Abstract
Hereditary hemochromatosis (HH) is classically associated with a Cys282Tyr (C282Y) mutation of the HFE gene. Non-C282Y HH is a heterogeneous group accounting for 15% of HH in Northern Europe. Pathogenic mutations of the transferrin receptor 2(TfR2) gene have been identified in 4 Italian pedigrees with the latter syndrome. The goal of this study was to perform a mutational analysis of the TfR2 and HFE genes in a cohort of non-C282Y iron overload patients of mixed ethnic backgrounds. Several sequence variants were identified within theTfR2 gene, including a homozygous missense change in exon 17, c2069 A→C, which changes a glutamine to a proline residue at position 690. This putative mutation was found in a severely affected Portuguese man and 2 family members with the same genotype. In summary, pathologic TfR2 mutations are present outside of Italy, accounting for a small proportion of non-C282Y HH.
Introduction
In 1996, Feder et al1 identified 85% of hereditary hemochromatosis (HH) patients of Northern European descent as being carriers of a C282Y mutation in the HFEgene. Since 1996, several other mutations of the HFEgene have been identified in association with HH2-4; however, with rare exception, these mutations occur in tandem with trans C282Y mutations and are infrequent causes of HH.
Mutations of the transferrin receptor 2 (TfR2) gene have been implicated as the genetic basis of a novel autosomal recessive adult-onset HH syndrome in 4 Italian pedigrees (Hemochromatosis, Type 3, OMIM 604250).5,6 The TfR2α transcript is a homolog of the transferrin receptor (TfR1) protein with 48% identity and 66% similarity in the extracellular portion of the protein.7TfR2 has both similar and distinct functional properties from TfR1, including a common affinity for diferric transferrin7 and a lack of affinity for the HFE protein,8 respectively. Nonsense or nonfunctional mutations of TfR2 are associated with HH5,6; however, the frequency and physiologic basis of this association are unclear.
The goal of the current study was to identify a genetic basis for iron overload in a cohort of patients with non-C282Y iron overload by direct sequencing of both the TfR2 and the HFE genes.
Study design
The study population consisted of all patients referred to the Molecular Diagnostic Laboratory of Children and Women's Health Centre in Vancouver, BC, Canada, for genetic testing for HH prior to May 5, 2000. From this database, we selected all patients with a transferrin saturation greater than or equal to 60%, an elevated serum ferritin level, and a negative test result for the C282Y HFE mutation. From the same laboratory, 93 control samples were obtained.
Testing for the HFE C282Y mutation was performed according to standard methods. Polymerase chain reaction (PCR) amplification and direct sequencing of all coding regions and all intron/exon boundaries of the HFE and TfR2 genes were performed on each patient at the British Columbia Genome Sequencing Centre (BCGSC).
Method details are available in the supplementary information stored on the BCGSC website (www.bcgsc.bc.ca/fg/hemoc). The project received approval from the ethical review boards of all participating institutions. Investigations were undertaken with the informed consent of all study participants.
Results and discussion
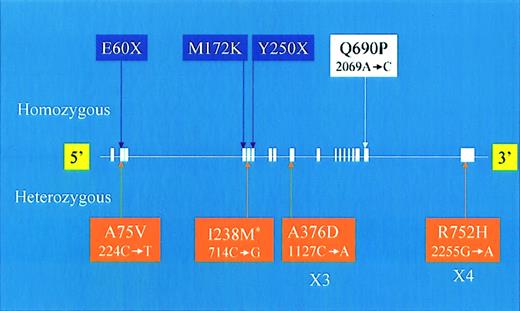
HFE and TfR2 sequencing was performed on DNA from 89 patients of mixed ethnic origin (patient demographic data available in the supplementary information). Of these patients, 15 had risk factors for secondary iron overload. Among this cohort, one probable pathogenic mutation of the TfR2 gene was identified, c2069 A→C, Q690P (Figure1). This novel mutation occurred in a single patient who was a child of a consanguineous marriage (Figure 2). The remaining patients either did not have a TfR2 or HFE genotype suggestive of HH (n = 74) or had genotypes associated with normal or mild iron overload status (HFE H63D/H63D, n = 11; HFE H63D/S65C, n = 3).9-11 In addition, 18 TfR2polymorphisms were identified, including 3 novel and one previously described12 amino acid coding changes (Figure 1 andwww.bcgsc.bc.ca/fg/hemoc). Five HFE single nucleotide polymorphisms were identified (www.bcgsc.bc.ca/fg/hemoc).
Summary of new and previously described coding sequence changes of the
TfR2 gene. Text box color code is as follows: purple indicates previously described homozygous mutations, white indicates a novel homozygous mutation, and orange indicates heterozygous sequence changes. The asterisk indicates a previously described heterozygous sequence change. For each sequence change identified in this study, both the amino acid (above) and cDNA nucleotide (below) changes are indicated.
Summary of new and previously described coding sequence changes of the
TfR2 gene. Text box color code is as follows: purple indicates previously described homozygous mutations, white indicates a novel homozygous mutation, and orange indicates heterozygous sequence changes. The asterisk indicates a previously described heterozygous sequence change. For each sequence change identified in this study, both the amino acid (above) and cDNA nucleotide (below) changes are indicated.
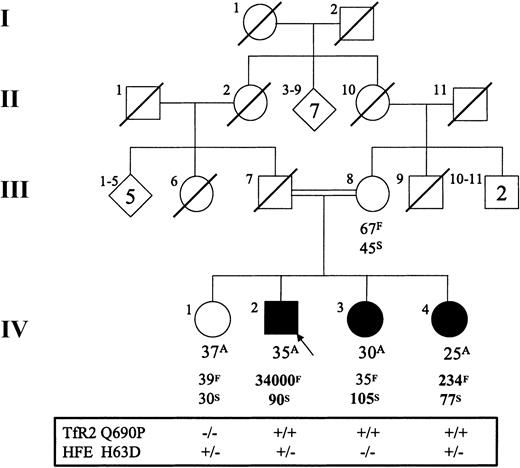
Q690P pedigree.
Age (A, years), ferritin (F, μg/L), transferrin saturation (S, %), and TfR2 Q690P and HFE H63D mutation status for the Portuguese family. “+” indicates the presence of the mutant allele, and “−” indicates its absence. The mother was heterozygous for both TfR2 Q690P and HFE H63D. Abnormal results are indicated in bold type. Values listed are those of the initial assessment for each patient.
Q690P pedigree.
Age (A, years), ferritin (F, μg/L), transferrin saturation (S, %), and TfR2 Q690P and HFE H63D mutation status for the Portuguese family. “+” indicates the presence of the mutant allele, and “−” indicates its absence. The mother was heterozygous for both TfR2 Q690P and HFE H63D. Abnormal results are indicated in bold type. Values listed are those of the initial assessment for each patient.
With respect to HFE mutations, a high percentage of patients carried the HFE H63D allele (48 of 89; 54%). This value is above that expected as compared to carrier rates for H63D ranging from 12% to 37% in European population studies.13 This overrepresentation of HFE H63D genotypes is in keeping with the hypothesis that HFE H63D mutations contribute to an iron overload phenotype, even in the absence of the HFE C282Y allele.14,15
The TfR2 Q690P mutation was identified in an individual of Portuguese descent. He presented at 29 years of age with fatigue, hypogonadotropic hypogonadism, hyperpigmentation, mild elevation of liver transaminases, and idiopathic thrombocytopenic purpura. He had markedly elevated serum iron indices (Figure 2) and has had more than 12 g of iron removed via phlebotomy in the past 4 years. Additional hematologic abnormalities included mild normocytic anemia and lymphopenia. He is heterozygous for the HFE H63D mutation. The Q690P mutation was not identified in our control group, 20 Portuguese controls, and 39 Portuguese patients with nonclassical HH.
The family of the Q690P proband was investigated for iron overload and the presence of the TfR2 Q690P mutation. The results of the family study are summarized in Figure 2 (additional data available atwww.bcgsc.bc.ca/fg/hemoc). Biochemical evidence of iron overload was detected in 2 female siblings (IV-3 and IV-4 in Figure 2), both of whom are homozygous for the TfR2 Q690P mutation. They had no clinical manifestations of disease aside from arthralgia, which affected sibling IV-3. Sibling IV-3 had a transferrin saturation level measured at greater than 100% with a normal serum ferritin and no evidence of hepatic iron overload as assessed by magnetic resonance imaging.16 Her hematologic profile revealed neutropenia but was otherwise normal. She has a wild-type HFE genotype. Sibling IV-4 had a transferrin saturation value of 77% and elevations of serum ferritin and hepatic iron stores (263 μmol/g). Her hematologic profile revealed a mild normocytic anemia and lymphopenia. She is heterozygous for the HFE H63D mutation. Sibling IV-1 and the mother of the proband showed no evidence of an iron overload phenotype or hematologic abnormalities. The mother is heterozygous for the TfR2 Q690P allele, while sibling IV-1 is wild type.
The impact of the Q690P mutation on TfR2 function can be estimated by analysis of the corresponding position in the homologous TfR1 protein (threonine 658). TfR1 thr658 is residue 18 of 23 amino acids forming the α helix 3 of the helical domain in the extracellular component of the protein.17 Sixteen of the 23 amino acids are identical or structurally conserved between TfR1 and TfR2.17 In TfR1, this α helix forms the proposed binding sites for both diferric transferrin and HFE.17,18 Specifically, mutagenesis of TfR1 α helix 3 amino acids 643, 646–648, and 650 all yield TfR1 proteins with significantly reduced affinity for transferrin.18,19 Therefore, disruption of the α helix 3 secondary structure via insertion of a proline residue at position 690 in TfR2 (position 658 in TfR1) would be expected to disrupt a key binding site for diferric transferrin. This loss of function could then manifest as an iron overload phenotype similar to that associated withTfR2 nonsense mutations.
The strong clinical expression of HH in the proband and family members with the Q690P homozygous mutation suggest that the TfR2 interaction with transferrin plays a critical role in normal iron homeostasis. However, because TfR2 is primarily expressed in hepatocytes and in erythroid precursors,20 much remains to be elucidated as to how an abnormal interaction with the transferrin protein results in systemic iron overload.
In our cohort of non-C282Y HH patients of predominately mixed European descent, only 1 of 89 was found to have a novel pathogenic mutation of either the TfR2 or HFE genes. The isolated nature of this finding is concordant with previous studies ofTfR212,21,22 orHFE15 23 in non-C282Y homozygous HH. However, the identification of the TfR2 Q690P mutation helps to establishTfR2 mutations as infrequent but important contributors to autosomal recessive adult-onset HH. This patient's family is the firstTfR2-associated HH pedigree to be described outside Italy.
We give special thanks to Duane Smailus and the Genome Sequencing Centre sequencing team for providing extensive technical support in this sequencing project. We also thank Dr Doug Horsman from the British Columbia Cancer Agency for his critical review of this manuscript. Most important, we thank the patients and family members for agreeing to participate in this study.
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2002-01-0133.
Supported by a fellowship grant from the National Science and Energy Research Council (NSERC) of Canada (G.V.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Gillian Lockitch, Dept of Pathology and Laboratory Medicine, Children's and Women's Health Centre of BC, 4480 Oak St, Vancouver, British Columbia, Canada, V6H 3V4; e-mail:glockitch@cw.bc.ca. In Europe: Maria De Sousa, Laboratory of Molecular Immunology, Institute for Molecular and Cell Biology, Rua do Campo Alegre, 823, 4150-180 Porto, Portugal; e-mail: mdesousa@ibmc.up.pt.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal