In chronic myelogenous leukemia (CML), the development of chromosomal abnormalities in addition to the Philadelphia chromosome (clonal evolution) is considered by many to be a feature of accelerated phase (AP). Imatinib mesylate (STI571), a selective inhibitor of the Bcr-Abl tyrosine kinase, has significant activity in AP CML. As clonal evolution could allow Bcr-Abl independent proliferation, we analyzed its impact on the outcome of 71 AP patients treated with 600 mg of imatinib mesylate. Fifteen patients had clonal evolution alone (AP-CE), 32 had AP features but no evidence of clonal evolution (HEM-AP), and 24 had AP features plus clonal evolution (HEM-AP + CE). Of the AP-CE patients, 73% had a major cytogenetic response, compared with 31% of the HEM-AP patients (P = .043) and 12.5% of the HEM-AP + CE patients (P = .007). Complete cytogenetic responses were seen in 60% of AP-CE patients, compared with 31% of HEM-AP patients (P = .19) and 8% of HEM-AP + CE patients (P < .001). With mean follow-up of 11.2 months, 35% of all patients failed treatment. The lowest estimated rate of treatment failure at 1 year, 0%, was seen in AP-CE patients, compared with rates of 31% for HEM-AP patients and 69% for HEM-AP + CE patients (P = .0004). After 1 year, 100% of AP-CE patients were still alive, compared with 85% of HEM-AP patients and 67.5% of HEM-AP + CE patients (P = .01). In conclusion, in patients with clonal evolution as the sole criterion of disease acceleration, good responses to imatinib are still possible. Once patients have other signs of acceleration, clonal evolution predicts lower response rates and a shorter time to treatment failure.
Introduction
Chronic myelogenous leukemia (CML) is a clonal hematopoietic stem cell disorder characterized by a specific chromosomal translocation, t(9;22), resulting in a shortened chromosome 22, commonly referred to as the Philadelphia (Ph) chromosome.1 The molecular consequence of this translocation is the generation of a Bcr-Abl fusion oncogene, resulting in the production of a constitutively activated Bcr-Abl tyrosine kinase.2 This kinase is capable of inducing leukemias in mice, implicating Bcr-Abl as the cause of this disease.3 The tyrosine kinase activity of the Bcr-Abl protein is essential to its transforming ability.4
Clinically, CML progresses through 3 distinct phases: chronic phase, accelerated phase, and blast crisis.5 The chronic phase of the disease is characterized by massive myeloid expansion with cells retaining the capacity to differentiate normally. Over time the capacity for terminal differentiation is lost, resulting in disease progression. Although Bcr-Abl is thought to be the initial disease-transforming event in CML, the acquisition of other molecular and cytogenetic abnormalities is likely to be responsible for disease progression.
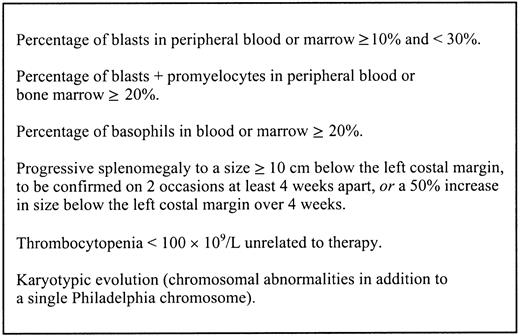
The accelerated phase is an intermediate stage in which patients show signs of disease progression without meeting the criteria for acute leukemia. This phase is manifested by increasing constitutional symptoms, progressive splenomegaly, and increasing refractoriness to standard therapy with progressive leucocytosis and/or thrombocytosis. Anemia and thrombocytopenia can also occur. Rising percentages of blasts and basophils in the bone marrow or peripheral blood are characteristic. Patients may develop more complex karyotypes, including trisomy 8, trisomy 19, and isochromosome 17q, with loss of p53 or additional copies of the Ph chromosome.6,7 In addition, molecular abnormalities such as p53 mutations can arise.8
Until recently, the median survival time of accelerated phase patients was less than 12 months. For most patients, treatment was unsatisfactory. Interferon was usually ineffective, and in many cases a palliative approach was pursued. Allogeneic stem cell marrow transplantation results in long-term disease-free survival in fewer than 40% of patients with accelerated phase disease and is unavailable to most patients owing to lack of a suitable donor or advanced age.9,10 High-dose chemotherapy followed by autologous transplantation, using stem cells harvested during the chronic phase, can establish a second chronic phase, but this is often short-lived.11 Similarly, intermediate-dose chemotherapy can restore a temporary chronic phase in some patients but has little impact on survival.10
Within the past 3 years, a promising new therapy has become available for the treatment of accelerated phase CML with the introduction of the Bcr-Abl tyrosine kinase inhibitor imatinib mesylate (formerly STI571) into clinical trials.12 In the pivotal phase 2 study of accelerated phase patients, the first patients enrolled were treated with imatinib mesylate at a dose of 400 mg.13 Once safety data were available from phase I testing, additional patients were treated at the higher dose of 600 mg. At this increased dose, a higher incidence of hematologic and cytogenetic responses was seen, as well as a significant improvement in time to progression and overall survival. Overall, 74% of accelerated phase patients treated with imatinib mesylate at a dose of 600 mg achieved a sustained hematologic response, which was complete in 41% of cases. Thirty percent of these patients achieved a major cytogenetic response, 21% of which were complete responses. Estimated 12-month progression-free and overall survival in patients receiving 600 mg are 68% and 80%, respectively.13
Whether the acquisition of additional cytogenetic abnormalities heralds a transformation to blast crisis and should be considered an accelerated phase feature has been controversial. A study at MD Anderson Cancer Center concluded that the prognostic significance of clonal evolution in CML is not uniform and is related to the specific abnormality (in particular, chromosome 17 abnormalities carry a worse prognosis), time to its development, its predominance in metaphases, and the presence of other accelerated features.14 Clonal evolution in the absence of other accelerated phase features has not adversely affected outcome following allogeneic stem cell transplantation.15 Similarly, a previous study using interferon and low-dose ara-C to treat accelerated phase patients demonstrated superior survival for patients in whom clonal evolution was the only criterion for disease acceleration (3-year survival rate 67% vs 22%; P < .01).16 Nevertheless, the World Health Organization classification of hematopoietic neoplasms does recognize clonal evolution as a criterion of accelerated phase.17 Although the initial, pivotal phase 2 study of imatinib mesylate in accelerated phase patients did not include clonal evolution as an accelerated phase criterion, a subsequent expanded access study did allow patients with clonal evolution to be entered into an accelerated phase study. Because imatinib mesylate targets Bcr-Abl tyrosine kinase alone and because additional oncogenic abnormalities could potentially allow Bcr-Abl–independent proliferation, we analyzed the impact of clonal evolution on the therapeutic response to imatinib mesylate in the treatment of accelerated phase CML patients.
Patients, materials, and methods
Patient samples and clinical and laboratory data
Between November 1, 1999, and May 31, 2001, 71 patients meeting the criteria for accelerated phase CML (Figure1) were treated at the Leukemia Center, Oregon Health and Science University (OHSU), in Novartis studies 109 and 114. Patients enrolled in study 109 satisfied the criteria of accelerated phase employed at MD Anderson Cancer Center, whereas later patients enrolled in study 114 met the less stringent criteria of the International Bone Marrow Transplant Registry, which are similar to those of Sokal et al (Table1).6,18 19 All were treated with imatinib mesylate, 600 mg daily. Patients were followed up every 2 weeks for the first 8 weeks for clinical assessment and underwent bone marrow evaluations, including metaphase cytogenetics and interphase fluorescence in situ hybridization (FISH) for Bcr-Abl, every 3 to 6 months. This study was approved by the institutional review board at OHSU.
International Bone Marrow Transplant Registry criteria for accelerated phase CML.
These criteria are presented as described by Speck et al.18
International Bone Marrow Transplant Registry criteria for accelerated phase CML.
These criteria are presented as described by Speck et al.18
Different criteria for accelerated phase CML
| MD Anderson Cancer Center6 . | Sokal et al19 . | International Bone Marrow Transplant Registry (used in present study)18 . |
|---|---|---|
| PB blasts ≥ 15% | PB or BM blasts ≥ 5% | PB or BM blasts ≥ 10% |
| PB blasts + promyelocytes ≥ 30% | — | PB or BM blasts + promyelocytes ≥ 20% |
| PB basophils ≥ 20% | PB basophils ≥ 20% | PB basophils + eosinophils ≥ 20% |
| Platelet count ≤ 100 × 109/L unrelated to therapy | Thrombopenia unrelated to therapy | Thrombopenia unresponsive to BU or HU therapy |
| Cytogenetic karyotypic evolution | Cytogenetic karyotypic evolution | Cytogenetic karyotypic evolution |
| — | Platelet ≥ 1000 × 109/L despite adequate therapy | Persistent thrombocytosis |
| — | Marrow collagen fibrosis | Myelofibrosis |
| — | Anemia unrelated to therapy | Anemia unresponsive to BU or HU therapy |
| — | Progressive splenomegaly | Progressive splenomegaly |
| — | Leukocyte doubling time < 5 d | Rapid doubling time of leukocytes < 5 d |
| — | Frequent Pelger-Huet-like neutrophils; nucleated erythrocytes; megakaryocyte nuclear fragments | Leukocyte count difficult to control with BU or HU therapy |
| — | Fever not otherwise explained | Development of chloromas |
| MD Anderson Cancer Center6 . | Sokal et al19 . | International Bone Marrow Transplant Registry (used in present study)18 . |
|---|---|---|
| PB blasts ≥ 15% | PB or BM blasts ≥ 5% | PB or BM blasts ≥ 10% |
| PB blasts + promyelocytes ≥ 30% | — | PB or BM blasts + promyelocytes ≥ 20% |
| PB basophils ≥ 20% | PB basophils ≥ 20% | PB basophils + eosinophils ≥ 20% |
| Platelet count ≤ 100 × 109/L unrelated to therapy | Thrombopenia unrelated to therapy | Thrombopenia unresponsive to BU or HU therapy |
| Cytogenetic karyotypic evolution | Cytogenetic karyotypic evolution | Cytogenetic karyotypic evolution |
| — | Platelet ≥ 1000 × 109/L despite adequate therapy | Persistent thrombocytosis |
| — | Marrow collagen fibrosis | Myelofibrosis |
| — | Anemia unrelated to therapy | Anemia unresponsive to BU or HU therapy |
| — | Progressive splenomegaly | Progressive splenomegaly |
| — | Leukocyte doubling time < 5 d | Rapid doubling time of leukocytes < 5 d |
| — | Frequent Pelger-Huet-like neutrophils; nucleated erythrocytes; megakaryocyte nuclear fragments | Leukocyte count difficult to control with BU or HU therapy |
| — | Fever not otherwise explained | Development of chloromas |
PB indicates peripheral blood; BM, bone marrow; BU, busulfan; and HU, hydroxyurea.
Chromosome preparations
Bone marrow aspirates were introduced into culture medium and 3 culture methods were used: one culture was harvested at 24 hours, one was synchronized with 10-7 M methotrexate and harvested at 24 hours, and one was supplemented with giant-cell tumor-conditioned medium and harvested at 48 hours. The harvest and slide preparations were done according to standard methods. Chromosomes were Giemsa-trypsin-Giemsa banded. Cytogenetic responses, based on metaphase analysis of at least 20 cells, were defined as complete (no cells positive for the Ph chromosome), partial (1%-35% of cells Ph positive), minor (36%-65% of cells Ph positive), and absent or no response (> 65% Ph positive). Major cytogenetic responses included complete and partial responses.
FISH
Cells fixed in 3:1 methanol acetic acid were dropped onto slides and treated to optimize spreading, similar to routine metaphase chromosome preparation. Slides were baked at 95°C for 5 to 6 minutes, incubated in 2 × SSC at 37°C for 30 minutes, dehydrated through an alcohol series (70%, 80%, and 95% for 2 minutes each), and dried. Direct-labeled probes for the t(9;22) translocation breakpoints (Ventana [Oncor] double fusion Bcr-Abl D-FISH set, catalog no. P5161-DC; Tucson, AZ) were co-denatured with the target DNA at 72°C for 2 minutes and allowed to renature overnight at 37°C. Slides were then rinsed in 0.5 × SSC at 72°C for 5 minutes and transferred to phosphate-nonadet buffer for 3 minutes. Preparations were counterstained with DAPI (4,6 diamidino-2-phenylindole) for visualization of red, green, and yellow probe signals and analyzed through a Zeiss Axiophot (Oberkochen, Germany); images were captured with a CytoVision system (Applied Imaging, Santa Clara, CA). At least 200 interphase cells were scored for signal patterns. FISH results for at least one metaphase cell were analyzed when possible, to aid in interpretation of complex signal patterns. The normal signal pattern for both probe sets is 2 red, 2 green.
The common abnormal pattern for the double fusion system (D-FISH) is 1 red, 1 green, and 2 yellow signals (representing both the derivative 9 and the derivative 22). Observation of a single interphase cell with 1 red, 1 green, and 2 yellow signals with the D-FISH kit is considered to be positive for the Ph rearrangement. Variations in the signal pattern may reflect underlying complex karyotypes.
Statistical analysis
Differences in response rates were analyzed with the Fisher exact test. Kaplan-Meier curves were constructed for time to treatment failure and overall survival and compared by means of the log-rank test. Time to treatment failure was defined as the time from the first dose of the drug to the date of discontinuation owing to resistant disease, disease progression to blast crisis, loss of response, toxicity, or death. Those without treatment failure were censored at the date of last contact. Disease progression was defined as transformation to blast crisis.
Results
Patient characteristics
The 71 patients meeting the criteria for accelerated phase CML who were included in this study were divided into 3 groups (Table2). One group had clonal evolution only (AP-CE, n = 15) (Table3), a second group had accelerated phase features but no evidence of clonal evolution (HEM-AP, n = 32), and a third group had accelerated phase features plus clonal evolution (HEM-AP + CE, n = 24) (Table4). Clonal evolution included duplication of the Ph chromosome as well as the acquisition of new chromosomal abnormalities. Of the 15 patients with AP-CE, 3 had duplication of the Ph chromosome and 13 had additional abnormalities. We evaluated the rate of complete and major cytogenetic responses with metaphase analysis; the decline in percentage of Bcr-Abl–positive bone marrow cells, using FISH; and time to treatment failure for the 3 different groups.
Characteristics of patients (n = 71)
| . | AP-CE (n = 15) . | HEM-AP (n = 32) . | HEM-AP + CE (n = 24) . |
|---|---|---|---|
| Sex, M/F | 11, 4 | 16, 16 | 16, 8 |
| Mean age, y (range) | 56 (38-76) | 60 (27-75) | 57 (22-74) |
| Mean CML duration, mo (range) | 45 (6-120) | 47 (6-108) | 58 (9-168) |
| Mean Hb, g/dL (range) | 12.1 (9.2-15.8) | 10.1 (2.7-15.4) | 10.3 (7.3-15.4) |
| Mean white blood cell count, × 109/L (range) | 15 (1.8-70.8) | 38.2 (2.5-173.9) | 48.5 (1.8-163) |
| Mean blasts, % (range) | 2.48 (0-7) | 12.8 (0-26) | 14 (1-25) |
| Mean basophils, % (range) | 4.1 (0-11) | 14.6 (0-76) | 13 (0-47) |
| Mean platelets, × 109/L (range) | 341 (129-826) | 512 (34-2252) | 342 (10-1018) |
| Mean duration prior interferon therapy, mo (range) | 17 (1-83) | 13 (1-79) | 25 (5-12) |
| Prior chemotherapy, no. | 7 | 13 | 9 |
| Prior stem cell transplant, no. | 3 autologous 4 allogeneic | 2 autologous | 3 autologous 2 allogeneic |
| Spleen larger than 10 cm, no. | 0 | 13 | 11 |
| Additional Ph, no. (%) | 3 (20) | — | 5 (21) |
| Chromosome 17 abnormalities, no. (%) | 3 (20) | — | 8 (33) |
| Other translocations, no. (%) | 4 (27) | — | 9 (37.5) |
| Mean metaphases with additional abnormalities, % (median) | 56 (50) | — | 63 (77) |
| . | AP-CE (n = 15) . | HEM-AP (n = 32) . | HEM-AP + CE (n = 24) . |
|---|---|---|---|
| Sex, M/F | 11, 4 | 16, 16 | 16, 8 |
| Mean age, y (range) | 56 (38-76) | 60 (27-75) | 57 (22-74) |
| Mean CML duration, mo (range) | 45 (6-120) | 47 (6-108) | 58 (9-168) |
| Mean Hb, g/dL (range) | 12.1 (9.2-15.8) | 10.1 (2.7-15.4) | 10.3 (7.3-15.4) |
| Mean white blood cell count, × 109/L (range) | 15 (1.8-70.8) | 38.2 (2.5-173.9) | 48.5 (1.8-163) |
| Mean blasts, % (range) | 2.48 (0-7) | 12.8 (0-26) | 14 (1-25) |
| Mean basophils, % (range) | 4.1 (0-11) | 14.6 (0-76) | 13 (0-47) |
| Mean platelets, × 109/L (range) | 341 (129-826) | 512 (34-2252) | 342 (10-1018) |
| Mean duration prior interferon therapy, mo (range) | 17 (1-83) | 13 (1-79) | 25 (5-12) |
| Prior chemotherapy, no. | 7 | 13 | 9 |
| Prior stem cell transplant, no. | 3 autologous 4 allogeneic | 2 autologous | 3 autologous 2 allogeneic |
| Spleen larger than 10 cm, no. | 0 | 13 | 11 |
| Additional Ph, no. (%) | 3 (20) | — | 5 (21) |
| Chromosome 17 abnormalities, no. (%) | 3 (20) | — | 8 (33) |
| Other translocations, no. (%) | 4 (27) | — | 9 (37.5) |
| Mean metaphases with additional abnormalities, % (median) | 56 (50) | — | 63 (77) |
Pretreatment karyotype and best response to imatinib in patients with clonal evolution without other accelerated phase features (AP-CE; n = 15)
| Case no. . | Pretreatment karyotype . | Best response . |
|---|---|---|
| 1 | 47, XY, t(9;22),+ t(9;22) [20] | (FISH 75.5%, Ph 100%) |
| 2 | 46, XX, t(9;22) [14]/47,idem,+ der(22)t(9;22) [6] | (FISH 10%, Ph 15%) |
| 3 | 47, XY, + 8, t(9;22)[7]/ 46, XY, t(9;22) [13] | (FISH 47%, Ph 100%) |
| 4 | 47, XY, + 8,t(9;22) [12]/ 47,XY, t(9;22),+ der(22)t(9;22) [7] | (FISH 3%, Ph 0% [11/11 negative]) |
| 5 | 46, XY, t(9;22;12) [2]/45,idem,der(16)t(16;17)(p13,3;q11,2), 17[7]/45,idem,der(17)t(17;17)(q25;p11.2), −17[3]45,idem, −17, der(19)t(17;19)(q11.2;p13.3)[2]/45,idem,der(4)t(4;17)(q16;q11.2), −17[2]/46,XY[3] | (FISH 0%, Ph 0%) |
| 6 | 46, XY, t(9;22) [4]/47,idem,+ 8,i(17)(q10)[1]/46,XY[1] | (FISH 0%, Ph 0%) |
| 7 | 46, XY,+ 8, t(9;22) [10], 46, XY[1] | (FISH 30.5%, Ph 90%) |
| 8 | 46, XX, t(9;22;14),del(12)(p1?3)[14] | (FISH 0%, Ph 0%) |
| 9 | 46, X,t(X;5)(p?11.4;q11.2),inv(1)(p22;q44), t(9;22) [20] | (FISH 5%, Ph 0%) |
| 10 | 46, XY, t(9;22) ([16]/46,idem,der(12)t(12;14)(q10;q10),add(13)(p11.2),− 14,+ mar[4] | (FISH 0%, Ph 0%) |
| 11 | 46, XY, t(8;10)(q?21;q?23),t(9;22) [10]/46,XY[10] | (FISH 0.3%, Ph 0%) |
| 12 | 46, XY, t(9;22) ?add(11)(p13)[2]/46,idem,?del(4)(p14p15.2)[4] | (FISH 2%, Ph 0%) |
| 13 | 46, XY, t(9;22) [15]/46,idem,del(7)(q22)[2] | (FISH 17.5%, Ph 0%) |
| 14 | 46, XY,t(9;22) [6]/46,idem,der(12)t(3;12)(q21;q11),iso(12)(q10)[7]/ 46,idem, add(4)(q12), der(9)t(4;9)(q11;p11), del(11)(q21)[3]/ 46,idem,add(4)(q12), der(9)t(4;9)(q11;p11),del(11)(q21),add(14)(q24)[3] | (FISH 24.5%, Ph 55%) |
| 15 | 46, XX,t(1;2)(q21;q21), t(9;22) [15]/46,idem,t(15;19)(q15;q13.3)[2]/46,idem,del(7)(q32),t(15;19) (q15;p13.3)[2]/46,XX[1] | (FISH 0%, Ph 0%) |
| Case no. . | Pretreatment karyotype . | Best response . |
|---|---|---|
| 1 | 47, XY, t(9;22),+ t(9;22) [20] | (FISH 75.5%, Ph 100%) |
| 2 | 46, XX, t(9;22) [14]/47,idem,+ der(22)t(9;22) [6] | (FISH 10%, Ph 15%) |
| 3 | 47, XY, + 8, t(9;22)[7]/ 46, XY, t(9;22) [13] | (FISH 47%, Ph 100%) |
| 4 | 47, XY, + 8,t(9;22) [12]/ 47,XY, t(9;22),+ der(22)t(9;22) [7] | (FISH 3%, Ph 0% [11/11 negative]) |
| 5 | 46, XY, t(9;22;12) [2]/45,idem,der(16)t(16;17)(p13,3;q11,2), 17[7]/45,idem,der(17)t(17;17)(q25;p11.2), −17[3]45,idem, −17, der(19)t(17;19)(q11.2;p13.3)[2]/45,idem,der(4)t(4;17)(q16;q11.2), −17[2]/46,XY[3] | (FISH 0%, Ph 0%) |
| 6 | 46, XY, t(9;22) [4]/47,idem,+ 8,i(17)(q10)[1]/46,XY[1] | (FISH 0%, Ph 0%) |
| 7 | 46, XY,+ 8, t(9;22) [10], 46, XY[1] | (FISH 30.5%, Ph 90%) |
| 8 | 46, XX, t(9;22;14),del(12)(p1?3)[14] | (FISH 0%, Ph 0%) |
| 9 | 46, X,t(X;5)(p?11.4;q11.2),inv(1)(p22;q44), t(9;22) [20] | (FISH 5%, Ph 0%) |
| 10 | 46, XY, t(9;22) ([16]/46,idem,der(12)t(12;14)(q10;q10),add(13)(p11.2),− 14,+ mar[4] | (FISH 0%, Ph 0%) |
| 11 | 46, XY, t(8;10)(q?21;q?23),t(9;22) [10]/46,XY[10] | (FISH 0.3%, Ph 0%) |
| 12 | 46, XY, t(9;22) ?add(11)(p13)[2]/46,idem,?del(4)(p14p15.2)[4] | (FISH 2%, Ph 0%) |
| 13 | 46, XY, t(9;22) [15]/46,idem,del(7)(q22)[2] | (FISH 17.5%, Ph 0%) |
| 14 | 46, XY,t(9;22) [6]/46,idem,der(12)t(3;12)(q21;q11),iso(12)(q10)[7]/ 46,idem, add(4)(q12), der(9)t(4;9)(q11;p11), del(11)(q21)[3]/ 46,idem,add(4)(q12), der(9)t(4;9)(q11;p11),del(11)(q21),add(14)(q24)[3] | (FISH 24.5%, Ph 55%) |
| 15 | 46, XX,t(1;2)(q21;q21), t(9;22) [15]/46,idem,t(15;19)(q15;q13.3)[2]/46,idem,del(7)(q32),t(15;19) (q15;p13.3)[2]/46,XX[1] | (FISH 0%, Ph 0%) |
Pretreatment karyotype and best response to imatinib in patients with clonal evolution and other accelerated phase features (HEM-AP + CE; n = 24)
| Case no. . | Pretreatment karyotype . | Best response . |
|---|---|---|
| 1 | 46, XY, t(9;22)[12]/46, XY, der(9)t(9;22),idicder(22q)[7]/ 47, XY, t(9;22),+ der(22)t(9;22)[1] | (FISH 51.5%, Ph 89%) |
| 2 | 46, XY, t(9;22) [16]/49,XY, t(9;22),+ 19,+ 21,+ der(22) t(9;22)[4] | (FISH 85.5%, Ph 100%, progression) |
| 3 | 46, XX, t(9;22) [16]/45,XX, t(9;22),t(15;18)(p11;q11),− 18[5] | (FISH 45.5%, Ph 100%) |
| 4 | 46, XY, t(9;22),t(12;14)(?q24;?q23)[22] | (FISH 12.5%, Ph 5%) |
| 5 | 46, XX, t(9;22),del(18)(q21.3)[1]/47,idem,+ 8[18]/47,idem,− 1,del(2)(q21),− 6,− 7,+ 8, t(9;22) (q34;q11.2),add(11)(q34),− 13,del(18)(q21.3),add(19)(q13),+ mar1,mar2,+ mar3,+ mar4[1] | (FISH 3.5%, Ph 0%) |
| 6 | 46, XY, t(3;21)(q26.2;q22),t(9;22) [23] | (FISH 36.5%, no metaphases for analysis) |
| 7 | 46, XX, t(9;22;12),t(3;der(12))(p22;q14),t(9;10)(q3?2;q2?2), del(13)(q14),del(11)(q13), t(11;17)(q23;p13)[20] | (progression) |
| 8 | 46, XY, t(9;22),− 17[1]/46-47,idem,+ r1,+ r2[cp18] | (FISH 61.5%, Ph 90%, progression) |
| 9 | 46, XY, t(3;7)(p21;p15),t(9;22),t(10;11)(p15;q23)[17]/46,XY,t(9;22) [5] | (FISH 25%, Ph 19%, progression) |
| 10 | 45, XY,der(7)t(7;17)(p13;q11),t(9;22),− 17[11]/46, XY, t(9;22) [9] | (FISH 90%, Ph 100%, progression) |
| 11 | 45, XX, add(6)(p25),der(9),t(9;22),− 22[18]/45,XX,der(9)t(9;22),− 14,add(16)(q22),add(17)(p12),+ 21,− 22[2] | (FISH 74%, Ph 100%, progression) |
| 12 | 46, XY, t(9;22) [18]/46,idem,iso(17)(q11.2)[2] | (FISH 83%, Ph 100%) |
| 13 | 46, XY, t(9;22),add(19)(q13)[20] | (FISH 43%, Ph 100%) |
| 14 | 46, XX, t(9;22)[16]/46,idem,t(10;12;11;16)(q23;q13;q11;q12)[5] | (FISH 60.5%, Ph 100%, progression) |
| 15 | 46, XX, t(3;19)(p21;p13),t(9;22) [20] | (FISH 63%, Ph 100%, progression) |
| 16 | 46, XY del(3)(q21), t(9;22) (q34;q11),add(17)(q25),add(21)(q22)[20] | (FISH 79%, Ph 95%, progression) |
| 17 | 46, XY, t(9;22;15) (q34;q11.2;q15),add(19)(p13.3)[5]/46,XY,t(9;22;15)[15] | (FISH 77%, Ph 100%) |
| 18 | 46, XY, t(9;22) [1]/45,XY, t(9;22) (q34;q11),der(14;17)(q10;q10)[19] | (progression) |
| 19 | 46, XY, t(9;22),− 17,add(19)(q13.4),+ mar[19]/46,XY, t(9;22),− 17,add(19)(q13.4)[1] | (FISH 83.5%, Ph 100%, progression) |
| 20 | 46, XX, t(9;22)[14]/45,XX,der(9)t(9;22),− 22[6] | (FISH 64%, Ph 100%, progression) |
| 21 | 46, XY, t(9;22)[17]/ 47,idem,+ der(22)t(9;22)[3] | (progression) |
| 22 | 46, XY, t(3;14)(p21;q32),t(9;22)[20] | (FISH 1.5%, Ph 0%) |
| 23 | 46, XY, t(9;22)[14]/45,X,− Y, t(9;22)[6] | (FISH 4%, Ph -no metaphases) |
| 24 | 46, XY,t(9;22)[8]/46,idem,add(1)(p36.3)[12] | (FISH 34.5%, Ph 100%, progression) |
| Case no. . | Pretreatment karyotype . | Best response . |
|---|---|---|
| 1 | 46, XY, t(9;22)[12]/46, XY, der(9)t(9;22),idicder(22q)[7]/ 47, XY, t(9;22),+ der(22)t(9;22)[1] | (FISH 51.5%, Ph 89%) |
| 2 | 46, XY, t(9;22) [16]/49,XY, t(9;22),+ 19,+ 21,+ der(22) t(9;22)[4] | (FISH 85.5%, Ph 100%, progression) |
| 3 | 46, XX, t(9;22) [16]/45,XX, t(9;22),t(15;18)(p11;q11),− 18[5] | (FISH 45.5%, Ph 100%) |
| 4 | 46, XY, t(9;22),t(12;14)(?q24;?q23)[22] | (FISH 12.5%, Ph 5%) |
| 5 | 46, XX, t(9;22),del(18)(q21.3)[1]/47,idem,+ 8[18]/47,idem,− 1,del(2)(q21),− 6,− 7,+ 8, t(9;22) (q34;q11.2),add(11)(q34),− 13,del(18)(q21.3),add(19)(q13),+ mar1,mar2,+ mar3,+ mar4[1] | (FISH 3.5%, Ph 0%) |
| 6 | 46, XY, t(3;21)(q26.2;q22),t(9;22) [23] | (FISH 36.5%, no metaphases for analysis) |
| 7 | 46, XX, t(9;22;12),t(3;der(12))(p22;q14),t(9;10)(q3?2;q2?2), del(13)(q14),del(11)(q13), t(11;17)(q23;p13)[20] | (progression) |
| 8 | 46, XY, t(9;22),− 17[1]/46-47,idem,+ r1,+ r2[cp18] | (FISH 61.5%, Ph 90%, progression) |
| 9 | 46, XY, t(3;7)(p21;p15),t(9;22),t(10;11)(p15;q23)[17]/46,XY,t(9;22) [5] | (FISH 25%, Ph 19%, progression) |
| 10 | 45, XY,der(7)t(7;17)(p13;q11),t(9;22),− 17[11]/46, XY, t(9;22) [9] | (FISH 90%, Ph 100%, progression) |
| 11 | 45, XX, add(6)(p25),der(9),t(9;22),− 22[18]/45,XX,der(9)t(9;22),− 14,add(16)(q22),add(17)(p12),+ 21,− 22[2] | (FISH 74%, Ph 100%, progression) |
| 12 | 46, XY, t(9;22) [18]/46,idem,iso(17)(q11.2)[2] | (FISH 83%, Ph 100%) |
| 13 | 46, XY, t(9;22),add(19)(q13)[20] | (FISH 43%, Ph 100%) |
| 14 | 46, XX, t(9;22)[16]/46,idem,t(10;12;11;16)(q23;q13;q11;q12)[5] | (FISH 60.5%, Ph 100%, progression) |
| 15 | 46, XX, t(3;19)(p21;p13),t(9;22) [20] | (FISH 63%, Ph 100%, progression) |
| 16 | 46, XY del(3)(q21), t(9;22) (q34;q11),add(17)(q25),add(21)(q22)[20] | (FISH 79%, Ph 95%, progression) |
| 17 | 46, XY, t(9;22;15) (q34;q11.2;q15),add(19)(p13.3)[5]/46,XY,t(9;22;15)[15] | (FISH 77%, Ph 100%) |
| 18 | 46, XY, t(9;22) [1]/45,XY, t(9;22) (q34;q11),der(14;17)(q10;q10)[19] | (progression) |
| 19 | 46, XY, t(9;22),− 17,add(19)(q13.4),+ mar[19]/46,XY, t(9;22),− 17,add(19)(q13.4)[1] | (FISH 83.5%, Ph 100%, progression) |
| 20 | 46, XX, t(9;22)[14]/45,XX,der(9)t(9;22),− 22[6] | (FISH 64%, Ph 100%, progression) |
| 21 | 46, XY, t(9;22)[17]/ 47,idem,+ der(22)t(9;22)[3] | (progression) |
| 22 | 46, XY, t(3;14)(p21;q32),t(9;22)[20] | (FISH 1.5%, Ph 0%) |
| 23 | 46, XY, t(9;22)[14]/45,X,− Y, t(9;22)[6] | (FISH 4%, Ph -no metaphases) |
| 24 | 46, XY,t(9;22)[8]/46,idem,add(1)(p36.3)[12] | (FISH 34.5%, Ph 100%, progression) |
Progression indicates transformation to blast crisis.
Impact of clonal evolution on cytogenetic response
Eleven (73%) of 15 AP-CE patients had a major cytogenetic response, compared with 10 (31%) of 32 HEM-AP patients (P = .0113) and 3 (12.5%) of 24 HEM-AP + CE patients (P < .001). Complete cytogenetic responses were seen in 9 (60%) of 15 AP-CE patients, compared with 10 (31%) of 32 HEM-AP patients (P = .109) and 2 (8%) of 24 HEM-AP + CE patients (P < .001). These responses were sustained for the duration of the study in all patients. Notably, one AP-CE patient was not considered a responder, owing to insufficient metaphases. This patient had 3% Bcr-Abl–positive cells by FISH, and 9 of 9 and 11 of 11 metaphases have been Ph negative on successive examinations. Inclusion of this patient as a complete cytogenetic responder increases the major and complete cytogenetic response rates in the AP-CE category to 80% and 67%, respectively. One additional HEM-AP + CE patient achieved low-level positivity for Bcr-Abl (4%) by FISH but never grew any metaphases for cytogenetic analysis.
In several cases there appear to be discrepancies between the percentages of metaphase and interphase cells positive for Ph andBcr-Abl, respectively (eg, cases 3 and 7 in Table 3 and cases 1, 3, 13, and 24 in Table 4). Our experience has been that the results from both tests correlate when the percentage ofBcr-Abl–positive cells by interphase FISH is less than 35%. Above this level, we have occasionally seen discrepant results. Potential reasons for these discrepancies are as follows: metaphase cytogenetics analyzes proliferating cells, while interphase cytogenetics analyzes nondividing cells. In addition, when cells are placed in metaphase culture, they are washed and may be grown for up to 48 hours in culture medium in the absence of imatinib. This may lead to the outgrowth of a highly proliferative fraction of Ph-positive cells.
Effect on time to treatment failure and overall survival
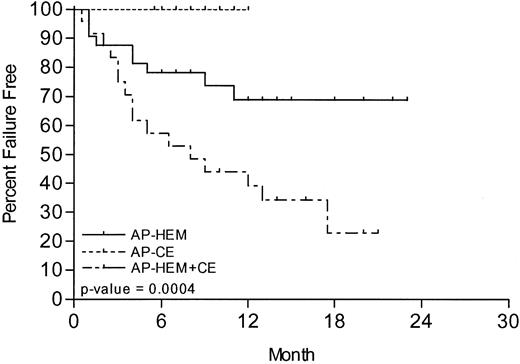
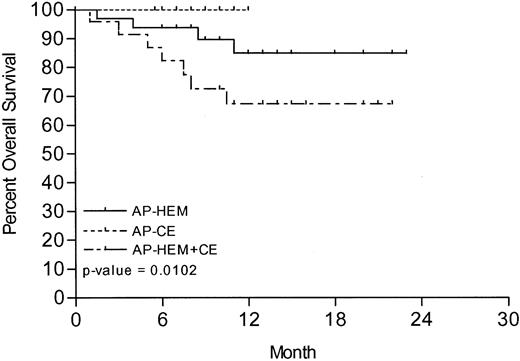
With a mean follow-up of 11.2 months (range, 1-23 months, SD ±5.3), 35% of all patients failed treatment. The lowest rate of treatment failure, 0% (0 of 15), was seen in AP-CE patients, compared with 28.12% (9 of 32) in HEM-AP patients and 66.7% (16 of 24) in HEM-AP + CE patients (P = .0004). The 1-year estimated rate of treatment failure was 0%, 31%, and 69% for these 3 groups, respectively (Figure 2). The median time to treatment failure in HEM-AP + CE patients was 8 months. Median time to treatment failure has not yet been reached in HEM-AP or AP-CE patients. Compared with HEM-AP + CE patients, both AP-CE and HEM-AP patient groups had a significantly lower rate of treatment failure (P = .0008 and P = .009, respectively). The difference in time to treatment failure between AP-CE and HEM-AP was less significant (P = .03). At the time of analysis, 81.7% of all patients were still alive. AP-CE patients have fared best, with 100% survival, compared with 87.5% of HEM-AP patients and 62.5% of HEM-AP + CE patients (P = .01; Figure 3). Compared with HEM-AP + CE patients, both AP-CE and HEM-AP patient groups had a significant survival advantage (P = .01 and P = .03, respectively), whereas there is currently no significant survival difference between the latter groups (P = .19). The 1-year estimate of survival was 100% in AP-CE patients, 85% in HEM-AP patients, and 67.5% in HEM-AP + CE patients (P = .01).
Kaplan-Meier curve of time to treatment failure in months from start of imatinib.
Kaplan-Meier curve of time to treatment failure in months from start of imatinib.
Kaplan-Meier curve of overall survival in months from start of imatinib.
Discussion
Cytogenetic clonal evolution may be a marker of disease progression in CML and is thought to reflect the genetic instability of the highly proliferative CML progenitors. Telomere shortening, which has been associated with disease progression, may contribute to this genetic instability.20 21 Regardless of the underlying mechanisms, the net result of additional genetic abnormalities is the potential for a more malignant phenotype and, possibly, less reliance on Bcr-Abl for proliferation and survival. We were interested in determining whether the presence of such abnormalities would affect the outcome of accelerated phase CML patients treated with imatinib mesylate, an agent that specifically targets the Bcr-Abl tyrosine kinase.
In this study we observed that patients with clonal evolution as the sole criterion of disease acceleration have excellent responses to imatinib mesylate. However, once patients have other accelerated phase features, the presence of clonal evolution predicts lower response rates and a shorter time to treatment failure. The poorer outcome in this group is perhaps not surprising, and there are now reports of clonal evolution as a mechanism of resistance to imatinib mesylate.22 The outcome of patients with clonal evolution alone compares favorably with the results in chronic phase CML patients treated with imatinib following interferon failure. Major and complete cytogenetic responses of 60% and 41%, respectively, have been reported in this patient population. In this phase 2 study, 400 mg of imatinib mesylate was used daily.23 Although obviously a small group, our AP-CE patients had major and complete responses of 73% and 60%, respectively.
Prior to July 2000, patients with clonal evolution alone were entered in chronic phase studies (hence the follow-up of this subgroup in this accelerated phase study is shorter than that of the other 2 groups). We recently analyzed the risk of relapse in 118 chronic patients treated with 400 mg of imatinib mesylate and found that 8 (8%) of 95 patients with the Ph chromosome alone relapsed, compared with 9 (39%) of 23 patients with additional chromosomal abnormalities (P< .001; M.E.O'D., B.J.D., unpublished data, 2002). This suggests that in the absence of accelerated phase features, clonal evolution combined with treatment with imatinib at a dose of 400 mg has an adverse impact on outcome (there was no significant difference in major cytogenetic response rates between these 2 groups). However, when AP-CE patients are treated as accelerated phase patients, with a more aggressive intent, superior results are obtained. Imatinib mesylate was administered at a dose of 600 mg, which has been shown to be superior to 400 mg in the pivotal study in accelerated phase patients.13 In addition, treatment was not withheld because of myelosuppression, unless this was sustained and associated with marrow hypocellularity of less than 10%. In addition, some patients were supported with myeloid growth factors, which allowed continued therapy without dose reductions.24 This suggests that with more aggressive therapy, patients with clonal evolution alone benefit greatly.
Clearly, several factors may have contributed to the high response rate seen. In the phase 2 chronic phase study, 5 baseline variables that independently predicted a high rate of major cytogenetic response were determined. These were (1) the absence of blasts in peripheral blood, (2) a hemoglobin (Hb) level higher than 12 g/dL, (3) the presence of fewer than 5% blasts in marrow, (4) a time from diagnosis of CML to start of treatment of less than one year, and (5) a history of cytogenetic relapse during interferon therapy.23 The 15 patients with clonal evolution alone in our study had a median Hb of 12.1, median peripheral blasts of 0%, and median marrow blasts of 1.7%, all favorable prognostic factors. However, the median duration of disease was 38 months, and only 2 of 15 patients had any prior cytogenetic response to interferon (the single most powerful predictor of response). Therefore, it is tempting to speculate that much of the benefit is due either to treatment with a higher dose or to increased dose intensity. Similarly, whether improved responses are seen with more aggressive management of myelosuppression will need to be analyzed. This could include treatment with myeloid growth factors or setting lower platelet thresholds for withholding therapy.
Our data also demonstrate that patients with accelerated phase CML and cytogenetic clonal evolution are at high risk of treatment failure when treated with imatinib mesylate alone and should be considered candidates for additional treatments, such as stem cell transplantation or combination regimens as appropriate. Finally, although this is a relatively small group of patients, the high rate of major and complete cytogenetic responses in AP-CE patients suggests that studies comparing 600 mg of imatinib mesylate with the currently recommended dose of 400 mg for chronic phase patients should be considered.
Prepublished online as Blood First Edition Paper, May 13, 2002; DOI 10.1182/blood-2002-03-0777.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael E. O'Dwyer, Department of Haematology, University College Hospital, Newcastle Road, Galway, Ireland; e-mail: michael.odwyer@whb.ie.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal