Preclinical and clinical evidence suggest a potential advantage for infusional therapy in lymphoma. Sixty-two analyzable patients with predominantly intermediate-grade non-Hodgkin lymphoma received cyclophosphamide (200 mg/m2 per day), doxorubicin (12.5 mg/m2 per day), and etoposide (60 mg/m2per day) (CDE) by continuous intravenous infusion for 4 days (96 hours) every 3 weeks for a maximum of 8 cycles. By the age-adjusted International Prognostic Index (IPI), 42% were at high risk and 58% were at high-intermediate risk. Complete response (CR) occurred in 30 (48%) patients (95% confidence interval [CI], 35%, 64%), and partial response occurred in 16 (26%) patients, yielding an overall response rate of 74% (95% CI, 62%, 84%). Failure-free survival (FFS) rates at 1 and 2 years were 55% (95% CI, 43%, 67%) and 50% (95% CI, 38%, 62%), respectively. When comparing the outcome for 62 patients receiving infusional CDE with historical data derived from 927 IPI-matched lymphoma patients using a Cox proportional hazards model, there was a nonsignificant trend favoring CDE in FFS (P = .12) and overall survival (P = .09). Severe or life-threatening toxicity included neutropenia (68%), anemia (57%), thrombocytopenia (44%), and infection (24%). Two patients (3%) died of treatment-related infectious complications. The primary end point of improving 1-year FFS from 55% to 70% was not achieved with infusional CDE given as initial therapy in patients with poor-risk intermediate-grade lymphoma. It is unlikely that infusional therapy as used in this study produces a 25% or greater relative improvement in FFS compared with standard therapy.
Introduction
Some preclinical and clinical evidence suggests a therapeutic advantage for administering cytotoxic therapy by way of a protracted rather than a brief intermittent schedule. For example, tumor cell lines exhibit less resistance when exposed to low concentrations of natural products (doxorubicin, etoposide, and vincristine) for prolonged periods compared with brief exposure to higher concentrations in vitro.1,2 Other evidence suggests that fractionated schedules of alkylating agents such as cyclophosphamide are also more effective in vitro and in vivo than single administration at a higher dose.3 Clinical evidence suggesting schedule dependence includes reports of the activity of infusional cyclophosphamide in refractory acute lymphocytic leukemia4 and infusional doxorubicin and vincristine in refractory myeloma.5 Furthermore, a randomized trial in extensive-disease small cell lung carcinoma demonstrated that intravenous etoposide induces a significantly higher objective response rate (89% vs 10%) when given as a daily 1-hour infusion for 5 days compared with an equivalent dose given as a single 24-hour infusion.6
These observations led to a series of trials performed at the Albert Einstein Comprehensive Cancer Center and the National Cancer Institute (NCI) evaluating infusional therapy for the treatment of non-Hodgkin lymphoma in patients who experienced relapse or disease progression during treatment with intravenous bolus cyclophosphamide and doxorubicin-based therapy. The Einstein group7 evaluated a 96-hour infusion of cyclophosphamide (187.5-200.0 mg/m2 per day), doxorubicin (12.5 mg/m2 per day), and etoposide (60 mg/m2 per day) (CDE) in 58 patients with relapsed intermediate-grade non-Hodgkin lymphoma and reported a 17% complete response (CR) rate and 52% objective response rate (complete and partial responses). Likewise, the NCI group evaluated a 96-hour infusion of etoposide (50 mg/m2 per day), doxorubicin (10 mg/m2 per day), vincristine (0.4 mg per day) plus intravenous bolus cyclophosphamide (750 mg/m2 after completion of the infusion), and prednisone (60 mg daily for 5 days) (EPOCH) in 125 assessable patients with relapsed or resistant lymphoma, of whom 24% achieved CR and 74% achieved objective response.8,9 A community-based multicenter phase 2 study in 96 assessable patients with relapsed Hodgkin disease and non-Hodgkin lymphoma reported a 25% CR rate and a 63% response rate for EPOCH,10 and several smaller studies using EPOCH11,12 or similar regimens13 reported comparable findings. Encouraging activity has also been reported for both CDE14-19 and EPOCH20 when used as first-line therapy for HIV-associated lymphoma. Although it is estimated that infusional CDE costs approximately twice as much to administer in an outpatient setting as CHOP,21 the cost would be justifiable if it were associated with even a modest benefit.
Based on these considerations, the Eastern Cooperative Oncology Group initiated a multi-institutional phase 2 study of infusional CDE as first-line therapy in patients with intermediate- and high-grade lymphoma expected to have a poor prognosis with standard therapy (E3493). Although the eligibility criteria permitted patients with high-grade lymphoma to enroll, most patients had intermediate-grade lymphoma (as classified by the National Cancer Institute Working Formulation).22 The trial included only patients 65 years of age or younger because another trial (E4494) evaluating standard therapy with or without rituximab was initiated at the same time in an elderly population. The age-adjusted International Prognostic Index23 was used to select patients for the study. Patients were required to have at least 2 of 3 adverse prognostic features, including elevated serum lactate dehydrogenase (LDH) level, stage III or IV disease, or poor performance status (ECOG 2, 3, or 4). The primary objective of the study was to determine whether infusional CDE improved 1-year failure-free survival (FFS) rate in this population from 55% to 70%.
Patients, materials, and methods
Patient selection
Patients were required to have biopsy- or cytology-proven intermediate- or high-grade non-Hodgkin lymphoma (categories D [follicular large cell], E [diffuse small cleaved cell], F [diffuse mixed small and large cell], G [diffuse large cell], H [large cell immunoblastic], and J small noncleaved cell] by the National Cancer Institute Working Formulation),22 to have measurable or evaluable disease, to be younger than 66 years and at least 18 years of age, and to have at least 2 of the following adverse prognostic features defined by the age-adjusted International Prognostic Index (IPI): stage III or IV disease, elevated serum LDH level, or ECOG performance status of 2, 3, or 4. Other eligibility criteria included serum creatinine less than 2-fold the upper limits of normal (or a measured or calculated creatinine clearance of at least 60 mL/min), no history of chemotherapy or irradiation, no known history of human immunodeficiency virus infection, no history of clinically significant heart disease, not pregnant or lactating, and willing to give written informed consent. Patients with category J lymphoma were required to have negative findings on cytologic examination of cerebrospinal fluid obtained by lumbar puncture to be eligible. In 4 patients, a protocol exemption was made to allow patients with anaplastic large cell lymphoma to enroll in the study, though they were required to meet all the other eligibility criteria because it was believed at that time that this condition was associated with a prognosis similar to diffuse large cell lymphoma.24
Chemotherapy
Patients received cyclophosphamide (200 mg/m2 per day), doxorubicin (12.5 mg/m2 per day), and etoposide (60 mg/m2 per day) by continuous intravenous infusion for 4 days (96 hours). The daily dose of cyclophosphamide and doxorubicin was admixed in the same bag of intravenous fluid (1 L) and infused through a central venous catheter, and etoposide was diluted in a separate liter of intravenous fluid and infused through a separate central venous or peripheral line. Doxorubicin and cyclophosphamide had previously been shown to be compatible when admixed in this fashion.25 Treatment was repeated every 21 or more days if the absolute neutrophil count (ANC) was at least 1500/μL, platelet count was at least 100 000/μL, and the patient had satisfactorily recovered from nonhematologic toxicity. Filgrastim (granulocyte–colony-stimulating factor [G-CSF]; Neupogen; Amgen, Thousand Oaks, CA) was given at a standard daily dose (5 μg/kg) subcutaneously beginning approximately 24 hours after the completion of the infusion and continued until the postnadir neutrophil count exceeded 10 000/μL. G-CSF was resumed before the next cycle if the neutrophil count became lower than 1500/μL, and it was discontinued 24 hours before the next cycle. The dose of each agent in CDE was reduced in subsequent cycles if there was severe neutropenia (neutrophil nadir less than 500/μL lasting more than 5 days) associated with fever requiring parenteral antibiotic therapy, severe thrombocytopenia (platelet nadir less than 20 000/μL), or severe (grade 3 or higher) mucositis. All toxicity was graded according the National Cancer Institute Common Toxicity Criteria. Doses were reduced by 25% for the first dose reduction and 10% for subsequent dose reductions. In patients with hepatic dysfunction, doxorubicin dose was modified based on the total bilirubin level. For those whose levels were greater than 5 mg/L or more per day, doxorubicin was withheld; for those whose levels were 3.0 to 4.9 mg/L per day, doxorubicin was reduced 75%; and for those whose levels were 1.6 to 2.9 mg/L per day, it was reduced 50%. Patients with renal dysfunction had etoposide dose reduction proportionate to the creatinine clearance (assuming that 60 mL/min was 100%). Patients continued treatment with CDE for 2 cycles beyond achievement of CR, for a minimum of 4 and a maximum of 8 cycles. Patients were removed from the study if there was progressive disease, stable disease after 4 cycles, or toxicity that in the judgment of the treating physician was prohibitive.
Staging evaluation and evaluation of response
All patients were required to undergo chest roentgenography, computed tomography of the abdomen and pelvis, and unilateral bone marrow biopsy within 6 weeks of registration. Radiographic studies demonstrating measurable or evaluable disease were required within 2 weeks of registration. All studies that demonstrated measurable or evaluable disease were repeated after cycle 4, cycle 6, and cycle 8 (if administered). Response was defined using the Eastern Cooperative Oncology Group response criteria.26 CR was defined as complete regression of palpable, radiographic, and histologic evidence of disease. Lymph nodes were required to decrease in size to less than 1 cm; larger lymph nodes required biopsy for confirmation of CR or had to be shown not to have enlarged on repeat evaluation no sooner than 3 months later. Partial response was defined as at least a 50% reduction in the sum of products of the dimensions of the measurable lesions that persisted for at least 4 weeks. Confirmation of CR or PR was required by repeating the physical examination or radiographic study no sooner than 4 weeks and documenting further shrinkage or stability. Patients were required to continue treatment for 2 cycles beyond achieving CR or for a maximum of 8 cycles.
Other required studies
All patients had complete blood counts taken twice weekly and liver function tests before each cycle of CDE. Representative pathologic materials were centrally reviewed by a single hematopathologist (TN), and all specimens were categorized by the National Cancer Institute Working Formulation, which was an accepted classification system at the time this study was conducted.
Statistical considerations
The primary objectives of the trial were to estimate the FFS rate, CR rate, and toxicity of this regimen. Accrual of 75 patients was planned to ensure 66 eligible patients. With this sample size, it was estimated that the trial would have 90% power (10% one-sided alpha level test) to detect a 15% improvement in 1-year FFS) from 55% to 70%. In terms of a parametric cure rate model,27 this corresponds to an improvement of the long-term plateau to 55% with no change in the exponential treatment failure (with an annual hazard rate of approximately 1.15) or to an improvement of the long-term plateau to 50% with a 25% decrease in the hazard rate among patients for whom the treatment fails. Exact 95% confidence intervals were computed for the objective response rate and the complete response rate. The Kaplan-Meier method28 was used to estimate failure-free survival, overall survival, duration of response, and disease-free survival. Failure-free survival was defined as the time between registration in the study and disease progression, relapse, or death from any cause. Duration of response was defined as the time between first documentation of response and relapse or last follow-up without relapse. Disease-free survival was defined as the time between first achievement of complete response and relapse or last follow-up without relapse. The initial design allowed for early stopping. In this design, if fewer than 16 patients of the first 30 eligible patients did not achieve complete response, the usefulness of the regimen was to be reconsidered. This design had a high likelihood (77% or higher) of ending early if the true complete response rate was less than that of standard regimens (45% or less) and a high likelihood (94% or higher) of continuing if the true complete response rate was at least 65%. In May 1996, the stopping rule was removed based on results in HIV-associated lymphoma with this regimen, suggesting a high response rate in populations at poor risk.19
The study population had more adverse prognostic features than initially anticipated. Therefore, an analysis was performed that was not specified a priori by the treatment protocol. Comparison was made with a matched population of patients with intermediate-grade non-Hodgkin lymphoma derived from the IPI database. Of the 27 possible combinations of the 5 IPI risk groups, patients treated in this trial fell into 13 of the 27 groups. Of the 1321 patients with 2 to 5 IPI factors, 927 had one of the 13 combinations of factors observed in E3493; therefore, they were selected as the control population. Failure-free survival and overall survival estimates were compared using a log-rank test with data up to 4.2 years (the maximum follow-up among survivors in this study). Selected patients in the IPI database with follow-up longer than 4.2 years were censored at 4.2 years. A Cox proportional hazards model was used to evaluate differences in failure-free survival and overall survival while accounting for the prognostic factors.
Results
Patient characteristics
Of 80 patients enrolled, 11 were pathology exclusions, 5 were ineligible, and 2 did not receive any therapy, leaving 62 analyzable patients. Reasons for pathology exclusion included insufficient material for review (n = 3), low-grade histology (n = 4), or other histologic types (n = 4) not specified in the eligibility criteria. Reasons for ineligibility included having only one adverse prognostic feature (n = 4) and failure to submit pathologic material (n = 1). Patients were enrolled between March 1995 and November 1997.
Characteristics of the 62 analyzable patients are shown in Table1. Adverse prognostic features by IPI included stage III-IV disease in 92% of the patients, elevated serum lactate dehydrogenase levels in 95%, poor performance status (ECOG 2, 3, or 4) in 55%, more than one extranodal site in 48%, and age older than 60 years in 24%. By the age-adjusted IPI index, 42% of the patients were at high risk and 58% were at high-intermediate risk. Histologic findings consisted of diffuse large cell lymphoma in 48 (77%) patients, follicular large cell lymphoma in 3 (5%) patients, diffuse mixed small and large cell lymphoma in 1 patient, diffuse immunoblastic lymphoma in 1 patient, diffuse small noncleaved cell lymphoma in 1 patient, and other subtypes that could not be classified by the Working Formulation in 8 (13%) patients. Other subtypes included anaplastic large cell lymphoma (n = 4), peripheral T-cell lymphoma (n = 1), high-grade Burkitt-like lymphoma (n = 2), and T-cell–rich diffuse large cell lymphoma (n = 1).
Patient characteristics
| Age, y | |
| Median | 52 |
| Range | 20-65 |
| Older than 60 (%) | 15 (24) |
| Sex (%) | |
| Male | 36 (58) |
| Female | 26 (42) |
| Stage (%) | |
| I | 2 (3) |
| II | 3 (5) |
| III | 19 (31) |
| IV | 38 (61) |
| Serum LDH (%) | |
| Normal | 3 (5) |
| Elevated | 59 (95) |
| ECOG performance status (%) | |
| 0/1 | 6/22 (45) |
| 2/3/4 | 25/8/1 (55) |
| Extranodal sites of disease (%) | |
| None | 9 (15) |
| One | 23 (37) |
| Two or more | 30 (48) |
| IPI (%) | |
| Low-intermediate, 2* | 18 (29) |
| High-intermediate, 3* | 21 (34) |
| High, 4-5* | 23 (37) |
| Age-adjusted IPI (%) | |
| High-intermediate, 2* | 36 (58) |
| High, 3* | 26 (42) |
| Histology (%) | |
| Follicular large cell | 3 (5) |
| Diffuse, mixed small and large cell | 1 (2) |
| Diffuse large cell or immunoblastic | 48/1 (79) |
| Diffuse small noncleaved cell | 1 (2) |
| Other types | 8 (13)† |
| Age, y | |
| Median | 52 |
| Range | 20-65 |
| Older than 60 (%) | 15 (24) |
| Sex (%) | |
| Male | 36 (58) |
| Female | 26 (42) |
| Stage (%) | |
| I | 2 (3) |
| II | 3 (5) |
| III | 19 (31) |
| IV | 38 (61) |
| Serum LDH (%) | |
| Normal | 3 (5) |
| Elevated | 59 (95) |
| ECOG performance status (%) | |
| 0/1 | 6/22 (45) |
| 2/3/4 | 25/8/1 (55) |
| Extranodal sites of disease (%) | |
| None | 9 (15) |
| One | 23 (37) |
| Two or more | 30 (48) |
| IPI (%) | |
| Low-intermediate, 2* | 18 (29) |
| High-intermediate, 3* | 21 (34) |
| High, 4-5* | 23 (37) |
| Age-adjusted IPI (%) | |
| High-intermediate, 2* | 36 (58) |
| High, 3* | 26 (42) |
| Histology (%) | |
| Follicular large cell | 3 (5) |
| Diffuse, mixed small and large cell | 1 (2) |
| Diffuse large cell or immunoblastic | 48/1 (79) |
| Diffuse small noncleaved cell | 1 (2) |
| Other types | 8 (13)† |
n = 62 patients.
Number of adverse prognostic factors.
Includes anaplastic large cell (n = 4), peripheral T cell (n = 1), high-grade Burkitt-like (n = 2), and T-cell–rich diffuse large cell lymphoma (n = 1).
Response data
Thirty patients had a complete response (48%; 95% CI, 35%, 64%), and 16 patients (26%) had a partial response, yielding an overall response rate of 74% (95% CI, 62%, 84%). Among the 46 responders, 20 (9 of the 30 patients in CR and 11 of the 16 patients in PR) had relapses. The median duration of response has not yet been reached at 25 months. Among those who have not had relapses (n = 26), the median follow-up time was 2.2 years. Among those who have not had relapses and are still alive (n = 22), the median follow-up time is 2.4 years. Four of 26 complete responders died of causes other than lymphoma (myocardial infarction, chronic lung disease, pulmonary embolus, and unknown cause).
Failure-free survival and overall survival
Thirty-one (50%) patients were alive at the time of this analysis after a median follow-up of 3.3 years (range, 2.1-4.2 years). Median FFS was 1.9 years, and median overall survival (OS) was 2.7 years. FFS rates at 1 year and 2 years were 55% (95% CI, 43%, 67%) and 50% (95% CI, 38%, 62%), respectively. OS rates at 1 year and 2 years were 74% (95% CI, 63%, 85%) and 58% (95% CI, 46%, 70%), respectively.
Outcome by International Prognostic Index
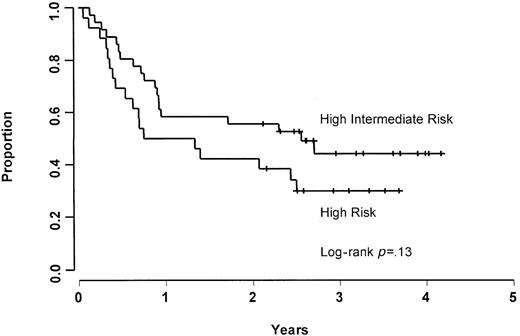
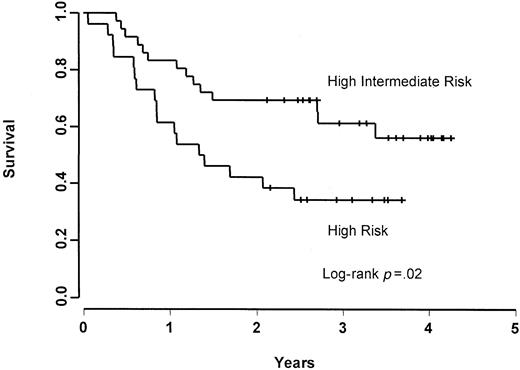
The CR, 2-year FFS, and 2-year OS rates were 56%, 56%, and 69%, respectively, for patients in the age-adjusted high-intermediate risk group, and 38%, 38%, and 42%, respectively, for patients in the age-adjusted high-risk group. Patients in the age-adjusted high-risk group were found to have a worse (though nonsignificant;P = .13) FFS rate (Figure 1) and a significantly inferior (P = .02) OS rate (Figure2) when compared with patients who were in the age-adjusted high-intermediate group. Using the age-unadjusted IPI, the CR, 2-year FFS, and 2-year OS rates were 67%, 61%, and 72%, respectively, in the low-intermediate group, 38%, 48%, and 62%, respectively, in the high-intermediate group, and 44%, 43%, and 43%, respectively, in the high-risk group.
Comparison with IPI-matched historical data
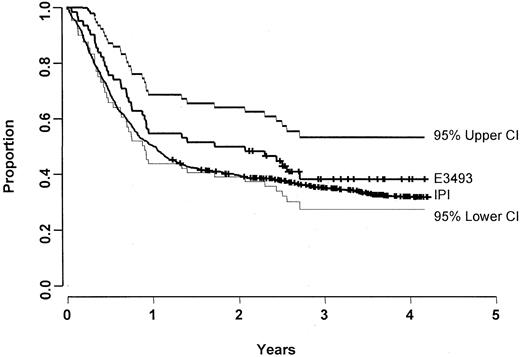
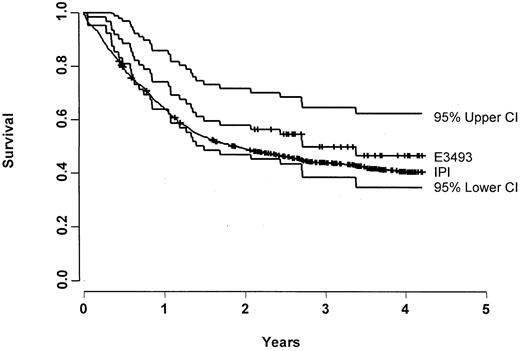
Characteristics and outcome of the 62 patients treated with CDE are compared with 927 IPI-matched controls in Table2. There were no significant differences in the characteristics of the study populations, though there was a trend for patients treated with CDE to be younger (as dictated by the eligibility criteria) and to have poorer performance status. There was no significant difference in FFS (median, 1.9 vs 1.0 years;P = .71) (Figure 3) or OS (median, 2.7 vs 1.9 years; P = .62) (Figure4). When compared with the IPI database, there was likewise no significant difference in FFS rate at 1 year (55% vs 50%) and 2 years (50% vs 39%). Results from the Cox proportional hazards model also showed no significant difference in FFS (P = .12) or OS (P = .09) after adjusting for prognostic factors.
Results and comparison with patients matched from IPI database
| . | Present study . | IPI database . | P . |
|---|---|---|---|
| No. | 62 | 927 | |
| Distribution of prognostic factors, % | |||
| Stage III-IV | 92 | 94 | .40 |
| Elevated LDH | 95 | 95 | .99 |
| PS 2 to 4 | 55 | 43 | .09 |
| Age older than 60 y | 24 | 37 | .06 |
| More than 1 extranodal site | 48 | 44 | .51 |
| CR rate, % (95% CI) | 48 (35, 64) | 52 (49, 55) | .60 |
| FFS | |||
| Median, y | 1.9 | 1.0 | .71 |
| 1 year, % (95% CI) | 55 (43, 67) | 50 (47, 53) | |
| 2 year, % (95% CI) | 50 (38, 62) | 39 (36, 42) | |
| Overall survival | |||
| Median, y | 2.7 | 1.9 | .62 |
| 1 year, % (95% CI) | 74 (63, 85) | 64 (61, 67) | |
| 2 year, % (95% CI) | 58 (46, 70) | 49 (46, 52) |
| . | Present study . | IPI database . | P . |
|---|---|---|---|
| No. | 62 | 927 | |
| Distribution of prognostic factors, % | |||
| Stage III-IV | 92 | 94 | .40 |
| Elevated LDH | 95 | 95 | .99 |
| PS 2 to 4 | 55 | 43 | .09 |
| Age older than 60 y | 24 | 37 | .06 |
| More than 1 extranodal site | 48 | 44 | .51 |
| CR rate, % (95% CI) | 48 (35, 64) | 52 (49, 55) | .60 |
| FFS | |||
| Median, y | 1.9 | 1.0 | .71 |
| 1 year, % (95% CI) | 55 (43, 67) | 50 (47, 53) | |
| 2 year, % (95% CI) | 50 (38, 62) | 39 (36, 42) | |
| Overall survival | |||
| Median, y | 2.7 | 1.9 | .62 |
| 1 year, % (95% CI) | 74 (63, 85) | 64 (61, 67) | |
| 2 year, % (95% CI) | 58 (46, 70) | 49 (46, 52) |
FFS for E3493 patients and its 95% CI (n = 62) along with the FFS for the IPI patients (n = 927).
FFS for E3493 patients and its 95% CI (n = 62) along with the FFS for the IPI patients (n = 927).
OS for E3493 patients and its 95% CI (n = 62) along with the OS for the IPI patients (n = 927).
OS for E3493 patients and its 95% CI (n = 62) along with the OS for the IPI patients (n = 927).
Treatment data and dose-intensity analysis
Among the 62 analyzable patients, 407 cycles of therapy were given. The median number of cycles given was 7 (range, 1-8 cycles). Treatment was discontinued early in 19 (31%) patients. Reasons for early treatment discontinuation included progressive disease (n = 12), patient withdrawal/refusal (n = 4), stable disease (n = 2), and excessive complications/toxicity (n = 1). Among the 4 patients who refused further therapy, 2 had 5 cycles, 1 had 6 cycles, and 1 had 7 cycles; all 4 are alive without progressive disease. Median relative dose intensity (expressed as a percentage of the designed dose) was 100% for cyclophosphamide, doxorubicin, and etoposide for all 8 cycles. The 25th percentile for all drugs was more than 95% for all cycles, indicating that most patients received the intended dose for all cycles. Mean relative dose intensity equaled or exceeded 95% of the intended doses for all cycles of cyclophosphamide and doxorubicin and 90% for all cycles of etoposide. Dose reduction was required for cyclophosphamide in 27% of all cycles given, for doxorubicin in 25% of all cycles given, and for etoposide in 29% of all cycles given. Dose reduction was required for cyclophosphamide in 45% of all patients, for doxorubicin in 48%, and for etoposide in 55%. The cyclophosphamide dose was in general reduced only for myelosuppression, whereas doxorubicin and etoposide doses might also have been reduced for nonhematologic toxicity (eg, mucositis) or hepatic/renal dysfunction.
Toxicity
Among all 78 patients who received therapy, 464 cycles of therapy were given. The median number of cycles was 6 (range, 1-8 cycles). Toxicity data for all 78 patients are shown in Table3. The most common severe (grade 3) or life-threatening (grade 4) toxicities included neutropenia (68%), anemia (57%), thrombocytopenia (44%), and infection (24%). With regard to all nonhematologic toxicities, the worst grade was 1 or 2 in 19 (24%) patients, grade 3 in 38 (49%) patients, grade 4 in 18 (23%) patients, and grade 5 (lethal) in 2 (3%) patients. One patient died of disseminated Mycobacterium kansasii infection 29 days after therapy was initiated with CDE. Another patient died of sepsis and multisystem failure 20 days after therapy was initiated.
Patients with toxicity
| . | Grade 1-2 . | Grade 3 . | Grade 4 . | Grade 5 . |
|---|---|---|---|---|
| Toxicity (%) | ||||
| Hematologic/infectious | ||||
| Neutropenia | 16 (21) | 8 (10) | 45 (58) | — |
| Leukopenia | 20 (26) | 21 (27) | 35 (45) | — |
| Infection | 28 (36) | 12 (15) | 7 (9) | 2 (3) |
| Anemia | 32 (41) | 38 (49) | 6 (8) | — |
| Thrombocytopenia | 32 (41) | 18 (23) | 16 (21) | — |
| Hemorrhage | 10 (3) | 2 (3) | 1 (1) | — |
| Gastrointestinal | ||||
| Nausea | 49 (63) | 8 (10) | 0 | — |
| Vomiting | 28 (36) | 3 (4) | 1 (1) | — |
| Stomatitis | 42 (54) | 3 (4) | 1 (1) | — |
| Diarrhea | 28 (36) | 0 | 1 (1) | — |
| Liver | 52 (67) | 2 (3) | 1 (1) | — |
| Cardiopulmonary | ||||
| Cardiac | 17 (22) | 2 (3) | 0 | — |
| Pulmonary | 23 (29) | 0 | 3 (4) | — |
| Other toxicities (%) | ||||
| Weight loss | 40 (51) | 0 | 0 | — |
| Weight gain | 11 (14) | 2 (3) | 0 | — |
| Fever (no infection) | 47 (60) | 1 (1) | 1 (1) | — |
| Metabolic | 22 (28) | 5 (6) | 2 (3) | — |
| Genitourinary | 10 (13) | 1 (1) | 0 | — |
| Neuromotor | 29 (37) | 4 (5) | 0 | — |
| Neurosensory | 10 (13) | 3 (4) | 1 (1) | — |
| Neuropsychologic | 12 (15) | 1 (1) | 0 | — |
| Neuroclinical | 6 (8) | 4 (5) | 0 | — |
| Fatigue | 22 (28) | 4 (5) | 0 | — |
| Skin | 16 (21) | 1 (1) | 0 | — |
| Phlebitis | 7 (9) | 2 (3) | 1 (1) | — |
| Worst-degree, nonhematologic (%) | 19 (24) | 38 (49) | 18 (23) | 2 (3) |
| . | Grade 1-2 . | Grade 3 . | Grade 4 . | Grade 5 . |
|---|---|---|---|---|
| Toxicity (%) | ||||
| Hematologic/infectious | ||||
| Neutropenia | 16 (21) | 8 (10) | 45 (58) | — |
| Leukopenia | 20 (26) | 21 (27) | 35 (45) | — |
| Infection | 28 (36) | 12 (15) | 7 (9) | 2 (3) |
| Anemia | 32 (41) | 38 (49) | 6 (8) | — |
| Thrombocytopenia | 32 (41) | 18 (23) | 16 (21) | — |
| Hemorrhage | 10 (3) | 2 (3) | 1 (1) | — |
| Gastrointestinal | ||||
| Nausea | 49 (63) | 8 (10) | 0 | — |
| Vomiting | 28 (36) | 3 (4) | 1 (1) | — |
| Stomatitis | 42 (54) | 3 (4) | 1 (1) | — |
| Diarrhea | 28 (36) | 0 | 1 (1) | — |
| Liver | 52 (67) | 2 (3) | 1 (1) | — |
| Cardiopulmonary | ||||
| Cardiac | 17 (22) | 2 (3) | 0 | — |
| Pulmonary | 23 (29) | 0 | 3 (4) | — |
| Other toxicities (%) | ||||
| Weight loss | 40 (51) | 0 | 0 | — |
| Weight gain | 11 (14) | 2 (3) | 0 | — |
| Fever (no infection) | 47 (60) | 1 (1) | 1 (1) | — |
| Metabolic | 22 (28) | 5 (6) | 2 (3) | — |
| Genitourinary | 10 (13) | 1 (1) | 0 | — |
| Neuromotor | 29 (37) | 4 (5) | 0 | — |
| Neurosensory | 10 (13) | 3 (4) | 1 (1) | — |
| Neuropsychologic | 12 (15) | 1 (1) | 0 | — |
| Neuroclinical | 6 (8) | 4 (5) | 0 | — |
| Fatigue | 22 (28) | 4 (5) | 0 | — |
| Skin | 16 (21) | 1 (1) | 0 | — |
| Phlebitis | 7 (9) | 2 (3) | 1 (1) | — |
| Worst-degree, nonhematologic (%) | 19 (24) | 38 (49) | 18 (23) | 2 (3) |
n = 78.
Based on NCI Common Toxicity Criteria.
Discussion
We report the results of a multi-institutional phase 2 trial of cyclophosphamide, doxorubicin, and etoposide plus filgrastim in 62 analyzable patients with poor-prognosis non-Hodgkin lymphoma. Approximately 80% of patients enrolled in the study had diffuse large cell lymphoma. Cytotoxic agents were given at conventional doses but were administered in an unconventional manner by protracted intravenous infusion over 96 hours. Seventy-four percent of patients had CR or PR, including 48% who had CR. Failure-free survival rates were 55% at 1 year and 50% at 2 years. We therefore failed to achieve the primary objective of the study, which was to improve the 1-year FFS rate from 55% to 70%.
Another commonly used 96-hour infusional regimen is EPOCH, reported to be highly active in patients with relapsed lymphoma and as initial therapy for HIV-associated lymphoma. EPOCH has not been extensively evaluated, however, as a first-line therapy in poor-prognosis lymphoma. The 58% incidence of grade 4 neutropenia in this study was higher than that reported for EPOCH (48%), as was the incidence of grade 3-4 thrombocytopenia (44% vs 24%) and infectious complications (26% vs 18%). Compared with EPOCH, the CDE regimen includes a higher intended dose per cycle of cyclophosphamide (800 vs 750 mg/m2), doxorubicin (50 vs 40 mg/m2), and etoposide (240 vs 200 mg/m2). Other differences include the administration of cyclophosphamide as a 96-hour infusion with CDE compared with an intravenous bolus with EPOCH, the inclusion of vincristine and prednisone in EPOCH (which are not included in CDE), and the routine use of filgrastim prophylaxis with CDE (which was not routinely used with EPOCH). Because of these dosing differences, the mean relative dose intensity in this study was higher for CDE than for EPOCH for cyclophosphamide (253 vs 177 mg/m2 per week), doxorubicin (16 vs 12 mg/m2 per week), and etoposide (75 vs 60 mg/m2 per week).9 Forty-five percent of patients treated with CDE required dose reduction for myelosuppression.
We succeeded in selecting a group of patients expected to have very poor prognosis with conventional therapy. Ninety-five percent had elevated serum LDH levels, 92% had stage III-IV disease (61% had stage IV), and 55% had poor performance status (very high compared with 18% in the IPI Prognostic Factor Project).23 Because of this imbalance, we compared FFS with IPI-matched historical data (selected from the entire IPI database), an analysis that was not planned when the study was initiated. In this analysis, there was no significant difference in FFS rates at 1 year (55% vs 50%) or 2 years (50% vs 39%). The Cox proportional hazards model revealed a trend favoring infusional therapy when compared with the IPI data, though the conventional criteria for statistical significance were not met.
One previously published report evaluated infusional therapy as first-line therapy for patients with intermediate grade non-Hodgkin lymphoma without HIV infection. The Southwest Oncology Group (SWOG) evaluated a 96-hour infusion of doxorubicin (12.5 mg/m2 per day) and vincristine (0.5 mg per day) plus intravenous bolus cyclophosphamide (750 mg/m2 on day 1) and oral dexamethasone (40 mg daily for 4 days) in patients with intermediate- or high-grade non-Hodgkin lymphoma used either alone (CVAD; n = 81) or in conjunction with the chemosensitizing (CS) P-glycoprotein modulators quinine (300 mg by mouth 3 times a day) and verapamil (240 mg twice a day) (CVAD+CS; n = 81).29 Patients were not selected for inclusion based on poor-risk IPI features, but approximately 40% to 50% were at high-intermediate or high risk by the age-unadjusted IPI. Toxicity and efficacy data were compared with a historical control group for 217 patients treated with CHOP in a prior SWOG study, of whom 44% were at intermediate-high or high risk. The relative dose intensity (RDI) for doxorubicin and cyclophosphamide was approximately 94% (of the intended dose) for CHOP, 90% for CVAD, and 81% for CVAD+CS. For vincristine the RDI for the 3 groups was 55%, 48%, and 42%, respectively. There was no significant difference among the 3 groups in CR rate (44% vs 39% vs 31%) or in 2-year FFS rate (46% vs 42% vs 41%), suggesting no advantage for infusional therapy. On the other hand, the CR rate and the 2-year FFS rate observed with infusional CDE in poor-prognosis patients compared favorably with the SWOG data when one considers that the SWOG report also included patients with low-risk disease.
Several other treatment approaches have been evaluated in patients with average or poor-risk intermediate- or high-grade non-Hodgkin lymphoma. Analysis of the U.S. Intergroup study found no evidence that multi-drug regimens were superior to CHOP, even in patients with poor-risk features by the IPI.30,31 Several studies have found no benefit for high-dose therapy plus stem cell transplantation compared with CHOP.32-34 A subset analysis of one study, however, showed a benefit for high-dose therapy in patients with IPI high-risk features.35 Another study demonstrated an advantage for high-dose sequential therapy, a treatment approach that was different than that used in other trials evaluating high-dose therapy.36 The role of high-dose therapy as initial treatment for poor-risk lymphoma remains investigational and is under evaluation in an Intergroup trial led by SWOG (S9704).
Other evidence suggests a possible advantage for a dose-intensive therapy that requires colony-stimulating factor (CSF) support without stem cells, though this has not been borne out in all studies. On the negative side, dose-escalated CHOP was found to be no more effective in poor-risk patients37 despite initial results that seemed promising.38 On the positive side, Gordon et al39 reported that a dose-escalated ProMACE-CytaBOM regimen (cyclophosphamide, doxorubicin, etoposide, prednisone, cytarabine, bleomycin, vincristine, methotrexate, and leucovorin) plus CSF resulted in a 69% CR rate and a 73% 4-year survival rate in 74 patients with intermediate-grade lymphoma treated in a multicenter ECOG study; the findings were nearly identical in the 46% of patients in the study who were at high-intermediate risk by age-adjusted IPI. Results in the high-intermediate risk group, therefore, compare favorably with the 56% CR rate and the 56% 2-year FFS observed for CDE and compared with historical IPI data. In the ProMACE-CytaBOM study, several drugs were given at a 2-fold higher dose than in the conventional ProMACE-CytaBOM regimen, including cyclophosphamide (1300 mg/m2 every 3 weeks), doxorubicin (50 mg/m2every 3 weeks), etoposide (240 mg/m2 every 3 weeks), and cytarabine (600 mg/m2 every 3 weeks). When comparing the normalized dose intensity for the agents common to both dose-escalated ProMACE-CytoBAM and CDE, the doses delivered were nearly identical for doxorubicin and etoposide and approximately 1.6-fold higher for cyclophosphamide in the ProMACE-CytaBOM regimen. The incidence of grade 4 leukopenia and nonhematologic toxicity were 74% and 27%, respectively, for dose-escalated ProMACE-CytaBOM compared with 45% and 23% for infusional CDE. In contrast, grade 4 toxicity (hematologic and nonhematologic) occurred in 31% of patients treated with CHOP (without CSF) in the Intergroup trial.30 Secondary myelodysplasia and leukemia have been the principal concern of further investigation of these dose-intensive approaches.37
Another approach that merits further evaluation is the combination of the anti-CD20 monoclonal antibody rituximab with standard chemotherapy. Interim analysis of a phase 3 trial performed by the Groupe d'Etude des Lymphomes de L'Adulte (GELA trial LNH 98-5) recently indicated significantly improved CR (76% vs 60%), event-free survival (69% vs 49%), and 1-year survival (83% vs 68%) rates in 328 elderly patients (60-80 years of age) treated with rituximab plus CHOP compared with CHOP alone,40 a finding that has been confirmed in the final report.41 Other trials of similar design are in progress, including a study performed by ECOG (E4494). E4494 also evaluates the role of maintenance rituximab in responders, an approach that was not evaluated in the GELA study. An unplanned interim analysis of E4494 was performed in October 2000, after accrual of 500 of the planned 630 patients and after approximately 25% of the events had occurred. An independent Data Safety Monitoring Committee recommended that the trial continue and complete its accrual goal. It is uncertain whether the benefit associated with rituximab, if confirmed, is attributed to an additive antineoplastic effect or to enhancement of the effect of cytotoxic therapy that has been observed in vitro.42 Should the latter phenomenon be true, infusional therapy may represent a better platform for concurrent administration with rituximab; the long half-life of rituximab,43 coupled with the protracted infusion of cytotoxic therapy, may maximize the potential for an additive or a synergistic effect. Preliminary evidence suggests that the combination of rituximab with infusional CDE or EPOCH is highly active in patients with HIV-associated lymphoma and relapsed lymphoma,44 45 and a study has been planned by the AIDS Malignancy Consortium to evaluate this approach in patients with HIV-associated lymphoma.
In conclusion, infusional CDE in patients with poor-risk, intermediate-grade non-Hodgkin lymphoma was not associated with a statistically significant improvement in 1-year FFS, which was the primary end point of our study. There were modest improvements in FFS and OS in the Cox proportional hazards model when compared with IPI-matched historical controls, though these differences were not statistically significant. It is unlikely that infusional CDE is associated with more than a 25% relative improvement in FFS in this population. However, this study has not ruled out the possibility of a benefit in patients with lower risk features or the possibility that infusional therapy may be a superior method of combining cytotoxic therapy with rituximab.
Conducted by the Eastern Cooperative Oncology Group (Robert L. Comis, Chair). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute. We thank Peter Wiernik, David Harrington, and Sandra Horning for their thoughtful review of the manuscript. We also thank Katherine Phillips, Richard Chung, and Candace Photopoulos for their expert data management and Amgen, Inc for providing filgrastim for this trial.
Supported in part by Public Health Service grants CA14958, CA23318, CA06594, CA13650, CA17145, CA66636, and CA21115 from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joseph A. Sparano, Department of Oncology, 2 South, Rm 47, Montefiore Medical Center, Albert Einstein College of Medicine, 1825 Eastchester Rd, Bronx, NY 10461-2373; e-mailsparano@jimmy.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal