Abstract
Thrombopoietin receptor c-mpl is expressed on hematopoietic progenitors and cells of the megakaryocytic lineage. The c-mpl promoter may, therefore, be useful for directing the expression of transgenes. We tested whether a 2-kb genomic DNA fragment comprising the putative c-mpl regulatory elements and most of the 5′-untranslated region of mouse c-mpl is able to direct the expression of a reporter gene to hematopoietic cells in transgenic mice. As a reporter gene we used the human placental alkaline phosphatase (PLAP). In adult transgenic mice, PLAP expression was specifically detected in megakaryocytes and platelets. Embryos showed PLAP reporter gene expression already in the yolk sac at embryonic day 6.5 (E6.5) and in blood islands at E7.5. At E9.5, expression was found in blood vessels of the yolk sac and the embryo proper, followed by high levels of expression in the fetal liver at E11.5. Expression in E6.5 yolk sac is compatible with a function of c-mpl and its ligand, thrombopoietin, in the earliest stages of embryonic hematopoiesis.
Introduction
Thrombopoietin (TPO) is the primary regulator of megakaryopoiesis and platelet production.1-4 In addition, TPO promotes the expansion of pluripotent stem cells and early hematopoietic progenitors without preference for a particular lineage.5 The effects of TPO are mediated by its cell surface receptor, c-mpl, which was initially described as the cellular homologue of the retroviral oncogene, myeloproliferative leukemia (v-mpl)6; c-mpl is a member of the cytokine receptor superfamily7,8 and its expression is restricted to cells of the megakaryocytic lineage and to CD34+ hematopoietic progenitors and stem cells.5
We tested whether a 2-kb genomic DNA fragment containing the promoter and regulatory sequences of the mouse c-mpl gene might be useful to direct expression to the megakaryocytic lineage in transgenic mice. A 2-kb genomic DNA fragment containing promoter and regulatory sequences of the mouse c-mpl gene was derived. As a reporter gene, we chose the human placental alkaline phosphatase (PLAP), a glycosylphosphatidylinositol (GPI)-linked cell surface protein. PLAP allows sensitive and specific detection of protein expression because of its heat-stable alkaline phosphatase (AP) activity.9 We found that the 2-kb c-mplpromoter fragment was sufficient to specifically direct expression of the PLAP reporter gene to the megakaryocytic lineage in the adult and to the sites of early hematopoiesis in the embryo.
Study design
DNA constructs
A 2-kb HindIII –ApaI genomic fragment containing the 5′ portion of the mouse c-mpl promoter was subcloned into pBluescript KSII (Stratagene) cut withHindIII and ApaI. The unique ApaI site was blunted using T4 polymerase and was ligated to a 120–base pair (bp) fragment generated by polymerase chain reaction using the primers 5′-GGCCCCAGAGGGGCAGAG-3′ and 5′-TGTGTGCCTGCCTTAGCC-3′ (Figure1A). This fragment corresponds to the region between positions −132 and −13 relative to the c-mpl translational start site and contains the 3′ portion of the c-mpl promoter and part of the 5′-untranslated region (Figure 1A). SV40 polyadenylation signal and the PLAP cDNA were added to yield the c-mpl–PLAP–SV40 transgene construct (Figure1B). This construct was excised as a 5-kbXhoI–NotI fragment and was used to generate transgenic mice in the B6D2F1 background by standard oocyte pronucleus injection.
Transgene construct and expression of PLAP mRNA and protein in adult mice.
(A) Mouse c-mpl gene (top) with sequences of the 5′-upstream region (enlarged) aligned with the c-mpl/PLAP transgene. Black box represents c-mpl exon 1. Start codons for c-mpl and PLAP are highlighted. The transgene retains most of the c-mpl 5′-untranslated region. (B) Transgenic construct and position of the riboprobe used for the detection of PLAP mRNA expression. Tissues of mice heterozygous for the PLAP transgene were analyzed: bm, bone marrow; ln, mesenteric lymph node; pa, pancreas; br, brain; lu, lung; li, liver; sp, spleen; ki, kidney; ht, heart; th, thymus. Riboprobes for PLAP, c-mpl, and HPRT were mixed to allow the simultaneous detection of PLAP, endogenous c-mpl, and HPRT mRNAs. (C) PLAP protein expression in platelets and megakaryocytes of wild-type (wt) and transgenic (TG) mice. Dark staining indicates PLAP activity. Note that platelets have aggregated in the blood smear from TG. Magnification × 500.
Transgene construct and expression of PLAP mRNA and protein in adult mice.
(A) Mouse c-mpl gene (top) with sequences of the 5′-upstream region (enlarged) aligned with the c-mpl/PLAP transgene. Black box represents c-mpl exon 1. Start codons for c-mpl and PLAP are highlighted. The transgene retains most of the c-mpl 5′-untranslated region. (B) Transgenic construct and position of the riboprobe used for the detection of PLAP mRNA expression. Tissues of mice heterozygous for the PLAP transgene were analyzed: bm, bone marrow; ln, mesenteric lymph node; pa, pancreas; br, brain; lu, lung; li, liver; sp, spleen; ki, kidney; ht, heart; th, thymus. Riboprobes for PLAP, c-mpl, and HPRT were mixed to allow the simultaneous detection of PLAP, endogenous c-mpl, and HPRT mRNAs. (C) PLAP protein expression in platelets and megakaryocytes of wild-type (wt) and transgenic (TG) mice. Dark staining indicates PLAP activity. Note that platelets have aggregated in the blood smear from TG. Magnification × 500.
Blood and bone marrow
Blood was obtained by cardiac puncture, and blood counts were taken with an automated blood counter (Technicon H-1; Miles, Tarrytown, NY). Femur bones were flushed with Iscoves modified Dulbecco medium supplemented with 10% fetal bovine serum (Gibco), and bone marrow cells were concentrated on a glass slide by cytospin centrifugation.
Staining for alkaline phosphatase
Blood or bone marrow cells were heated to 65°C for 5 minutes, fixed in 4% paraformaldehyde, washed in HEPES buffered saline (HBS) (150 mM NaCl, 20 mM HEPES), and incubated for 5 to 10 minutes at room temperature in AP staining solution containing 0.17 mg/mL BCIP and 0.33 mg/mL NBT, 100 mM Tris, pH 9.5, 100 mM NaCl, and 5 mM MgCl2. Alkaline phosphatase activity results in blue staining.10
RNA isolation and ribonuclease protection assay
RNA from mouse tissues was isolated by the acid phenol method.11 To assess the expression of PLAP mRNA, a ribonuclease protection assay was performed.12 Total RNA (30 μg) was simultaneously hybridized with the following 3 riboprobes: (1) a riboprobe specific for PLAP (generated with the polymerase chain reaction primers 5′-TGTTCGACGACGCCATTGAG-3′ (sense) and 5′-CTTCGTCCAGGGGCACTGCT-3′ (antisense) that resulted in the protection of a 290-nucleotide (nt) fragment; (2) a riboprobe for mouse c-mpl (nucleotides −6 to +233 of the mouse c-mplcDNA sequence7) that protects a 239-nt fragment was added; and (3) a riboprobe for mouse hypoxanthine-guanine phosphoribosyl transferase (HPRT) to normalize for RNA loading that protected a 162-nt fragment.13
Analysis of transgene expression during embryogenesis
Timed pregnancies were obtained by natural mating in a 6 am to 7 pm light cycle. Plugs were checked in the morning, and 12 am was taken as the time of fertilization. Pregnant females were killed at embryonic days 6.5, 7.5, 8.5, 9.5, 10.5, and 11.5. Embryos with their yolk sacs were isolated in phosphate-buffered saline and immediately fixed for 4 hours in 4% paraformaldehyde. Endogenous AP activity was inactivated by heating the embryos to 65°C for 1 hour. PLAP activity in whole mounts was detected by AP staining for 4 hours.
Results and discussion
PLAP cDNA, as a reporter gene, was placed under the control of a 2-kb fragment containing the promoter and the regulatory sequences of the mouse c-mpl gene (Figure 1). Because the transcriptional start site of mouse c-mpl has not been precisely mapped, we included most of the c-mpl 5′-untranslated region (Figure1A). Transgenic mice were generated and analyzed for expression of the PLAP reporter gene. Of 2 transgenic lines obtained, one showed expression and was chosen for further analysis. Using an RNase protection assay, we detected the expression of PLAP mRNA in bone marrow and spleen at levels comparable with endogenous c-mpl(Figure 1B). Furthermore, PLAP protein was detectable in megakaryocytes and platelets from transgenic mice but not in wild-type controls (Figure 1C). Mice expressing the reporter gene had normal peripheral blood counts (not shown); thus, expression of the PLAP protein had no unexpected adverse effects.
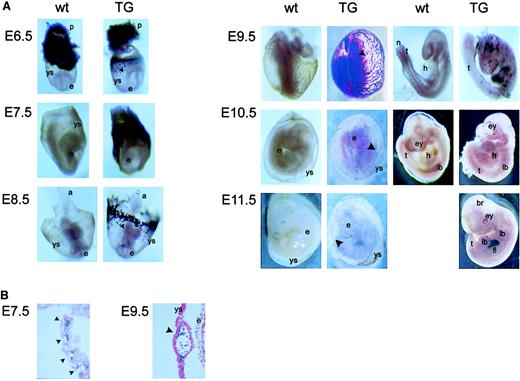
The alkaline phosphatase activity of PLAP allowed us to follow expression of the PLAP reporter gene during embryonic development. Already at 6.5 days after coitus (E6.5), PLAP was expressed as a ring in the yolk sac (Figure 2A). At E7.5 this staining was even more pronounced, and sections through the yolk sac revealed PLAP-positive cells within blood islands (Figure 2B). From E8.5 to E9.5, a major expansion of PLAP-positive cells was detectable in the extra-embryonic blood vessels, along with the appearance of staining within the embryo proper. Cross-sections through blood vessels of day E9.5 yolk sac showed staining of cells associated with the endothelium and with blood cells in the lumen (Figure 2B). Furthermore, PLAP-positive cells were also detectable in blood vessels of the embryo, including the aorta. At E10.5, staining of the extra-embryonic blood vessels markedly decreased, PLAP activity was found in a punctate pattern throughout the embryo, and strong expression of PLAP was detected in the fetal liver of E11.5 embryos.
Transgene expression in mouse embryos.
(A) Wild-type (wt) and transgenic (TG) embryos from E6.5 through E11.5 were collected and assayed in situ for PLAP enzymatic activity (blue staining). Embryos from E9.5 to E11.5 are shown with yolk sac (left column) and without yolk sac (right column). a indicates allantois; br, brain; e, embryo proper; ey, eye; fl, fetal liver; h, heart; lb, limb bud; n, notochord; p, placenta; t, tail; ys, yolk sac. Filled arrowheads point to blood islands or blood vessels in the yolk sac. Magnification × 10-20. (B) Paraffin sections at E7.5 and E9.5 show transgene expression in blood islands or blood vessels of the yolk sac. Annotations are as in panel A. Magnification × 200.
Transgene expression in mouse embryos.
(A) Wild-type (wt) and transgenic (TG) embryos from E6.5 through E11.5 were collected and assayed in situ for PLAP enzymatic activity (blue staining). Embryos from E9.5 to E11.5 are shown with yolk sac (left column) and without yolk sac (right column). a indicates allantois; br, brain; e, embryo proper; ey, eye; fl, fetal liver; h, heart; lb, limb bud; n, notochord; p, placenta; t, tail; ys, yolk sac. Filled arrowheads point to blood islands or blood vessels in the yolk sac. Magnification × 10-20. (B) Paraffin sections at E7.5 and E9.5 show transgene expression in blood islands or blood vessels of the yolk sac. Annotations are as in panel A. Magnification × 200.
In summary, embryonic expression of the PLAP reporter driven by the 2-kb c-mpl promoter followed the known sites of embryonic hematopoiesis, starting in blood islands and later appearing in the fetal liver. TPO/c-mpl signaling was implicated as playing a role in early yolk sac hematopoiesis,14,15 and a recent abstract also suggests a function in the hemangioblast.16Our finding of PLAP expression as early as day 6.5 further strengthens this possibility. At later stages, expression was found in blood cells and in endothelial cells. In accordance, the expression of c-mpl was previously reported in a mouse liver–derived endothelial cell line, LEC-1,17 and in human umbilical cord vein–derived endothelial cells.18 Additional transgenic lines will be necessary to confirm these results and to rule out the effects of integration site on the pattern and the levels of expression. We expect that this c-mpl promoter will be useful for directing the expression of other transgenes to sites of hematopoiesis in embryos and to the megakaryocytic lineage in adult mice.
We thank Dr Christoph Berger for help with the mouse embryo dissections and the interpretation of embryonic expression data.
Prepublished online as Blood First Edition Paper, June 28, 2002; DOI 10.1182/blood-2002-01-0281.
Supported by grants from the Swiss National Science Foundation to R.C.S.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Radek C. Skoda, Department of Research, Experimental Hematology, Basel University Hospitals, Hebelstrasse 20, 4031 Basel, Switzerland; e-mail: radek.skoda@unibas.ch.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal