation. (B) Anti–SHP-1 Western blot analysis of cell lysates shown in panel A. Lane 1, no transfection; lane 2, transfection with FcγRIIA and EGFP-SYK; lane 3, transfected with empty vector plasmids; lane 4, transfected with FcγRIIA, EGFP-SYK, and SHP-1.
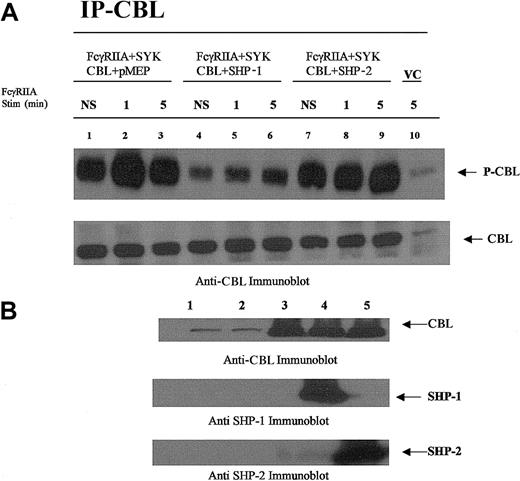
CBL is dephosphorylated by SHP-1 and not SHP-2.
CBL immunoprecipitated from COS7 cells transfected with plasmids encoding the expression of FcγRIIA, CBL, SYK, SHP-1, or SHP-2 followed by FcγRIIA stimulation. (A) CBL immunoprecipitated from transfected COS7 cells was resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with antiphosphotyrosine-specific antibodies (upper panel) and anti-CBL antisera (lower panel). Plasmid transfection conditions are shown above lanes. Transfected COS7 cells were either not stimulated (NS) or stimulated with IgG-opsonized sRBCs for times indicated. VC indicates cell lysates prepared from empty vector–transfected COS7 cells. (B) Anti-CBL, anti–SHP-1, and –SHP-2 immunoblot analysis of lysates prepared from resting COS7 cells shown in panel A. Lane 1, no transfection; lane 2, empty vector–transfected cells (control); lane 3, CBL transfected in the absence of SHP-1 or SHP-2; lane 4, CBL cotransfected with SHP-1; lane 5, CBL cotransfected with SHP-2.
CBL is dephosphorylated by SHP-1 and not SHP-2.
CBL immunoprecipitated from COS7 cells transfected with plasmids encoding the expression of FcγRIIA, CBL, SYK, SHP-1, or SHP-2 followed by FcγRIIA stimulation. (A) CBL immunoprecipitated from transfected COS7 cells was resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with antiphosphotyrosine-specific antibodies (upper panel) and anti-CBL antisera (lower panel). Plasmid transfection conditions are shown above lanes. Transfected COS7 cells were either not stimulated (NS) or stimulated with IgG-opsonized sRBCs for times indicated. VC indicates cell lysates prepared from empty vector–transfected COS7 cells. (B) Anti-CBL, anti–SHP-1, and –SHP-2 immunoblot analysis of lysates prepared from resting COS7 cells shown in panel A. Lane 1, no transfection; lane 2, empty vector–transfected cells (control); lane 3, CBL transfected in the absence of SHP-1 or SHP-2; lane 4, CBL cotransfected with SHP-1; lane 5, CBL cotransfected with SHP-2.
Discussion
Because of overlapping binding affinities, Fcγ receptors must function in concert during the process of phagocytosis. During Fcγ receptor–mediated phagocytosis by macrophages, all the 3 types of Fcγ receptors are cocrosslinked by the Fc portion of IgG coating the surface of the foreign invaders. Of these receptors, FcγRI and FcγRIII are involved in activation of nonreceptor tyrosine kinases such as HCK, LYN, and SYK. In contrast, FcγRIIB has an ITIM in its cytoplasmic domain, which is known to play an inhibitory role during signal transduction by virtue of its association with protein and lipid phosphatases such as SHP-1 and SHIP. These phosphatases are recruited directly to the signalsomes generated by activating receptors. In this work we have focused on the potential role for one key protein tyrosine phosphatase, SHP-1, in the complex process of phagocytic signal transduction.
Activation of macrophages through Fcγ receptors leads to activation of protein tyrosine kinases from the Src and SYK families.1,3 SYK, with its 2 amino terminal SH2 domains, becomes associated with phosphorylated ITAM present in the signaling subunit of the activated Fc receptors.3,4 Consistent with this model, immunofluorescence studies have demonstrated translocation of SYK to regions where FcγR-mediated phagocytosis40 and the essential role of SYK in phagocytosis have been demonstrated by failure of SYK-deficient macrophages to engulf IgG-coated particles.8 These macrophages exhibited normal response to complement and lipopolysaccharide. Furthermore, chimeric transmembrane Fc receptors bearing SYK tyrosine kinase domains can autonomously trigger phagocytosis and redistribution of filamentous actin in COS cells.9 SYK is not required for actin polymerization but is involved in closure of the phagosome.8 To determine the role of SYK J774A.1 cells, we overexpressed a construct containing the 2 SH2 domains of SYK (Figure 1A). This SYK mutant acts as a dominant-negative mutant by blocking the signal mediated by endogenous SYK and ITAM. Expression of dominant-negative SYK abrogated phagocytosis of sensitized sRBCs. Our results support the role of SYK and SRC tyrosine kinases in positive modulation of Fcγ receptor–mediated phagocytosis.
In order to determine the role played by Src family kinases in phagocytosis, we treated J774A.1 cells with PP1,41 a selective inhibitor of Src kinases, before and during stimulation with sensitized sRBCs (Figure 2A). The data demonstrated that PP1 abrogates the Fcγ receptor–mediated phagocytosis and CBL tyrosine phosphorylation in a concentration-dependent manner. Crowley et al8 have shown that macrophages derived from mice deficient for HCK, FGR, and LYN exhibit a delay in the Fcγ receptor–mediated phagocytosis as compared with the inhibition of the phenomenon with PP1 treatment as observed by us. Other workers42 have implicated Hck in the process of degranulation related to phagocytosis, as HCK translocates toward the phagosome from secretory granules during neutrophil activation. Our results strongly suggest the involvement of Src family kinases in the tyrosine phosphorylation of CBL, an event that is essential for the formation of the ITAM/SYK/CBL complex to initiate the phagocytic response. Our current hypothesis is that SYK and/or SRC mediates the tyrosine phosphorylation of CBL at positions 774 and 731, which is essential for the recruitment of CRKL and the p85 subunit of PI-3 kinase, respectively, to the receptor complex. Preliminary data from our laboratory obtained by using a CBL (Y731F) mutant strongly supports this model (P.D., D.L.D., unpublished observation, June 2001). We are currently using this model to implicate specific substrates for SRC and SYK family kinases involved in phagocytosis.
PI-3 kinase has also been clearly implicated in Fc receptor–dependent signaling. Stimulation of Fc receptors leads to the association of PI-3 kinase with the receptor complexes43and to an increase in its kinase activity.44 Araki et al45 reported that wortmannin, a potent inhibitor of PI 3-kinase, allowed formation of pseudopodia around the sRBCs but prevented the closure of phagosomes around the sheep erythrocytes in macrophages. Chimeric receptors composed of FcγR extracellular and transmembrane domains fused to p85 subunit of PI-3 kinase, when transfected into COS cells, are sufficient to trigger the process of phagocytosis upon activation. In our system, wortmannin inhibited the FcγR-mediated phagocytosis in a concentration-dependent manner, supporting a role for PI-3 kinase in J774 phagocytosis (Figure 2B). More recent data from our laboratory have directly addressed a specific role for PI-3 kinase in Fcγ receptor–mediated phagocytosis.34
The Fc receptor–dependent signaling events downstream of tyrosine kinases are predicted to involve recruitment of a platform of adapter proteins, nucleotide exchange proteins, and GTPases. Results from these experiments (Figure 3A-D) indicate that CBL is tyrosine-phosphorylated in J774A.1 cells in response to stimulation with sensitized sRBCs. This event is significantly inhibited with PP1, an inhibitor of Src family kinases (Figure 3A, compare lanes 3 and 6), and to a lesser extent by dominant-negative SYK (Figure 3C), in parallel to the inhibition of phagocytosis by these agents (Figures 1A,2A). This suggest a potential role for CBL phosphorylation in phagocytosis. CBL is phosphorylated on stimulation of FcγR in myeloid cells.36,37,46,47Notably, by virtue of its association with adapter proteins such as CRKL38 and Grb2,36 it can mediate the signal downstream to nucleotide exchange factors such as C3G and Sos and then to GTPases such as RAP, RAS, and RAC. There is also significant evidence that48 CBL is a negative regulator for SYK. These observations suggest that CBL can act as a central molecule to control traffic along the FcγR signaling pathway. Recent data from Sato et al49 have implicated CBL in the control of phagocytosis in myeloid cells in a PI-3 kinase–dependent manner. Our data are consistent with results of Beitz et al,50 indicating that dominant-negative SYK blocks CBL tyrosine phosphorylation in B cells, altering the CBL-p85 interaction and PI-3 kinase activation. Importantly, many of these regulatory interactions involve phosphotyrosine-dependent interactions between CBL and other effectors of signal relay (eg, PI-3 kinase binding to residue Y731 in CBL), raising the idea that tyrosine phosphatases may regulate the interaction between SYK, CBL, and PI-3 kinase via the p85 regulatory subunit. Because CBL regulatory interactions involve phosphotyrosine-dependent interactions between CBL and other effectors of signal relay (eg, CRKL, PI-3 kinase binding to residue Y731 in CBL), these data suggest that tyrosine phosphatases may directly regulate the interaction between CBL and its associated effectors.
To begin to address this possibility, we evaluated the potential consequences of overexpression of SHP-1 in the J774A.1 macrophage cell line (Figure 4A-D). Strikingly, overexpression of SHP-1 led to abrogation of phagocytosis, association of SHP-1 with CBL, dephosphorylation of CBL, and abrogation of CBL-CRKL interaction only in cells expressing catalytically active SHP-1. In contrast, the phosphatase SHP-2 has no effect on ITAM-induced phagocytosis (Figure6A) and was not associated with the dephosphorylation of CBL (Figure8). These findings clearly indicate a selective inhibitory role for SHP-1 in the regulation of IgG-mediated phagocytosis separate from SHP-2 function.
There are very few reports describing putative substrates for SHP-1. Recently Brockdorff et al51 have proposed that activated SHP-1 is involved in dephosphorylation of Zap-70 and SYK and in subsequent inhibition of T-cell receptor signaling. Earlier reports from our laboratory52 have shown the involvement of SLP-76 in FcγRI–mediated signal transduction. To date, our data suggest that SLP-76 is not a major substrate for SHP-1 in this system. In contrast, our findings indicate that CBL is a key substrate for SHP-1 and that dephosphorylation of CBL abrogates the CBL-CRKL interaction. The CBL-CRKL interaction is mediated through YxxP motifs within the CBL C terminus at tyrosine 774.38 The data suggest that SHP-1 targets the tyrosine at position 774 for dephosphorylation under conditions following receptor activation, resulting in loss of CBL-CRKL interaction. This observation is consistent with the observation that SHP-1 abrogates the ITAM-induced activation of Rac, a biochemical event that has been implicated in control of actin polymerization events and phagocytosis.5 Moreover, our results establish a specificity for SHP-1 in the regulation of Fcγ receptor ITAM signaling, in that SHP-2 does not suppress signaling through the myeloid ITAM signalsome. The SHP-2 phosphatase has been confirmed to play a role in gp130 cytokine receptor (eg, IL-6 receptor) signaling.53 Moreover, these data suggest a specific role for SHP-1 (vs SHP-2) in the regulation of the small GTPase Rac and strengthens the biochemical link between SHP-1 and ITAM signal relay. The data argue for a divergence of phosphatase action between ITAM receptors and other receptors such as gp130 linked receptor cytokine signaling that involves the SHP-2 phosphatase.
Taken together, our data are consistent with the model that during Fcγ receptor–mediated phagocytosis, FcγRI and FcγRIII receptors use ITAM, SYK, Src family kinases, and PI-3-kinase to generate activating signals that mediate phagocytosis. SHP-1 constitutes a negative feedback loop for this response, mediated either through FcγRII or through direct recruitment to the signalsome. These data provide the first direct evidence that SHP-1 negatively regulates phagocytosis, which it accomplishes at least in part by altering the phosphorylation state of CBL and by inhibiting conversion of GDP-Rac to its activated GTP-bound state.
We would like to thank Drs Bernard Moss, Andrew Scharenberg, J. P. Kinet, Rebecca Chan, and G. S. Feng for reagents provided, and Dr Robert C. Seeger for his considerable support of A.M.K. during the performance of this work.
Supported by a grant from the American Cancer Society (RPG-98-244- 01-LBC) to D.L.D. A.M.K. is a recipient of the Childrens Hospital Los Angeles Career Development Fellowship. D.J.R is the recipient of a McDonnell Scholar Award, a Leukemia and Lymphoma Society Scholar Award, and the Joan J. Drake Grant for Excellence in Cancer Research. This work was supported in part by National Institutes of Health grants HD37091and CA81140 and by the American Cancer Society.
A.M.K. and P.D. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Donald L. Durden, Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, 1044 W Walnut St, Rm 468, Indianapolis, IN 46202; e-mail: ddurden@iupui.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal