Extending the principle of conventional acute lymphoblastic leukemia (ALL) therapy to transplantation, 77 adult patients receiving autografts in first remission after melphalan with or without total body irradiation were scheduled to receive 6-mercaptopurine (6MP), methotrexate (MTX), and vincristine-prednisone (VP) for 2 years after transplantation to reduce relapse. Seventy-one percent of patients received 6MP, 57% received MTX, and 38% received VP. Thirty patients had a relapse at 1.5 to 80 months (median, 12.5 months), 15 in the first year and 7 beyond 3 years. The cumulative incidence of relapse at 10 years was 42% (95% CI, 31%-55%). The 10-year probabilities of disease-free survival (DFS) and overall (OS) survival were 50% (95% CI, 38%-62%) and 53% (95% CI, 41%-65%), respectively. Age older than 30 years, more than 4 weeks to attain remission, and high-risk karyotypes, for example, t(9;22) or t(4;11), were adverse features contributing to the identification of 3 prognostic risk groups with 0, 1, and 2 adverse features, respectively: standard (47%), intermediate (36%), and high (17%). The 10-year cumulative incidences of relapse (20%, 48%, 85%; P < .0001) and probabilities of DFS (72%, 41%, 10%; P = .0003) were significantly different among these groups. In Cox analysis of the 71 patients alive and well 120 days after transplantation, those receiving 2 or 3 maintenance chemotherapy agents had significantly lower relapse rates and superior DFS compared with those receiving 0 or 1 agent. Our data suggest that maintenance chemotherapy improves the outcome of patients with ALL undergoing autografting. However, it is unlikely that autograft-based strategies are optimal for the high-risk group of patients who should be considered for alternative-donor allograft procedures.
Introduction
Improvement in the outcome of adults with acute lymphoblastic leukemia (ALL) has lagged behind developments in childhood ALL,1,2 although the use of intensive regimens3 has resulted in better outcome. The results of further intensification of therapy by using autotransplantation in adults with ALL have also been generally disappointing with relapse being the most common eventuality.3-16
We have extended the principle of prolonged maintenance chemotherapy in ALL to the autograft setting by administering 6-mercaptopurine (6MP), methotrexate (MTX), and vincristine-prednisone (VP) after autotransplantation in an attempt to decrease relapse rates.17,18 The pilot results have been encouraging.17 18 We now provide long-term follow-up on a group of 77 patients with a minimum follow-up of 2.5 years. This is also the largest single-center series of autotransplantation in first complete remission (CR) ALL.
Patients and methods
Patients
Prospectively gathered data19 on 77 consecutive patients older than 15 years of age who underwent autografting for ALL in first CR between July 1984 and December 1998 at the Leukaemia Unit of the Royal Marsden Hospital were analyzed. Table1 shows the patient characteristics.
Patient characteristics
| . | All . | Marrow . | Blood . | P . |
|---|---|---|---|---|
| No. | 77 | 35* | 42 | |
| Female (%) | 30 (39) | 10 (29) | 20 (48) | .089 |
| Age, y (range) | 26 (16-59) | 25 (17-53) | 32 (16-59) | .01 |
| Presentation leukocyte count (range) | 7.6 (0.7-900) | 12.2 (0.9-900) | 4.4 (0.7-602) | .65 |
| Karyotype (%) | .25 | |||
| t(9;22)† | 6 (8) | 4 (11) | 2 (5) | |
| t(4;11) | 3 (4) | 1 (3) | 2 (5) | |
| Other clonal | 26 (34) | 9 (26) | 17 (40) | |
| Normal | 27 (35) | 11 (31) | 16 (38) | |
| Not available | 15 (19) | 10 (29) | 5 (12) | |
| CNS disease at presentation (%) | 2 (3) | 1 (3) | 1 (2) | .89 |
| Immunophenotype (%) | .11 | |||
| Common | 50 (65) | 18 (51) | 32 (76) | |
| T | 14 (18) | 9 (26) | 5 (12) | |
| Null | 8 (10) | 4 (11) | 4 (10) | |
| B | 3 (4) | 3 (9) | 0 | |
| Unknown | 2 (3) | 1 (3) | 1 (2) | |
| Induction therapy (%) | < .0001 | |||
| MRC UKALL X (or similar) | 49 (63) | 34 (97) | 15 (36) | |
| MRC UKALL XII (or similar) | 28 (37) | 1 (3) | 27 (64) | |
| CR-transplantation interval, wk (range) | 16 (1-90) | 15 (5-69) | 18 (1-90) | .96 |
| Conditioning regimen (%) | < .0001 | |||
| Melphalan-TBI | 35 (45) | 35 (100) | ||
| Melphalan alone | 42 (55) | 42 (100) |
| . | All . | Marrow . | Blood . | P . |
|---|---|---|---|---|
| No. | 77 | 35* | 42 | |
| Female (%) | 30 (39) | 10 (29) | 20 (48) | .089 |
| Age, y (range) | 26 (16-59) | 25 (17-53) | 32 (16-59) | .01 |
| Presentation leukocyte count (range) | 7.6 (0.7-900) | 12.2 (0.9-900) | 4.4 (0.7-602) | .65 |
| Karyotype (%) | .25 | |||
| t(9;22)† | 6 (8) | 4 (11) | 2 (5) | |
| t(4;11) | 3 (4) | 1 (3) | 2 (5) | |
| Other clonal | 26 (34) | 9 (26) | 17 (40) | |
| Normal | 27 (35) | 11 (31) | 16 (38) | |
| Not available | 15 (19) | 10 (29) | 5 (12) | |
| CNS disease at presentation (%) | 2 (3) | 1 (3) | 1 (2) | .89 |
| Immunophenotype (%) | .11 | |||
| Common | 50 (65) | 18 (51) | 32 (76) | |
| T | 14 (18) | 9 (26) | 5 (12) | |
| Null | 8 (10) | 4 (11) | 4 (10) | |
| B | 3 (4) | 3 (9) | 0 | |
| Unknown | 2 (3) | 1 (3) | 1 (2) | |
| Induction therapy (%) | < .0001 | |||
| MRC UKALL X (or similar) | 49 (63) | 34 (97) | 15 (36) | |
| MRC UKALL XII (or similar) | 28 (37) | 1 (3) | 27 (64) | |
| CR-transplantation interval, wk (range) | 16 (1-90) | 15 (5-69) | 18 (1-90) | .96 |
| Conditioning regimen (%) | < .0001 | |||
| Melphalan-TBI | 35 (45) | 35 (100) | ||
| Melphalan alone | 42 (55) | 42 (100) |
Three patients with lymphoblastic lymphoma and bone marrow involvement who were included in our prior report have been excluded here.
Six patients had Ph+ disease detected on conventional cytogenetic studies (G-banding).
Reverse transcription–polymerase chain reaction was not performed routinely. It is therefore possible that the actual proportion of patients with Ph+ disease may be higher.
Thirty-five patients received autologous bone marrow transplants (ABMTs) after conditioning with melphalan and total body irradiation (TBI) between July 1984 and December 1992. The subsequent 42 patients received autologous peripheral blood stem cell transplants (PBSCTs) between January 1993 and April 1994 after conditioning with high-dose melphalan alone. The ABMT population represented patients without HLA-identical sibling donors because adults with suitable sibling donors underwent allogeneic bone marrow transplantation in first remission until December 1992. After December 1992, transplantation with PBSCs was offered as the procedure of first choice irrespective of the availability of matched sibling donors as part of our “sequential high-dose therapy” approach.20 21 The idea behind sequential high-dose therapy was to perform a low-morbidity autograft as the first step, followed by a salvage allograft in second remission in relapsing patients. Exceptions to the sequential high-dose therapy approach included patients taking more than 8 weeks to attain CR and those with central nervous system (CNS) disease and t(9;22), who had allografts in first CR if an HLA-matched sibling donor was available.
Patients with lymphoid blast crisis of chronic myeloid leukemia were not included. Patients with biphenotypic disease (presence of myeloid markers) received different initial chemotherapy and were excluded. All research protocols were approved by the Royal Marsden Hospital institutional review board. All patients gave informed consent for the transplantation.
Induction chemotherapy
Forty-nine patients received induction (weeks 1-4) and early intensification therapy (weeks 5-6) according to the United Kingdom Medical Research Council's (MRC) adult UKALL X Regimen B combination chemotherapy protocol or similar combination induction-intensification. Twenty-eight patients received phase I (weeks 1-4) and phase II (weeks 5-9) induction therapy according to the United Kingdom MRC's adult UKALL XII chemotherapy protocol, which is more intense. Induction chemotherapy was administered either at the Royal Marsden Hospital or at other regional hospitals from where the patients were referred for autografting in first CR.
CNS prophylaxis
Patients treated with melphalan-TBI as part of the conditioning received an additional 800 cGy cranial (no CNS disease at presentation) or craniospinal (CNS disease at presentation) irradiation in 5 daily fractions the week before the TBI. Patients treated with melphalan alone received 2400 cGy cranial irradiation in 15 fractions after hematologic recovery from intensification or phase II induction and before leukapheresis. No patient received testicular irradiation.
Patients without CNS disease received 6 injections of intrathecal MTX (usually 15 mg) over the treatment period prior to the transplantation, and those with CNS disease received triple intrathecal chemotherapy with MTX (usually 15 mg), cytarabine (usually 30 mg), and hydrocortisone (usually 100 mg) until 6 consecutive spinal fluid samples were free of disease. No intrathecal chemotherapy was administered after transplantation.22
Bone marrow harvest
Bone marrow (BM) was harvested from the posterior iliac crests under general anesthesia, usually 6 to 8 weeks after completion of the intensification therapy. Seven patients received BM that was purged in vitro with Campath-1M,23 whereas the remainder received unmanipulated BM. Back-up BM was harvested from the first 7 PBSCT recipients prior to leukapheresis and cryopreserved in case of secondary failure of engraftment with maintenance chemotherapy. This was not used, and the practice was subsequently discontinued.
PBSC harvest
The first 7 recipients of PBSCs received granulocyte colony-stimulating factor (G-CSF; filgrastim) at the dose of 125 μg/m2 subcutaneously every 12 hours starting 2 weeks after the BM harvest for a period of 7 days. Stem cells were harvested on days 5 to 8 (4 consecutive days). The next 35 patients received 12 to 16 μg/kg filgrastim subcutaneously every 24 hours on days 1 to 4, and stem cells were harvested on days 4 and 5. Leukapheresis was performed on a Cobe Spectra (Cobe Industries, Lakewood, CO) continuous-flow cell separator with 150% to 200% of the patient's calculated blood volume being processed at each session.
Cryopreservation and infusion
Cells were cryopreserved with 10% dimethyl sulfoxide using a controlled-rate freezer and were stored in the vapor phase of liquid nitrogen. The cells were rapidly thawed in a water bath at 37°C by the bedside and infused within 2 weeks of collection.
High-dose therapy and transplantation
The conditioning regimen for the ABMT group was 110 mg/m2 melphalan on day −1 and single-fraction TBI on day 0, and 950 cGy (n = 2), 1050 cGy (n = 31), or 1150 cGy (n = 2). TBI was delivered from opposed 60Co sources at a low dose rate (4 cGy/min). Autologous BM was infused on day 0. PBSCT recipients received a single dose of 200 mg/m2 melphalan with hydration on day −1. All cryopreserved cells (excluding back-up BM) were infused on day 0, 24 hours after the administration of melphalan. No growth factors were administered after transplantation.
Supportive care
All patients were treated in protective isolation in rooms with positive-pressure ventilation. Blood products transfused were not screened for cytomegalovirus (CMV) antibody in CMV-seropositive patients. Antibiotic prophylaxis and therapy varied in accordance with prevalent practices and research programs. Broad-spectrum antibiotic therapy was started for fever in the neutropenic phase. Irradiated random platelets were transfused to maintain the platelet count at 20 × 109/L and packed cells to maintain the hemoglobin at 100 g/L.
Maintenance chemotherapy
Maintenance chemotherapy with daily 6MP was usually begun when the leukocytes reached 3 × 109/L and the platelets 100 × 109/L, and was continued for 2 years. Commencement of chemotherapy was not delayed by any factor except for poor hematologic recovery, relapse, or ongoing medical problems (eg, interstitial pneumonitis), which could potentially be exacerbated by starting chemotherapy. Therapy was started with 25 mg 6MP and this was increased weekly or fortnightly in 25- to 50-mg steps. Weekly oral MTX at a dose of 5 mg (increasing in 2.5- to 5-mg steps) was added when the dose of 6MP reached 75 mg. All maintenance chemotherapy was discontinued 2 years after starting the first agent (6MP).
Drug doses were adjusted to maintain the absolute neutrophil count over 1 × 109/L. Initially, monthly vincristine (1.4 mg/m2; maximum 2 mg) with prednisone (40 mg/m2for a week) was administered to patients who could not tolerate myelosuppressive chemotherapy. After 1995, this was added routinely to all patients.
The target doses of 6-MP and MTX were 80 mg/m2 daily and 20 mg/m2 weekly, respectively. Patients received folic acid and prophylaxis for Pneumocystis carinii pneumonia with oral trimethoprim-sulfamethoxazole (if on 50% of the target 6-MP dose) or aerosolized pentamidine (if on < 50% of target 6-MP dose) while on maintenance chemotherapy. Acyclovir was administered to prevent reactivation of Varicella zoster virus.
The average dose of 6-MP and MTX administered was determined by calculating the actual total amount of drug administered after the transplantation and dividing this amount by the number of days (6-MP) or weeks (MTX) from the date of the transplantation to the last date of the chemotherapy. The latter was the day on which chemotherapy was electively discontinued after completion of 2 years or the day of relapse or transplant-related mortality (TRM). The body surface area used for calculations was that at the time of the transplantation procedure.
Statistical analysis
The χ2 test was used to compare categoric variables, and the Wilcoxon rank-sum test was used to compare continuous variables. The probabilities of disease-free survival (DFS) and overall survival (OS) were estimated by the Kaplan-Meier method, and compared using the log-rank test. The cumulative incidence of TRM and relapse was estimated using each type of event as a competing risk for the other.24 25 The significance of differences in TRM and relapse was calculated using the likelihood-ratio statistic for proportional-hazards regression models. Two patients dying in CR of causes obviously unrelated to the transplantation and the underlying disease (homicide and pre-existing ischemic heart disease at 8 and 36 months, respectively) were censored at the time of death for OS and DFS, and were considered competing events for calculating the cumulative incidence of TRM and relapse.
The disease risk stratification was based on age, cytogenetics, and the time taken to attain CR (Tables 2 and3), and was a modification of the German classification proposed by Hoelzer et al.26 The following factors were analyzed in Cox proportional-hazards regression models for effect on relapse, DFS and OS: disease risk (standard versus intermediate versus high), sex, CR-transplantation interval (< 4 versus ≥ 4 months), stem cell source (marrow versus blood), type of induction therapy (UKALL X or similar versus UKALL XII or similar), white blood cell (WBC) count at presentation (< 30 versus ≥ 30 × 109/L), intensity of maintenance therapy (0/1 agent versus 2/3 agents), and immunophenotype (null versus others). The conditioning regimen was not included in the model because of 100% concordance between the stem cell source and conditioning.
Adverse disease factors and risk stratification
| . | No. . | % . | Marrow, n = 35 (%) . | Blood, n = 42 (%) . | P . |
|---|---|---|---|---|---|
| Adverse factor | |||||
| Age, older than 30 y | 36 | 47 | 14 (40) | 22 (52) | .28 |
| t(4;11) or t(9;22) | 9 | 12 | 5 (14) | 4 (10) | .52 |
| Time to CR, more than 4 wk | 9 | 12 | 5 (14) | 4 (10) | .52 |
| Risk stratification | |||||
| Standard, 0 adverse factors | 36 | 47 | 19 (54) | 17 (40) | .39 |
| Intermediate, 1 adverse factor | 28 | 36 | 10 (29) | 18 (43) | |
| High, 2 adverse factors | 13 | 17 | 6 (17) | 7 (17) |
| . | No. . | % . | Marrow, n = 35 (%) . | Blood, n = 42 (%) . | P . |
|---|---|---|---|---|---|
| Adverse factor | |||||
| Age, older than 30 y | 36 | 47 | 14 (40) | 22 (52) | .28 |
| t(4;11) or t(9;22) | 9 | 12 | 5 (14) | 4 (10) | .52 |
| Time to CR, more than 4 wk | 9 | 12 | 5 (14) | 4 (10) | .52 |
| Risk stratification | |||||
| Standard, 0 adverse factors | 36 | 47 | 19 (54) | 17 (40) | .39 |
| Intermediate, 1 adverse factor | 28 | 36 | 10 (29) | 18 (43) | |
| High, 2 adverse factors | 13 | 17 | 6 (17) | 7 (17) |
Outcome of patients with adverse features
| . | No. . | % . | TRM . | Relapse . | DFS . | OS . |
|---|---|---|---|---|---|---|
| Age | ||||||
| Older than 30 y | 36 | 47 | 3% | 63% | 34% | 38% |
| 30 y or less | 41 | 53 | 12% | 23% | 64% | 66% |
| P | .18 | .0007 | .007 | .01 | ||
| Karyotype | ||||||
| t(4;11) or t(9;22) | 9 | 12 | 0% | 89% | 11% | 22% |
| Other | 68 | 88 | 9% | 35% | 55% | 57% |
| P | .99 | .0003 | .002 | .03 | ||
| Time to CR | ||||||
| More than 4 wk | 9 | 12 | 22% | 56% | 14% | 14% |
| 4 wk or less | 68 | 88 | 6% | 39% | 55% | 59% |
| P | .11 | .17 | .05 | .04 |
| . | No. . | % . | TRM . | Relapse . | DFS . | OS . |
|---|---|---|---|---|---|---|
| Age | ||||||
| Older than 30 y | 36 | 47 | 3% | 63% | 34% | 38% |
| 30 y or less | 41 | 53 | 12% | 23% | 64% | 66% |
| P | .18 | .0007 | .007 | .01 | ||
| Karyotype | ||||||
| t(4;11) or t(9;22) | 9 | 12 | 0% | 89% | 11% | 22% |
| Other | 68 | 88 | 9% | 35% | 55% | 57% |
| P | .99 | .0003 | .002 | .03 | ||
| Time to CR | ||||||
| More than 4 wk | 9 | 12 | 22% | 56% | 14% | 14% |
| 4 wk or less | 68 | 88 | 6% | 39% | 55% | 59% |
| P | .11 | .17 | .05 | .04 |
The relapse and TRM represent cumulative incidences at 10 years, and the DFS and OS figures, actuarial 10-year probabilities.
Patient follow-up data are current through August 1, 2001, when the median, minimum, and maximum follow-up duration for surviving patients was 6.9, 2.5 (excluding the patient who was murdered 8 months after autografting), and 17 years, respectively.
Results
Neutrophil recovery to 0.5 × 109/L was complete and sustained in all patients, occurring earlier in PBSCT recipients than ABMT recipients. The stem cell source did not influence platelet recovery significantly.
TRM and morbidity
Six patients died due to transplant-related causes 2 to 8 months (median, 5.5 months) after the ABMT procedure, and none after the PBSCT procedure (P = .007; Fisher exact test). All deaths were due to interstitial pneumonitis and were most likely the result of TBI, which was used for ABMT but not for PBSCT (Table 1). The cumulative 10-year incidence of TRM was 8% (95% CI, 4%-17%).
Maintenance chemotherapy
Table 4 shows the time to starting each maintenance chemotherapy agent and the proportion of patients starting it for the entire group as well as for ABMT and PBSCT recipients separately. The only significant difference—the higher proportion of PBSCT recipients receiving VP—is the result of changed treatment strategy (routine use of VP rather than only in patients with poor marrow function). None of the 22 patients who did not receive 6MP received MTX, whereas 5 received VP.
Time to starting maintenance chemotherapy
| Drug . | No. (%) . | Marrow (%) . | Blood (%) . | P . | Time to starting drug, d (range) . | Probability of being on drug 1 y after transplantation . | |||
|---|---|---|---|---|---|---|---|---|---|
| All, % . | Marrow, % . | Blood, % . | P . | ||||||
| 6MP | 55 (71) | 25 (71) | 30 (71) | 1 | 61 (15-678) | 74 | 84 | 67 | .32 |
| MTX | 44 (57) | 18 (51) | 26 (62) | .36 | 114 (34-891) | 51 | 60 | 46 | .99 |
| VP | 29 (38) | 8 (23) | 21 (50) | .015 | 83 (24-692) | 38 | 28 | 47 | .03 |
| Drug . | No. (%) . | Marrow (%) . | Blood (%) . | P . | Time to starting drug, d (range) . | Probability of being on drug 1 y after transplantation . | |||
|---|---|---|---|---|---|---|---|---|---|
| All, % . | Marrow, % . | Blood, % . | P . | ||||||
| 6MP | 55 (71) | 25 (71) | 30 (71) | 1 | 61 (15-678) | 74 | 84 | 67 | .32 |
| MTX | 44 (57) | 18 (51) | 26 (62) | .36 | 114 (34-891) | 51 | 60 | 46 | .99 |
| VP | 29 (38) | 8 (23) | 21 (50) | .015 | 83 (24-692) | 38 | 28 | 47 | .03 |
Seventeen patients received no maintenance chemotherapy at all because of early TRM within 4 months (n = 3), pulmonary problems subsequently terminating in TRM beyond 4 months (n = 2), relapse within 4 months (n = 3), sluggish hematologic recovery terminating in relapse beyond 4 months (n = 3), unknown/patient preference (n = 5), and noncompliance/lifestyle (n = 1; this patient eventually became a homicide victim). Nine patients received only a single drug (5 VP and 4 6MP), 34 patients received 2 drugs (27 6MP-MTX and 7 6MP-VP), whereas 17 patients received all 3 drugs.
The average daily dose of 6MP administered was 3 to 109 mg/m2 (median, 33 mg/m2). The average weekly dose of MTX administered was 0.2 to 18.1 mg/m2 (median, 2.8 mg/m2). Maintenance chemotherapy was tolerated well, and other than transient myelosuppression requiring dose reduction or temporary cessation of therapy, no other side effects were seen.
Relapse and therapy after relapse
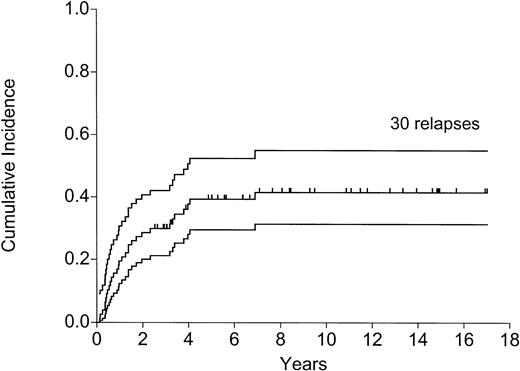
Thirty patients (39%) had a relapse at 1.5 to 80 months (median, 12.5 months); 15 in the first year, 7 in the second year, 1 in the third, 5 in the fourth, 1 in the fifth, and 1 at almost 7 years. All relapses were confirmed to be immunophenotypically and cytogenetically (where applicable) identical to the original disease. No case of second or secondary leukemia was seen. Figure 1shows the cumulative incidence of relapse.
Eighteen patients died of relapsed disease or toxicity of salvage chemotherapy. Eleven patients underwent an allograft from an HLA-matched sibling (n = 6) or a matched unrelated donor (n = 5) in second CR, and 1 is awaiting allograft. Nine died of transplant-related toxicity (n = 7) or relapse (n = 2), and 2 are alive and well in second CR at 7 years (HLA-identical sibling) and 1.5 years (unrelated donor).
OS and DFS
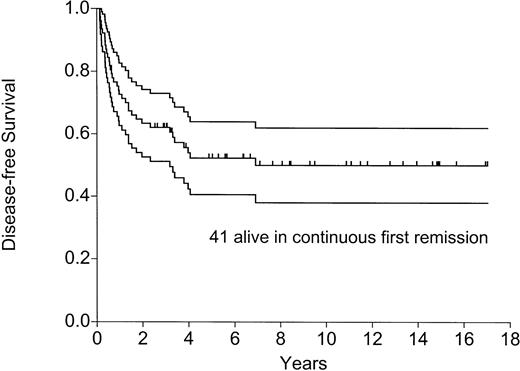
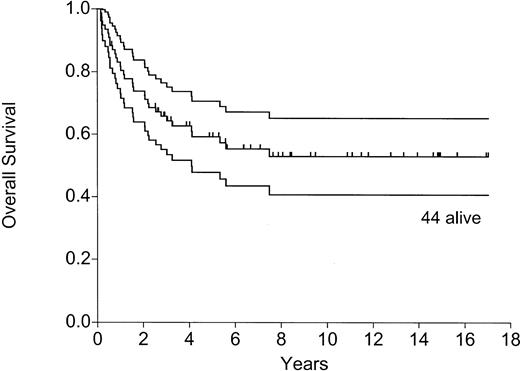
As of August 1, 2001, 44 of 77 patients were alive and in remission 8 months to 17 years (median, 6.9 years) after the transplantation—41 in continuous first CR, 2 in second CR after allogeneic BMT, and 1 in second CR after chemotherapy (awaiting allograft). Figures 2 and3 show the actuarial probabilities of DFS and OS. All patients are off maintenance chemotherapy.
Identification of risk groups
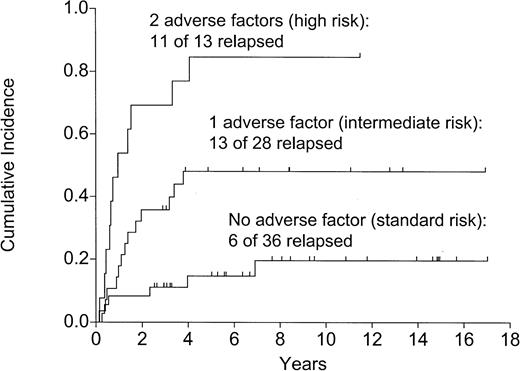
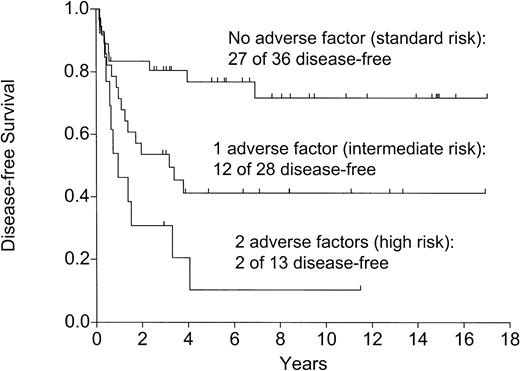
The 3 adverse risk factors, age older than 30 years, t(9;22), t(4;11), and more than 4 weeks to attain CR (Table 2), were identified to be high-risk factors for relapse, DFS, and OS (Table 3). As Table 2shows, these factors could be combined to produce 3 risk groups. The outcome of these 3 groups in terms of relapse (Figure4), DFS (Figure5), and OS (P = .003) was highly significantly disparate.
Relapse: effect of the number of adverse factors (P< .0001).
Relapse: effect of the number of adverse factors (P< .0001).
Disease-free survival: effect of the number of adverse factors (P=.0003).
Disease-free survival: effect of the number of adverse factors (P=.0003).
The effect of maintenance chemotherapy
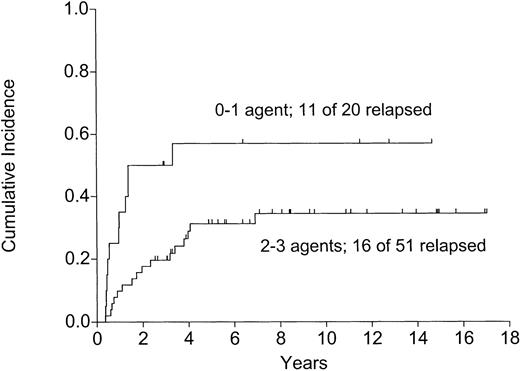
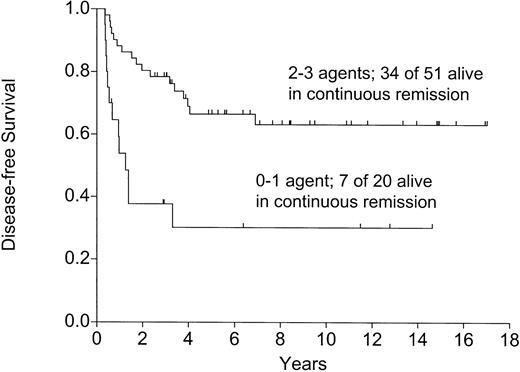
Among patients alive and well at 120 days, those getting 2 or 3 maintenance chemotherapy agents had a significantly lower relapse rate (Figure 6), higher DFS (Figure7), and OS (P = .0008). This time point was chosen to eliminate the 6 patients dying early, 3 of relapse and 3 of TRM, before becoming eligible to receive maintenance chemotherapy.
The effect of maintenance chemotherapy on relapse in patients alive and well on day 120 following the autograft (P=.004).
The effect of maintenance chemotherapy on relapse in patients alive and well on day 120 following the autograft (P=.004).
The effect of maintenance chemotherapy on disease-free survival in patients alive and well on day 120 following the autograft (P=.0005).
The effect of maintenance chemotherapy on disease-free survival in patients alive and well on day 120 following the autograft (P=.0005).
In the same population of patients, there was no significant difference in the outcome of those getting no maintenance chemotherapy and those getting any maintenance chemotherapy (data not shown). There was no obvious relationship between the amount of chemotherapy given and outcome (data not shown).
Cox analysis
Table 5 shows the results of the multivariable analysis adjusted by survival on day 120 after transplantation. Standard-risk disease, longer CR-autograft intervals, and more intense maintenance chemotherapy were associated with lower relapse and higher DFS. OS was beneficially affected by standard-risk disease and more intense maintenance chemotherapy.
Cox analysis of factors affecting outcome independently among patients alive and disease-free on day 120
| Outcome . | Covariate . | Favorable . | Adverse . | Risk ratio, 95% CI (range) . | P . |
|---|---|---|---|---|---|
| Relapse | Disease risk | Standard | Intermediate | 3.3 (1.1-9.8) | .034 |
| High | 11.1 (3.5-35.1) | < .0001 | |||
| CR-transplantation interval | ≥ 120 d | < 120 d | 2.6 (1.1-6.2) | .031 | |
| Maintenance therapy | 2-3 agents | 0-1 agent | 2.7 (1.0-7.2) | .049 | |
| DFS | Disease risk | Standard | Intermediate | 3.1 (1.2-7.9) | .018 |
| High | 7.2 (2.5-20.6) | < .0001 | |||
| CR-transplantation interval | ≥ 120 d | < 120 d | 2.3 (1.0-5.1) | .038 | |
| Maintenance therapy | 2-3 agents | 0-1 agent | 2.5 (1.0-6.0) | .046 | |
| OS | Disease risk | Standard | Intermediate | 3.0 (1.2-7.7) | .024 |
| High | 3.5 (1.2-10.5) | .024 | |||
| Maintenance therapy | 2-3 agents | 0-1 agent | 2.9 (1.2-7.1) | .022 |
| Outcome . | Covariate . | Favorable . | Adverse . | Risk ratio, 95% CI (range) . | P . |
|---|---|---|---|---|---|
| Relapse | Disease risk | Standard | Intermediate | 3.3 (1.1-9.8) | .034 |
| High | 11.1 (3.5-35.1) | < .0001 | |||
| CR-transplantation interval | ≥ 120 d | < 120 d | 2.6 (1.1-6.2) | .031 | |
| Maintenance therapy | 2-3 agents | 0-1 agent | 2.7 (1.0-7.2) | .049 | |
| DFS | Disease risk | Standard | Intermediate | 3.1 (1.2-7.9) | .018 |
| High | 7.2 (2.5-20.6) | < .0001 | |||
| CR-transplantation interval | ≥ 120 d | < 120 d | 2.3 (1.0-5.1) | .038 | |
| Maintenance therapy | 2-3 agents | 0-1 agent | 2.5 (1.0-6.0) | .046 | |
| OS | Disease risk | Standard | Intermediate | 3.0 (1.2-7.7) | .024 |
| High | 3.5 (1.2-10.5) | .024 | |||
| Maintenance therapy | 2-3 agents | 0-1 agent | 2.9 (1.2-7.1) | .022 |
Effect of maintenance chemotherapy by risk group
Table 6 shows that receiving more intensive maintenance chemotherapy made a significant difference in outcome in the standard-risk group and a less-marked difference to patients in the intermediate-risk group. On the other hand, it made no difference to patients in the high-risk group at all.
The effect of maintenance chemotherapy by risk group
| Risk . | No. of maintenance chemotherapy agents . | n . | Relapse, % (range) . | DFS, % (range) . | OS, % (range) . |
|---|---|---|---|---|---|
| Standard | 0-1 | 7 | 29 (9-92) | 43 (6-80) | 36 (0-75) |
| 2-3 | 29 | 17 (7-44) | 79 (62-96) | 83 (67-99) | |
| P | .07 | .002 | .0007 | ||
| Intermediate | 0-1 | 12 | 50 (28-88) | 25 (5-50) | 33 (7-60) |
| 2-3 | 16 | 45 (26-79) | 55 (29-80) | 52 (25-78) | |
| P | .09 | .008 | .03 | ||
| High | 0-1 | 7 | 86 (63-100) | 14 (0-40) | 14 (0-40) |
| 2-3 | 6 | 83 (58-100) | 0 | 25 (0-65) | |
| P | .75 | .75 | .45 |
| Risk . | No. of maintenance chemotherapy agents . | n . | Relapse, % (range) . | DFS, % (range) . | OS, % (range) . |
|---|---|---|---|---|---|
| Standard | 0-1 | 7 | 29 (9-92) | 43 (6-80) | 36 (0-75) |
| 2-3 | 29 | 17 (7-44) | 79 (62-96) | 83 (67-99) | |
| P | .07 | .002 | .0007 | ||
| Intermediate | 0-1 | 12 | 50 (28-88) | 25 (5-50) | 33 (7-60) |
| 2-3 | 16 | 45 (26-79) | 55 (29-80) | 52 (25-78) | |
| P | .09 | .008 | .03 | ||
| High | 0-1 | 7 | 86 (63-100) | 14 (0-40) | 14 (0-40) |
| 2-3 | 6 | 83 (58-100) | 0 | 25 (0-65) | |
| P | .75 | .75 | .45 |
The figures represent 10-year cumulative incidence of relapse and the 10-year probabilities of DFS and OS.
Discussion
Our data suggest that standard ALL-type maintenance chemotherapy can be administered safely after autotransplantation in patients with ALL. Although only a randomized study can answer the question of its benefit, if any, 2 observations in this series of patients suggest that maintenance chemotherapy may contribute to an improved outcome in terms of reduced relapse and increased survival. The first is that higher intensity of maintenance therapy was clearly associated with better outcome. The second is that the results reported here are better than most reported using the more conventional approach of autograft without any posttransplantation intervention.
An obvious shortcoming of the first observation here is that autografted patients with compromised myeloid reserve have a higher risk of disease recurrence. These are also the patients who are likely to be unable to start chemotherapy after transplantation. This obstacle was partially overcome by excluding patients having relapse early. However, even after excluding the 3 patients who had a relapse within 4 months, 4 additional patients continued to have poor counts and eventually had relapse without being able to start any maintenance therapy at all.
Another indirect observation that may support using posttransplantation therapy in ALL comes from the International Bone Marrow Transplant Registry, which showed that the use of methotrexate after transplantation as graft-versus-host disease (GVHD) prophylaxis decreased relapse in patients undergoing allografting for ALL independently of clinically obvious GVHD, perhaps secondary to a direct antileukemic effect.27
In a French cooperative group study that showed no impact of ABMT on survival compared to chemotherapy alone, late relapses were significantly less common among autografted patients,12and relapse relatively early after the transplantation was an important cause of treatment failure. It is conceivable that posttransplantation maintenance chemotherapy in a setting like this may reduce the incidence of early posttransplantation relapse and improve DFS by eliminating clonogenic leukemic cells reinfused with the graft, or more likely, have remained viable in the patient despite the conditioning regimen.
Other posttransplantation treatments that have been used to decrease relapse rates after autotransplantation in ALL have included intensive chemotherapy,28 interleukin 2 infusions,11and cell-mediated immunotherapy with haploidentical T lymphocytes.29 Reports describing these approaches have suffered from small patient numbers and inadequate follow-up; making any assessment difficult.
Patients with HLA-identical siblings in the ABMT era received allografts at the Royal Marsden Hospital. Those with HLA-identical siblings in the PBSCT era underwent autografting and the allograft was reserved for salvage therapy of relapse. The reasoning behind this was avoidance of toxic therapy (allograft) unless absolutely essential. Although treatment-related mortality was indeed reduced with the elimination of TBI and the use of blood-derived stem cells, this did not necessarily make the outcome of subsequent salvage therapy any easier or more successful; only 2 of 11 patients receiving allografts survive, with high TRM rates that were no different from rates we had observed in the past.30 These patients were conditioned using conventional-intensity regimens. It is possible that the outcome may have been better if reduced-intensity regimens had been used. In any case, we feel that an allograft from an HLA-identical sibling is probably the best treatment option in adult patients with ALL who require a transplantation in first CR, especially with the possibility of augmented graft-versus-leukemia reactions from the use of blood-derived stem cells31,32 and limited TRM with the use of an adequate number of CD34+ cells for the transplant.33
What is the role of the pretransplantation conventional chemotherapy administered for induction and intensification/consolidation? It was disappointing to see that there was no difference in the outcome of patients receiving less intensive therapy initially (UKALL X and similar) compared with those getting more intensive therapy (UKALL XII). This is unlike in acute myeloid leukemia (AML) where in vivo purging before transplantation, achieved by administering at least 2 cycles of consolidation chemotherapy, makes a significant impact on outcome.34 Because graft source and conditioning regimen were changed simultaneously, and there was a strong association between these and the initial induction therapy, the relative contribution of each to outcome cannot be assessed. A beneficial effect of TBI of reduction of relapse cannot be ruled out because the higher-intensity induction chemotherapy that non-TBI patients received could have offset the disadvantage of not having received TBI. It is also possible that the difference between less and more intensive initial therapy is negated to some extent by posttransplantation maintenance therapy.
There has been a strong trend toward the use of alternative-donor transplants rather than autografts in ALL because of poor results of autotransplantation in ALL. However, high-risk allografts may not be the right approach for all patients. A recent registry comparison of unrelated donor transplants and autografts in first and second remission ALL found that although relapse rates were lower with allografts, the benefits of this were negated by significantly higher TRM. Because of this, survival was comparable with the 2 modalities of transplantation.35 In view of this, it is clearly important to identify patients who are likely to do poorly with an autograft and offer them alternative-donor transplantation. Those without features that increase the risk of relapse to prohibitive levels may be better off with an autograft. Our data provide some clues toward who these patients may be.
The data in Table 6 suffer from very small patient numbers in the various subgroups and must be interpreted with caution. They suggest that the outcome of patients with high-risk disease is dismal with autotransplantation whether posttransplantation chemotherapy is administered or not. This is the group of patients most likely to benefit from high-risk allograft approaches.35 What should be done for patients with standard-risk disease? It is tempting to suggest an autograft as the first approach even in patients who have an HLA-identical sibling donor because of the excellent outcome seen here. Relapsing patients can then be salvaged by an allograft in the second remission. However, we do not have adequate data to suggest an optimum approach in this situation, especially because of the poor outcome of allografted patients in this series. Patients with intermediate-risk disease ought to be allografted if an HLA-identical sibling is available. In the absence of a sibling donor, an autograft should be the preferred mode of therapy, to be followed by a salvage, high-risk, alternative-donor allograft in case of relapse.
Within the limitations of the data in Table 6 (small patient numbers), the suggestion that patients with the best disease also respond further to the maximum extent with posttransplantation therapy is interesting and is in keeping with observations in AML that patients with good-risk karyotypes gain the most from further intensification of therapy; either with chemotherapy36 or with autotransplantation.37
We conclude that autotransplantation followed by maintenance chemotherapy is an excellent way of intensifying therapy in adult patients with ALL unless the disease is high-risk. This is a strategy that should ideally be explored in a randomized trial. In the absence of such a study, it appears safe enough to use routinely in practice to improve outcome of autografted ALL patients.
Prepublished online as Blood First Edition Paper, May 13, 2002; DOI 10.1182/blood-2002-03-0776.
Supported by the Bud Flanagan Leukaemia Fund, David Adams Leukaemia Fund, Cancer Research Campaign, and Institute of Cancer Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ray Powles, Head, Leukaemia and Myeloma Units, Royal Marsden Hospital, Downs Rd, Sutton, Surrey SM2 5PT, United Kingdom.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal