In vitro studies as well as clinical trials indicate that the cytokines granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) enhance the ability of neutrophils (polymorphonuclear leukocytes) to eliminate microbial organisms. Toll-like receptor (TLR) proteins, homologs of the Drosophila protein Toll, have been found on the surface of mammalian cells and are important in the responses of macrophages to bacterial, viral, and fungal antigens. TLR4 is critical for the response to lipopolysaccharide (LPS) of gram-negative bacteria, while TLR2 is important for response to gram-positive bacteria, bacterial peptides, and yeast zymosan. We demonstrate that TLR2, but very little TLR4, is present on the surface of human neutrophils. In addition we demonstrate that GM-CSF and G-CSF dramatically up-regulate TLR2 and CD14 surface expression. GM-CSF treatment also up-regulates TLR2 and CD14 mRNA levels in neutrophils. In addition to increasing receptor expression, GM-CSF treatment enhanced the interleukin 8 (IL-8) secretion and superoxide priming responses of neutrophils to stimulation with TLR2 ligands, including zymosan, peptidoglycan, and lipoarabinomannan. The human monocyte response to crude bacterial LPS is composed of a TLR4-specific response to the pure LPS component and a TLR2-dependent response to associated lipopeptides. The removal of TLR2 lipopeptide components from LPS by phenol re-extraction substantially reduced both the IL-8 and superoxide response of the stimulated neutrophils, indicating that, unlike monocytes, the neutrophil response is preferentially directed to TLR2 ligands. Thus, our studies demonstrate that GM-CSF dramatically enhances the functional response of neutrophils to TLR2 ligands, including LPS-associated lipopeptides.
Introduction
Neutrophils provide the rapid deployment and effector arm of the innate immune system. Approximately 1011 per day transit through the human circulation en route to tissue, where they form the first line of cellular defense against invading microorganisms.1,2 As potent agents of the inflammatory response, they also play a major role in the inflammation and tissue damage of a wide variety of noninfectious diseases, such as arthritis, inflammatory bowel disease, and ischemia-reperfusion injury.3-5
These motile, phagocytic cells respond to a wide variety of particulate and soluble stimuli.6,7 Exposure to other agents, most of which do not activate neutrophils directly, elicits a priming reaction that enhances subsequent function in response to other activating stimuli or to higher doses of the priming agent.8 Priming stimuli include bacterial lipopolysaccharide (LPS), cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF), and low doses of chemotactic molecules such as formylated peptides, including f-Met-Leu-Phe (f-MLP) and C5a.8-10 Primed neutrophils exhibit enhanced expression of integrins and selectins, inhibition of apoptosis, and membrane assembly of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex.8,10 11
Many functionally important receptors for microbial ligands on neutrophils have not yet been identified. Recently, a family of receptor proteins, the Toll-like receptors (TLRs), has been identified in mammals.12-14 TLRs mediate cellular responses to a large array of microbial ligands. At present, 10 different TLR proteins have been cloned.15,16 TLR2 is the receptor for a variety of microbial ligands, including gram-positive bacteria, peptidoglycan, yeast zymosan, and mycobacterial ara-lipoarabinomannan (araLAM).15,16 TLR4 is a receptor for gram-negative bacteria, LPS, and some viruses.15-17 TLR4 and TLR2, like other TLR family members, have a conserved intracellular signaling motif. This signaling motif, which is also found in the intracellular domain of the IL-1 receptor (IL-1R), is responsible for nuclear factor-κB (NF-κB) activation/translocation after TLR or IL-1R receptor engagement and is an essential signaling pathway for IL-1β and tumor necrosis factor-α (TNF-α) secretion.18 RNA transcripts encoding TLR family members TLR2 and TLR4 have been detected in human peripheral blood neutrophils.19
A second important receptor for microbial ligands is CD14. CD14 is a glycosylphosphatidylinositol (GPI)–anchored protein expressed at high levels on the surface of circulating monocytes.20 CD14 has also been detected in neutrophils, where it primarily resides within granules.21-23 A soluble form of CD14 is present in serum; membrane and soluble CD14 function as coreceptors for microbial ligands, including LPS, zymosan, peptiodoglycan, and araLAM.24 In this paper, we examine the role of TLR2, TLR4, and CD14 protein expression and receptor function in human peripheral blood neutrophils and demonstrate a functional role for TLR2 in neutrophil responses to microbial ligands.
We demonstrate that (1) TLR2 and CD14 are expressed on the surface of neutrophils as well as on the surface of monocytes; (2) cell surface expression of both TLR2 and CD14 on neutrophils is modulated by external factors, TLR2 and CD14 expression being up-regulated by GM-CSF, LPS, and G-CSF, while only minimal effects on monocyte receptor expression are seen; and (3) the neutrophil response to bacterial and yeast cell wall components is enhanced by GM-CSF, while this cytokine has minimal effects on the monocyte responses to the same stimuli.
Materials and methods
Cell culture and reagents
Human embryonic kidney HEK293 cells (ATCC, Rockville, MD) were grown in RPMI-1640 medium (Gibco BRL, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (Atlanta Biologicals, Norcross, GA). LPS from Escherichia coli serotype 0111:B4, f-Met-Leu-Phe, and zymosan were purchased from Sigma (St Louis, MO). Zymosan preparations consisted of yeast cell walls (ghost cells) with an average particle diameter of 3 microns. Peptidoglycan was obtained from ICN (Costa Mesa, CA). AraLAM was provided by Dr John Belisle (Fort Collins, CO) under National Institute of Allergy and Infectious Diseases (NIAID) contract NO1-AI-75320 entitled “Tuberculosis Research Materials and Vaccine Testing.” Phenol extraction of the commercial (stock) preparation of LPS was performed as described by Manthey et al.25 GM-CSF (Leukine) was obtained from Immunex (Seattle, WA), and G-CSF (Neupogen) was obtained from Amgen (Thousand Oaks, CA).
Isolation and staining of neutrophils and peripheral blood mononuclear cells
Neutrophils were isolated from normal human peripheral blood by dextran sedimentation and centrifugation through Ficoll-Hypaque (Amersham Biosciences, Piscataway, NJ). Neutrophils were recovered from the pellet and mononuclear cells were recovered from the interface, as previously described,26 using a protocol that minimizes activation of the neutrophils during isolation. Cells were cultured in RPMI-1640 supplemented with 10% fetal calf serum and antibiotics. Cells were cultured in 24-well culture dishes at a density of 107 per well (neutrophils) or 106 per well (mononuclear cells). Culture supernatants were collected 18 hours after stimulation, and IL-8 levels were determined by enzyme-linked immunosorbent assay (ELISA) (Pierce-Endogen, Rockford, IL). All reagents, serum, buffers, and media were free of LPS (< 0.01 ng/mL by Limulus amoebocyte lysate assay, Sigma). For fluorescence-activated cell-sorter scanner (FACS) staining, neutrophils and monocytes were incubated with the minimal priming doses of LPS, which were determined to be 0.25 ng/mL for neutrophils and 1 ng/mL LPS for monocytes, based on pilot dose-response experiments. In all other analyses, both neutrophils and monocytes were incubated with a maximal activating dose of 10 ng/mL LPS.
CD14, TLR2, and TLR4 receptor expression were determined by flow cytometry. Cells were stained with anti-CD14 mAb (clone MY4; Coulter Immunology, Hialeah, FL), anti-TLR2 (clone TL2.1; gift of Dr Espevik, Trodheim, Norway), anti-TLR4 (clone HTA125; eBiosciences, San Diego, CA), or isotype control antibodies (Sigma). Antibody binding was detected using a phycoerythrin (PE)–labeled goat anti–mouse IgG antiserum (Sigma). In some experiments, directly PE-labeled anti-TLR2 (TL2.1) and anti-TLR4 (HTA125) antibodies purchased from eBiosciences were used. Cells were fixed in RBC Lysing Solution (Becton-Dickinson, San Jose, CA) and analyzed using a FACScan analyzer. For each time point and assay condition, at least 10 000 cells were analyzed. Statistical analysis was performed by the Kolmogorov-Smirnoff algorithm analysis and by Probability Binning Chi(T) analysis using FlowJo software for univariant analysis of population distributions of FACS data (Tree Star, San Carlos, CA). For Probability Binning Chi(T) analysis, a minimum value of ChiT(X) = 100 for comparison of 2 populations was applied for significance at the P < .001 level. For Kolmogorov-Smirnoff analysis, a minimum of 30% positive events in samples compared to controls by Super-enhanced Dmax Subtraction at the P < .001 level for each comparison of 2 populations was applied (Tree Star, San Carlos, CA).
Generation of stable TLR- and CD14-expressing clones
HEK293 cells were transfected with plasmids encoding puromycin resistance (gift of Dr Richard Kitchens, University of Texas Southwestern Medical Center), human TLR2 or TLR4 (Tularik, San Francisco, CA; FLAG-epitope tagged at the N-terminus), and/or human CD14 using Escort reagent (Sigma) according to manufacturer's protocol. Forty-eight hours later, 5 μg/mL puromycin was added to the cultures. Clones of puromycin-resistant cells were isolated and analyzed by FACS for surface expression of proteins, using anti-FLAG mAb to detect TLR proteins and anti-CD14 mAb followed by a PE-labeled goat anti–mouse IgG (Sigma). Clones expressing equivalent levels of TLR proteins were selected for further study. Cells were plated in 24-well culture dishes, and 24 hours later the medium was replaced with fresh medium containing LPS or no stimulant. After 18 hours of stimulation, culture supernatants were harvested, and IL-8 levels in the supernatants were measured using a commercial IL-8 ELISA assay kit (Endogen).
Northern blot analysis
Neutrophils were incubated with or without GM-CSF (10-100 U/mL) for 2 hours. RNA was extracted by the guanidine HCl method.26 RNA from control transfected cell lines was similarly extracted. Northern blot analysis was performed according to standard procedures,27 using 32P-labeled cDNAs for the human TLR2 and CD14 genes as hybridization probes. Sequential cycles of filter stripping and reprobing were performed as previously described.28 Equal loading of lanes was demonstrated by examination of gels after ethidium-bromide staining and by rehybridization with a 5.8-kilobase HindIII restriction fragment of rat 18S ribosomal cDNA.29
Superoxide release
Superoxide release was measured by a modified superoxide dismutase (SOD) inhibitable cytochrome c reduction assay.30 Neutrophils (8 × 105 per tube) were incubated for 90 minutes in Hanks balanced salt solution (HBSS) without phenol red, with or without GM-CSF 10 U/mL, then further incubated 30 minutes at 37°C with no stimulus or with one of the following TLR ligands: zymosan (1 μg/mL), araLAM (1 μg/mL), peptidoglycan (1 μg/mL), commercial (stock) LPS (10 ng/mL), or phenol-extracted LPS (10 ng/mL). Superoxide generation was then measured by the addition of cytochrome c (50 μM) and formyl-methionyl-leucyl-phenylalanine (f-MLP, 10−7 M) in the presence of dihydrocytochalasin B (10-5 M). One reference tube for each experimental group also received SOD (60 U/mL). After 15 minutes of incubation at 37°C, the reaction was stopped by placing the tubes on ice and the cells removed by centrifugation for 1 minute at 15 000g. The light absorbance of the supernatants was measured at 550 nm, and the amount of superoxide released was calculated from the difference in A550between the assay and SOD-containing tubes, using an extinction coefficient of 0.21 nM−1 cm−1. The results are expressed as nmol of superoxide released per minute per 106 cells.
Results
Neutrophils express TLR2 and CD14
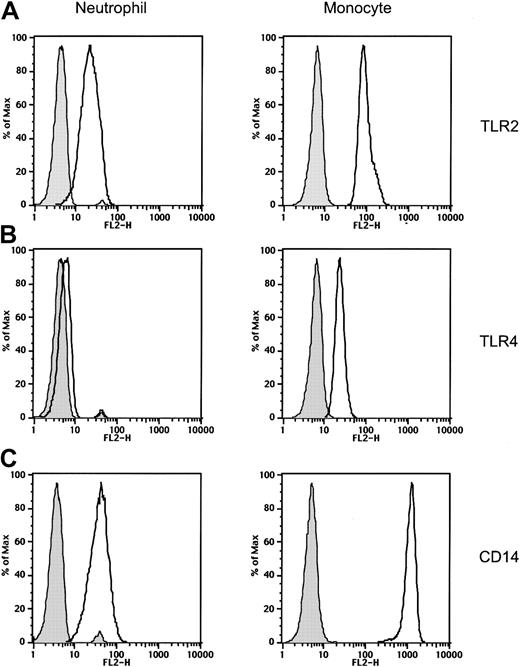
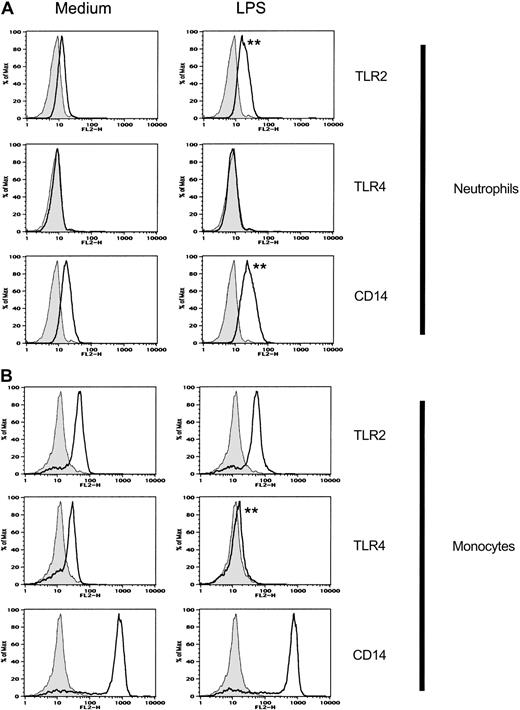
We examined the expression of the Toll-like receptors TLR2 and TLR4, as well as CD14, on normal human peripheral blood neutrophils and compared neutrophil receptor expression to the expression of these receptors on monocytes. TLR2 and CD14 were expressed at moderate levels on neutrophils. The levels of TLR2 and CD14 expressed on monocytes were higher than the levels of these receptors expressed on neutrophils from the same donor (Figure 1). TLR4 was only weakly expressed on freshly isolated neutrophils (Figure 1) and was undetectable on neutrophils cultured in medium for 2 hours (data not shown; and Figure 2). In contrast, we readily detected TLR4 expression on monocytes isolated at the same time from the same donor (Figure 1), even after culturing the cells in medium (data not shown). This pattern of low TLR2 and CD14 expression and weak or undetectable TLR4 expression on neutrophils compared to levels of the same proteins on monocytes was observed with all of the donors we examined. (n = 7 donors tested on 14 separate occasions. Concurrent flow cytometric analysis of neutrophils and monocytes from individual donors is shown in Figures 1 and 5.)
Expression of Toll-like receptors and CD14 on neutrophils and monocytes.
Expression of TLR2, TLR4, and CD14 on peripheral blood neutrophils and monocytes was measured by fluorescence analysis of cells stained with anti-TLR2, anti-TLR4, anti-CD14, or isotype-control mAbs followed by PE-labeled goat anti–mouse Ig Ab. Isotype-control Ab–stained cells are shown as gray histograms. Specific mAb–stained cells are shown as black line histograms. Geometric mean fluorescence intensity (SD). Neutrophils: isotype control, 4.7 (0.3); TLR2, 22.6 (0.5); TLR4, 6.3 (0.3); and CD14, 42.1 (0.5). Monocytes: isotype control, 6.3 (0.3); TLR2, 96.6 (0.3); TLR4, 23.4 (0.2); and CD14, 1183 (0.3).
Expression of Toll-like receptors and CD14 on neutrophils and monocytes.
Expression of TLR2, TLR4, and CD14 on peripheral blood neutrophils and monocytes was measured by fluorescence analysis of cells stained with anti-TLR2, anti-TLR4, anti-CD14, or isotype-control mAbs followed by PE-labeled goat anti–mouse Ig Ab. Isotype-control Ab–stained cells are shown as gray histograms. Specific mAb–stained cells are shown as black line histograms. Geometric mean fluorescence intensity (SD). Neutrophils: isotype control, 4.7 (0.3); TLR2, 22.6 (0.5); TLR4, 6.3 (0.3); and CD14, 42.1 (0.5). Monocytes: isotype control, 6.3 (0.3); TLR2, 96.6 (0.3); TLR4, 23.4 (0.2); and CD14, 1183 (0.3).
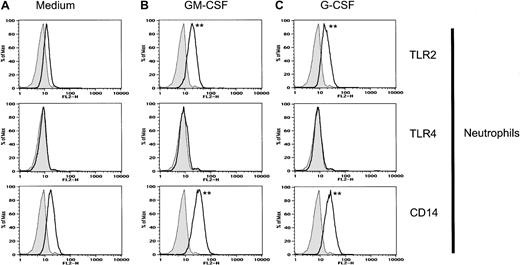
GM-CSF and G-CSF enhance TLR2 and CD14 expression on neutrophils.
Neutrophils were incubated with GM-CSF (100 U/mL), G-CSF (100 U/mL), or medium alone for 2 hours prior to staining and FACS analysis. Isotype-control Ab–stained cells are shown as gray histograms. Specific mAb–stained cells are shown as black line histograms. Geometric mean fluorescence intensity (SD). Neutrophils treated with medium alone: isotype control, 7.9 (0.4); TLR2, 12.3 (0.3); TLR4, 8.5 (0.3); and CD14, 17.5 (0.3). Neutrophils treated with GM-CSF: isotype control, 7.9 (0.4); TLR2, 19.4 (0.4); TLR4, 9.2 (0.4); and CD14, 33.8 (0.4). Neutrophils treated with G-CSF: isotype control, 7.9 (0.4); TLR2, 18.0 (0.4); TLR4, 8.8 (0.3); and CD14, 24.7 (0.4). Significance of difference between treated cells and untreated controls: **P < .001 by Probability Binning ChiT analysis (ChiT(X) > 450); P < .001 by Kolmogorov-Smirnoff analysis.
GM-CSF and G-CSF enhance TLR2 and CD14 expression on neutrophils.
Neutrophils were incubated with GM-CSF (100 U/mL), G-CSF (100 U/mL), or medium alone for 2 hours prior to staining and FACS analysis. Isotype-control Ab–stained cells are shown as gray histograms. Specific mAb–stained cells are shown as black line histograms. Geometric mean fluorescence intensity (SD). Neutrophils treated with medium alone: isotype control, 7.9 (0.4); TLR2, 12.3 (0.3); TLR4, 8.5 (0.3); and CD14, 17.5 (0.3). Neutrophils treated with GM-CSF: isotype control, 7.9 (0.4); TLR2, 19.4 (0.4); TLR4, 9.2 (0.4); and CD14, 33.8 (0.4). Neutrophils treated with G-CSF: isotype control, 7.9 (0.4); TLR2, 18.0 (0.4); TLR4, 8.8 (0.3); and CD14, 24.7 (0.4). Significance of difference between treated cells and untreated controls: **P < .001 by Probability Binning ChiT analysis (ChiT(X) > 450); P < .001 by Kolmogorov-Smirnoff analysis.
GM-CSF increases TLR2 and CD14 expression on neutrophils
GM-CSF has been shown to prime neutrophils for responses to LPS.31 32 Therefore, we examined the effect of GM-CSF treatment on neutrophil expression of TLR2, TLR4, and CD14. Neutrophils were isolated from peripheral blood and incubated with 100 U/mL GM-CSF for 2 hours. GM-CSF treatment increased TLR2 and CD14 expression on neutrophils (Figure 2) but did not affect TLR4 expression. In contrast, GM-CSF had little effect on TLR2, TLR4, or CD14 expression on monocytes (data not shown).
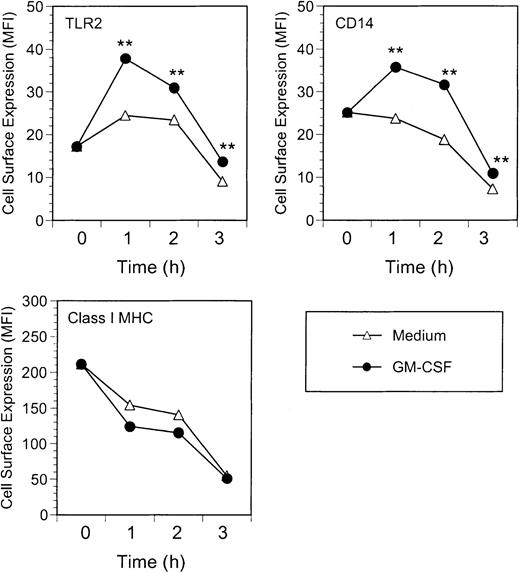
Kinetic analysis indicated that GM-CSF enhancement of TLR2 and CD14 expression on neutrophils was detectable within 1 hour of GM-CSF treatment and was maintained over a 3-hour period (Figure3). Class I major histocompatibility complex (MHC) expression was unaffected by GM-CSF treatment. Neutrophils incubated without GM-CSF had stable expression of TLR2 over the first 2 hours, although CD14 expression declined slightly at the 2-hour time point. At 3 hours of incubation, expression of all 3 surface receptors (Class I MHC, TLR2, and CD14) was reduced. Nevertheless, GM-CSF–treated neutrophils maintained higher levels of TLR2 and CD14 than medium-treated cells, even at 3 hours (Figure 3).
Kinetics of GM-CSF–induced TLR2 and CD14 expression.
Neutrophils were incubated with GM-CSF (100 U/mL) or medium alone for up to 3 hours at 37°C. At the indicated times, cells were stained and analyzed for expression of TLR2, CD14, and Class I MHC. Data are expressed as geometric mean fluorescence intensity of cells stained at t = 0 (immediately after isolation) or following incubation for 1, 2, or 3 hours prior to staining. Significance of difference between treated cells and untreated controls: **P < .001 by Probability Binning ChiT analysis (ChiT(X) > 330);P < .001 by Kolmogorov-Smirnoff analysis.
Kinetics of GM-CSF–induced TLR2 and CD14 expression.
Neutrophils were incubated with GM-CSF (100 U/mL) or medium alone for up to 3 hours at 37°C. At the indicated times, cells were stained and analyzed for expression of TLR2, CD14, and Class I MHC. Data are expressed as geometric mean fluorescence intensity of cells stained at t = 0 (immediately after isolation) or following incubation for 1, 2, or 3 hours prior to staining. Significance of difference between treated cells and untreated controls: **P < .001 by Probability Binning ChiT analysis (ChiT(X) > 330);P < .001 by Kolmogorov-Smirnoff analysis.
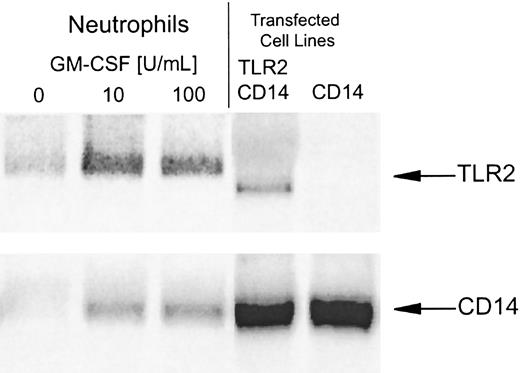
Northern blot analysis demonstrated that neutrophils have low or undetectable levels of TLR2 and CD14 mRNA when isolated from peripheral blood (Figure 4). Both TLR2 and CD14 mRNA levels increased after treatment with GM-CSF (Figure 4), suggesting that the increase in TLR2 and CD14 expression observed by flow cytometry was due to increased gene transcription. TLR4 mRNA was not detected in either untreated or GM-CSF–treated neutrophils, consistent with the lack of TLR4 protein detectable by flow cytometry of these cells (data not shown; and Figures 1, 2, and 5).
GM-CSF increases TLR2 and CD14 mRNA levels in neutrophils.
Neutrophils were incubated with GM-CSF (10-100 U/mL) or medium alone for 2 hours, and mRNA was extracted for Northern blot analysis. Positive control mRNA was extracted from Chinese hamster ovary (CHO) cells stably transfected with human CD14 alone or CD14 in combination with human TLR2. Samples, each containing 10 μg RNA, were electrophoresed and blotted on nylon-nitrocellulose membranes. (Equal loading was confirmed by ethidium-bromide staining and hybridization of the blots with an 18S ribosomal cDNA probe.) Northern blots were probed with labeled human TLR2 (upper blot), CD14 (lower blot), and TLR4 (not shown) cDNAs.
GM-CSF increases TLR2 and CD14 mRNA levels in neutrophils.
Neutrophils were incubated with GM-CSF (10-100 U/mL) or medium alone for 2 hours, and mRNA was extracted for Northern blot analysis. Positive control mRNA was extracted from Chinese hamster ovary (CHO) cells stably transfected with human CD14 alone or CD14 in combination with human TLR2. Samples, each containing 10 μg RNA, were electrophoresed and blotted on nylon-nitrocellulose membranes. (Equal loading was confirmed by ethidium-bromide staining and hybridization of the blots with an 18S ribosomal cDNA probe.) Northern blots were probed with labeled human TLR2 (upper blot), CD14 (lower blot), and TLR4 (not shown) cDNAs.
LPS enhances TLR2 and CD14 but not TLR4 expression on neutrophils.
Neutrophils and monocytes were incubated with LPS or medium alone for 2 hours prior to staining and FACS analysis. Isotype-control Ab–stained cells are shown as gray histograms. Specific mAb–stained cells are shown as black line histograms. Geometric mean fluorescence intensity (SD). Neutrophils treated with medium alone: isotype control, 7.9 (0.4); TLR2, 12.3 (0.3); TLR4, 8.5 (0.3); and CD14, 17.5 (0.3). Neutrophils treated with LPS: isotype control, 7.9 (0.4); TLR2, 18.2 (0.4); TLR4, 8.4 (0.3); and CD14, 27.1 (0.5). Monocytes treated with medium alone: isotype control, 12.9 (0.4); TLR2, 42.3 (0.5); TLR4, 23.5 (0.6); and CD14, 633 (1.6). Monocytes treated with LPS: isotype control, 12.9 (0.4); TLR2, 48.6 (0.6); TLR4, 14.8 (0.5); CD14, 566 (1.8). Significance of difference between treated cells and untreated controls: **P < .001 by Probability Binning ChiT analysis (ChiT(X) > 500); P < .001 by Kolmogorov-Smirnoff analysis.
LPS enhances TLR2 and CD14 but not TLR4 expression on neutrophils.
Neutrophils and monocytes were incubated with LPS or medium alone for 2 hours prior to staining and FACS analysis. Isotype-control Ab–stained cells are shown as gray histograms. Specific mAb–stained cells are shown as black line histograms. Geometric mean fluorescence intensity (SD). Neutrophils treated with medium alone: isotype control, 7.9 (0.4); TLR2, 12.3 (0.3); TLR4, 8.5 (0.3); and CD14, 17.5 (0.3). Neutrophils treated with LPS: isotype control, 7.9 (0.4); TLR2, 18.2 (0.4); TLR4, 8.4 (0.3); and CD14, 27.1 (0.5). Monocytes treated with medium alone: isotype control, 12.9 (0.4); TLR2, 42.3 (0.5); TLR4, 23.5 (0.6); and CD14, 633 (1.6). Monocytes treated with LPS: isotype control, 12.9 (0.4); TLR2, 48.6 (0.6); TLR4, 14.8 (0.5); CD14, 566 (1.8). Significance of difference between treated cells and untreated controls: **P < .001 by Probability Binning ChiT analysis (ChiT(X) > 500); P < .001 by Kolmogorov-Smirnoff analysis.
G-CSF increases TLR2 and CD14 expression on neutrophils
G-CSF, like GM-CSF, has been shown to prime neutrophils for responses to LPS.8,31 32 Therefore, we examined the effect of G-CSF treatment on neutrophil receptor expression. G-CSF increased TLR2 and CD14 expression on neutrophils (Figure 2). TLR4 remained low or undetectable on treated neutrophils (Figure 2). These results suggest that neutrophil priming by GM-CSF and G-CSF may be due, at least in part, to increased TLR2 and CD14 receptor expression.
LPS increases TLR2 and CD14 expression on neutrophils and down-regulates TLR4 expression on monocytes
TLR4 expression is known to be regulated by LPS.19,33 34 We examined the effect of LPS treatment on TLR2, TLR4, and CD14 receptor expression on neutrophils and mononuclear cells incubated with LPS for 2 hours (Figure5). LPS increased TLR2 expression on neutrophils and, to a lesser degree, on monocytes. CD14 expression on neutrophils was also increased by LPS treatment (Figure 5). In contrast, LPS treatment down-regulated TLR4 expression on monocytes. Neutrophils had low or undetectable TLR4 expression both before and after LPS treatment (Figure 5).
GM-CSF enhances neutrophil responses to TLR2 ligands
TLR2 is an essential receptor for cellular responses to gram-positive bacteria, mycobacteria, and yeast.16,35,36Transfection studies of human and rodent cells and studies of TLR2 knockout mice have identified several microbial components that activate host-cell responses via TLR2 (and CD14). These include peptidoglycan, a cell wall component of gram-positive bacteria; zymosan, a cell wall component of yeast; and araLAM, a component of Mycobacterium tuberculosis.16,35 36
Our experiments showed that GM-CSF enhanced neutrophil expression of TLR2 (Figures 2-4). This prompted us to examine the effect of GM-CSF treatment on neutrophil responses to TLR2 ligands. Neutrophils, isolated from peripheral blood, were treated with increasing doses of GM-CSF and stimulated with peptidoglycan, zymosan, or araLAM (Figures6-8). GM-CSF treatment induced a dose-dependent increase in the IL-8 response of neutrophils to peptidoglycan (Figure6), zymosan (Figure7), and araLAM (Figure8), suggesting that increased TLR2 expression after GM-CSF treatment may enhance neutrophil responsiveness to TLR2 ligands. The receptor specificity of each of these ligands was analyzed by stimulating TLR- (and CD14) expressing HEK293 transfected cell lines and measuring IL-8 secretion. Peptidoglycan, zymosan, and araLAM each stimulated transfected cell lines in a TLR2-dependent manner (Figures 6-8, panel B).
Neutrophil activation by the TLR2 ligand, peptidoglycan, is enhanced by GM-CSF treatment.
(A) GM-CSF enhances peptidoglycan-stimulated IL-8 secretion from neutrophils but not from monocytes. Neutrophils and monocytes were incubated with or without GM-CSF (1-100 U/mL) and stimulated with (○) peptidoglycan (10 μg/mL) or (▪) medium alone. IL-8 secretion was measured 18 hours later by ELISA. (B) Peptidoglycan stimulates TLR2-expressing cells. HEK293 clones expressing TLR2 or no TLR (none) were incubated with (▪) peptidoglycan (10 μg/mL), (░) phenol-extracted LPS (10 ng/mL), or (■) medium alone. Secretion of IL-8 was measured 18 hours later by ELISA.
Neutrophil activation by the TLR2 ligand, peptidoglycan, is enhanced by GM-CSF treatment.
(A) GM-CSF enhances peptidoglycan-stimulated IL-8 secretion from neutrophils but not from monocytes. Neutrophils and monocytes were incubated with or without GM-CSF (1-100 U/mL) and stimulated with (○) peptidoglycan (10 μg/mL) or (▪) medium alone. IL-8 secretion was measured 18 hours later by ELISA. (B) Peptidoglycan stimulates TLR2-expressing cells. HEK293 clones expressing TLR2 or no TLR (none) were incubated with (▪) peptidoglycan (10 μg/mL), (░) phenol-extracted LPS (10 ng/mL), or (■) medium alone. Secretion of IL-8 was measured 18 hours later by ELISA.
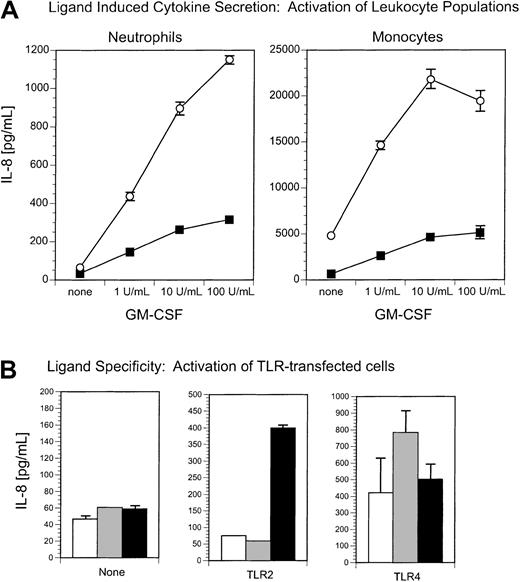
Neutrophil activation by the TLR2 ligand, zymosan, is enhanced by GM-CSF treatment.
(A) GM-CSF enhances zymosan-stimulated IL-8 secretion from neutrophils but not from monocytes. Neutrophils and monocytes were incubated with or without GM-CSF (1-100 U/mL) and stimulated with (○) zymosan (10 μg/mL) or (▪) medium alone. IL-8 secretion was measured 18 hours later by ELISA. (B) Zymosan stimulates TLR2-expressing cells but not TLR4-expressing cells. HEK293 clones expressing TLR2, TLR4, or no TLR (none) were incubated with (▪) zymosan (10 μg/mL), (░) phenol-extracted LPS (10 ng/mL), or (■) medium alone. Secretion of IL-8 was measured 18 hours later by ELISA.
Neutrophil activation by the TLR2 ligand, zymosan, is enhanced by GM-CSF treatment.
(A) GM-CSF enhances zymosan-stimulated IL-8 secretion from neutrophils but not from monocytes. Neutrophils and monocytes were incubated with or without GM-CSF (1-100 U/mL) and stimulated with (○) zymosan (10 μg/mL) or (▪) medium alone. IL-8 secretion was measured 18 hours later by ELISA. (B) Zymosan stimulates TLR2-expressing cells but not TLR4-expressing cells. HEK293 clones expressing TLR2, TLR4, or no TLR (none) were incubated with (▪) zymosan (10 μg/mL), (░) phenol-extracted LPS (10 ng/mL), or (■) medium alone. Secretion of IL-8 was measured 18 hours later by ELISA.
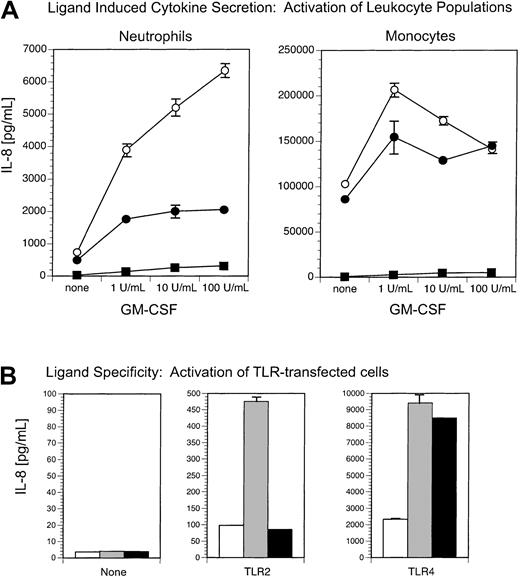
Neutrophil and monocyte activation by the TLR2 ligand, araLAM, is enhanced by GM-CSF treatment.
(A) GM-CSF enhances araLAM-stimulated IL-8 secretion from both neutrophils and monocytes. Neutrophils and monocytes were incubated with or without GM-CSF (1-100 U/mL) and stimulated with (○) araLAM (1 μg/mL) or (▪) medium alone. IL-8 secretion was measured 18 hours later by ELISA. (B) araLAM stimulates TLR2-expressing cells but not TLR4-expressing cells. HEK293 clones expressing TLR2, TLR4, or no TLR (none) were incubated with (▪) araLAM (1 μg/mL), (░) phenol-extracted LPS (10 ng/mL), or (■) medium alone. Secretion of IL-8 was measured 18 hours later by ELISA.
Neutrophil and monocyte activation by the TLR2 ligand, araLAM, is enhanced by GM-CSF treatment.
(A) GM-CSF enhances araLAM-stimulated IL-8 secretion from both neutrophils and monocytes. Neutrophils and monocytes were incubated with or without GM-CSF (1-100 U/mL) and stimulated with (○) araLAM (1 μg/mL) or (▪) medium alone. IL-8 secretion was measured 18 hours later by ELISA. (B) araLAM stimulates TLR2-expressing cells but not TLR4-expressing cells. HEK293 clones expressing TLR2, TLR4, or no TLR (none) were incubated with (▪) araLAM (1 μg/mL), (░) phenol-extracted LPS (10 ng/mL), or (■) medium alone. Secretion of IL-8 was measured 18 hours later by ELISA.
Monocytes constitutively express higher levels of TLR2 than neutrophils (Figures 1 and 5). Consistent with their higher levels of TLR2 (and CD14) expression, monocytes are activated by the TLR2 ligands, peptidoglycan (Figure 6), and zymosan (Figure 7) at near optimal levels, even in the absence of GM-CSF treatment. In contrast to its effect on monocyte responses to zymosan and peptidoglycan, GM-CSF treatment enhanced the response of monocytes, as well as neutrophils, to araLAM (Figure 8). These results suggest that in addition to TLR2, GM-CSF may increase expression of an araLAM-specific receptor, perhaps a TLR involved in heterodimer formation with TLR2.
GM-CSF enhances neutrophil responses to the TLR2-activating component of commercial LPS
Genetic studies have shown that LPS-induced activation is dependent on TLR4 and CD14 expression.37-40 While many studies have demonstrated neutrophil responses to LPS, our experiments indicate that neutrophils express low levels of TLR4. How then do neutrophils respond to LPS? The answer, in part, may lie in the source of the LPS. Pure, protein-free LPS is a ligand for TLR4 and CD14, but not for TLR2.41,42 However, the LPS used in most studies of neutrophil priming is commercially prepared. Several studies have demonstrated that commercial LPS is contaminated with a TLR2-stimulating substance, perhaps a lipopeptide, which can be removed by extensive phenol extraction.41 42 We therefore examined the ability of commercial LPS (with TLR2- and TLR4-stimulating components) and phenol-extracted LPS (TLR4 stimulating only) to induce IL-8 secretion from neutrophils and monocytes.
Commercial LPS activated neutrophils and stimulated IL-8 secretion, and this activity was enhanced by GM-CSF treatment (Figure9). When the TLR2-stimulating component of the LPS was removed by phenol extraction, the resulting LPS preparation had a substantially reduced ability to stimulate neutrophils (Figure 9). In contrast, monocytes responded equally well to commercial unpurified and to phenol-extracted LPS (Figure 9). The receptor specificity of the LPS preparations was analyzed using TLR- (and CD14) expressing HEK293 transfected cell lines. Commercial unpurified LPS stimulated both TLR2- and TLR4-expressing cells, while phenol-extracted LPS stimulated TLR4-expressing cells but had no stimulating activity for TLR2-expressing cells (Figure 9B). These results suggest that the GM-CSF–enhanced neutrophil response to LPS was directed to the TLR2-stimulating component(s) of the commercial LPS preparation rather than the TLR4-stimulating component.
Neutrophil activation by commercial LPS is enhanced by GM-CSF treatment.
(A) GM-CSF enhances commercial LPS-stimulated IL-8 secretion from neutrophils but only weakly enhances neutrophil responsiveness to phenol-extracted LPS. Neutrophils and monocytes were incubated with or without GM-CSF (1-100 U/mL) and stimulated with (○) commercial (stock) LPS (10 ng/mL), (●) phenol-extracted LPS (10 ng/mL), or (▪) medium alone. IL-8 secretion was measured 18 hours later by ELISA. (B) Commercial (stock) LPS stimulates both TLR2-expressing cells and TLR4-expressing cells, but phenol-extracted LPS stimulates only TLR4-expressing cells. HEK293 clones expressing TLR2, TLR4, or no TLR (none) were incubated with (░) commercial (stock) LPS (10 ng/mL), (▪) phenol-extracted LPS (10 ng/mL), or (■) medium alone. Secretion of IL-8 was measured 18 hours later by ELISA.
Neutrophil activation by commercial LPS is enhanced by GM-CSF treatment.
(A) GM-CSF enhances commercial LPS-stimulated IL-8 secretion from neutrophils but only weakly enhances neutrophil responsiveness to phenol-extracted LPS. Neutrophils and monocytes were incubated with or without GM-CSF (1-100 U/mL) and stimulated with (○) commercial (stock) LPS (10 ng/mL), (●) phenol-extracted LPS (10 ng/mL), or (▪) medium alone. IL-8 secretion was measured 18 hours later by ELISA. (B) Commercial (stock) LPS stimulates both TLR2-expressing cells and TLR4-expressing cells, but phenol-extracted LPS stimulates only TLR4-expressing cells. HEK293 clones expressing TLR2, TLR4, or no TLR (none) were incubated with (░) commercial (stock) LPS (10 ng/mL), (▪) phenol-extracted LPS (10 ng/mL), or (■) medium alone. Secretion of IL-8 was measured 18 hours later by ELISA.
GM-CSF enhances neutrophil superoxide generation in response to TLR2-activating ligands
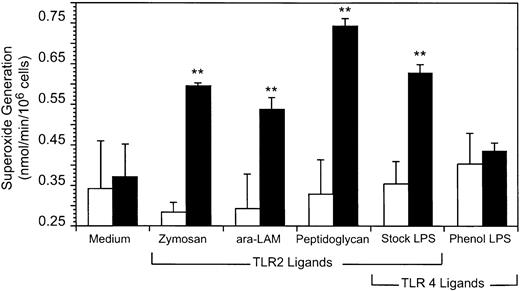
Neutrophils generate superoxide within minutes of stimulation.8 9 In contrast, IL-8 secretion does not reach detectable levels until hours after stimulation. Because of the prolonged time course for cytokine secretion, GM-CSF enhancement of IL-8 secretion could reflect both the increase in TLR2 and CD14 expression early in the response and further changes in gene expression during the course of the 18 hours of incubation with TLR ligands. We therefore were interested in studying an immediate, early response to TLR ligand stimulation after GM-CSF induction of TLR2. We examined the ability of TLR2 and TLR4 ligands to enhance superoxide generation from neutrophils (Figure 10). The ligands used were araLAM (TLR2), peptidoglycan (TLR2), zymosan (TLR2), commercial LPS (TLR2 and TLR4 ligand), or phenol-extracted LPS (TLR4 ligand). Superoxide generation was significantly increased in GM-CSF–treated neutrophils stimulated with TLR2 ligands, that is, zymosan, araLAM, and peptidoglycan (P < .005). Interestingly, TLR2 ligands did not increase superoxide generation from neutrophils in the absence of GM-CSF treatment (Figure 10), that is, the TLR2-specific response was highly dependent on pre-exposure to GM-CSF.
GM-CSF enhances superoxide generation from neutrophils stimulated with TLR2 ligands.
Neutrophils were pretreated with (▪) GM-CSF or (■) medium alone for 90 minutes and stimulated with zymosan, araLAM, peptidoglycan, or LPS as described in “Materials and methods.” Superoxide generation was determined by a SOD inhibitable cytochrome c reduction assay of cells incubated with cytochrome c, f-MLP, and cytochalasin B for 15 minutes. The mean and SD of 3 replicate determinations are shown. **t test, P < .005 for comparison of untreated versus GM-CSF–treated cells stimulated with zymosan, araLAM, peptidoglycan, or commercial (stock) LPS (which contains a contaminating TLR2 ligand). t test,P = .13 (not significant) for comparison of untreated versus GM-CSF–treated cells stimulated with phenol-extracted LPS (which retains TLR4-stimulating activity, but from which the TLR2 activity has been removed).
GM-CSF enhances superoxide generation from neutrophils stimulated with TLR2 ligands.
Neutrophils were pretreated with (▪) GM-CSF or (■) medium alone for 90 minutes and stimulated with zymosan, araLAM, peptidoglycan, or LPS as described in “Materials and methods.” Superoxide generation was determined by a SOD inhibitable cytochrome c reduction assay of cells incubated with cytochrome c, f-MLP, and cytochalasin B for 15 minutes. The mean and SD of 3 replicate determinations are shown. **t test, P < .005 for comparison of untreated versus GM-CSF–treated cells stimulated with zymosan, araLAM, peptidoglycan, or commercial (stock) LPS (which contains a contaminating TLR2 ligand). t test,P = .13 (not significant) for comparison of untreated versus GM-CSF–treated cells stimulated with phenol-extracted LPS (which retains TLR4-stimulating activity, but from which the TLR2 activity has been removed).
We have also noted that GM-CSF–treated neutrophils release very high levels of superoxide when treated with anti-TLR2 monoclonal antibody compared to cells incubated with an isotype-matched control antibody (data not shown). The response to anti-TLR2 treatment was dependent on pre-exposure to GM-CSF, similar to the response to TLR2 ligands.
The TLR4-specific ligand, phenol LPS, did not significantly increase superoxide generation from neutrophils, nor was this response significantly increased by GM-CSF treatment of the cells (P = .13; Figure 10). In contrast, the neutrophils responded strongly to priming with the TLR2/TLR4 ligand containing commercial (stock) LPS after GM-CSF treatment (P < .005; Figure 10). These results suggest that the generation of superoxide from neutrophils is preferentially primed by TLR2 ligand containing microbial stimulants and that GM-CSF enhances the generation of superoxide by TLR2 but not TLR4 ligands. Our results further suggest that the neutrophil priming activity in LPS is primarily due to a contaminating TLR2-stimulating activity in commercial LPS and not to the TLR4-specific pure LPS core structure.
Discussion
The innate immune response genes of vertebrates, invertebrates, and plants are remarkably conserved. Members of the Toll-like receptor gene family have been identified in Drosophila, where they are important components of antibacterial and antifungal immunity. A family of Toll-like receptors (TLRs) has been identified in human cells.12-14 TLR4 plays an essential role in the ability of cells to respond to LPS.37-40 This has been demonstrated in both mouse and human cells.37-39 A point mutation in the C3H/HeJ TLR4 gene is responsible for the resistance of these mice to LPS.37-39
In addition to TLR4, several other TLR proteins have been implicated in the response to a diverse group of microbial ligands.43,44TLR2 has been shown to confer responsiveness to gram-positive bacteria and their cell wall peptidoglycan.45-49 TLR2 is also an important recognition receptor for yeast cell wall zymosan, mycobacterial araLAM, and spirochete lipopeptides.45-47,50-56 These observations are based on both transfection studies and analysis of the responses of TLR2 knockout mice.49,57 Studies with knockout animals also have implicated TLR9 in the response to bacterial CpG DNA.58
Although TLR2 was initially described as an LPS receptor, it is now becoming apparent that TLR2 recognizes a distinct pattern of microbial products from those recognized by TLR4.16,35,36 The confusion over whether TLR2, TLR4, or both function as primary LPS receptors arose because commercial preparations of LPS contain a phenol-extractable TLR2-stimulating component, perhaps a lipopeptide, which stimulates cells via TLR2.59-61 When this component is removed by exhaustive phenol extraction of the LPS, the remaining activity is directed to TLR4.41 42
The emerging picture of TLR-ligand interactions is that individual TLR proteins recognize a set of microbial products.16,35,36Some TLR proteins may act cooperatively in the response to particular microbial ligands.62-64 Several studies suggest that TLR2 signals cooperatively with TLR1 or TLR6.62-64 The cooperation of TLRs may add greater specificity or a broader range of ligand recognition capacity to the TLR proteins as well as enhance their signal transduction capacity.
We were interested in determining if TLRs were expressed by neutrophils and investigating the role of TLRs in neutrophil activation by bacterial products. The role of TLR and CD14 receptors in monocyte responses to bacteria has been well documented.16,35,36Although neutrophils have been shown to contain CD14 and to use this receptor in their response to LPS,21-23 the role of TLRs in neutrophil responses remained to be established. We have found TLR2 and CD14 expressed on normal human neutrophils. In contrast, TLR4 was expressed only weakly by neutrophils. In studies of neutrophil priming, we have demonstrated that expression of TLR2 and CD14 was up-regulated by GM-CSF or LPS treatment. In similar studies recently published by Flo et al,65 the authors failed to detect an increase in TLR2 expression after GM-CSF treatment, however, these authors did report that CD14 expression was increased after GM-CSF, similar to our results.65 Muzio et al19 noted an increase in TLR2 mRNA after LPS treatment of neutrophils, similar to the increase in TLR2 expression we found in our studies. In contrast to our studies, Muzio et al19 also detected TLR4 mRNA expression in neutrophils. The basis for the disparity between different studies may reflect differences in the method of neutrophil isolation used in each study, differences in donor sensitivity to GM-CSF, differences in the level of TLR2 or TLR4 expression in different donors, or differences in the purity of the cell population being analyzed.
Our GM-CSF treatment experiments were performed using a neutrophil isolation protocol specifically developed to avoid activation of these cells during isolation.26 We found that GM-CSF induced increases in expression of TLR2 in 3 of 4 donors in repeated experiments. (Neutrophils from one individual were refractory to GM-CSF induction of TLR2.) Visintin et al66 noted that TLR1 and TLR4 levels are highly variable between donors, with estimates of monocyte TLR4 surface expression ranging from 400 to 3200 molecules per cell, and levels of TLR1 ranging from 0 to 5400 molecules per cell. Nevertheless, our experiments are the first to demonstrate that GM-CSF treatment dramatically enhances the functional response of neutrophils to TLR2 ligands.
In addition to increased TLR2 and CD14 expression after GM-CSF treatment, we have shown enhancement of the neutrophil response to peptidoglycan, zymosan, and araLAM, microbial ligands known to stimulate monocytes via TLR2 and CD14 receptors. That is, treatment of neutrophils with GM-CSF–enhanced IL-8 secretion and superoxide generation in response to TLR2 ligands. It is important to note that GM-CSF enhancement of neutrophil responses was receptor-specific, that is, the response to TLR2 but not TLR4 ligands was dramatically increased in GM-CSF treatment of neutrophils.
The results of our protein expression and functional studies suggest that GM-CSF primes for enhanced neutrophil responses to microbial ligands in part by increasing the levels of TLR2 and CD14 expression on the cell surface. The results further suggest that the primary neutrophil-stimulating activity of LPS preparations is due to the contaminating TLR2-specific ligand found in commercial LPS preparations. Removal of the TLR2-stimulating component by phenol re-extraction significantly diminishes the neutrophil-stimulating activity of the LPS, but does not affect the TLR4-stimulating activity of the LPS and only slightly decreases the monocyte-stimulating activity of the LPS. Thus, monocytes respond strongly to the TLR4-specific, pure LPS while neutrophils preferentially respond to the TLR2-ligand contaminated, partially purified commercial LPS. Nevertheless, TLR4 may play a role in neutrophil responses. Neutrophils do respond to phenol LPS (pure TLR4 ligand) for both IL-8 secretion and superoxide generation, albeit at lower levels than to commercial LPS. It is interesting to note that although the neutrophil IL-8 secretion response to phenol LPS was enhanced by GM-CSF treatment, the response to commercial LPS showed a greater dose-dependence on GM-CSF treatment than the response to phenol LPS, again suggesting that GM-CSF preferentially enhances TLR2-dependent responses.
Our studies suggest that TLR2 expression by neutrophils controls their response to microbial ligands and that this response is dramatically enhanced by GM-CSF treatment. These data provide a mechanism by which the use of GM-CSF and G-CSF enhances the activity of neutrophils in host defense against bacterial and fungal infection.8 67-70
Supported by grants RO1 GM 63244 (R.W.F.) and RO1 DK 54369 (P.E.N.) from the National Institutes of Health, Bethesda, MD; and a grant from the Arthritis Foundation, Atlanta, GA (P.E.N.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Evelyn A. Kurt-Jones, Department of Medicine, University of Massachusetts Medical School, 364 Plantation St, Lazare Research Building Rm 226, Worcester, MA 01605; e-mail:evelyn.kurt-jones@umassmed.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal