Abstract
The development of chronic myeloid leukemia (CML) is dependent on the deregulated tyrosine kinase of the oncoprotein BCR-ABL. STI571 (imatinib mesylate), an abl tyrosine kinase inhibitor, has proven remarkably effective for the treatment of CML. However, resistance to STI571 because of enhanced expression or mutation of theBCR-ABL gene has been detected in patients. In the current study we show that the farnesyl transferase inhibitor (FTI) SCH66336 (lonafarnib) inhibits the proliferation of STI571-resistant BCR-ABL–positive cell lines and hematopoietic colony formation from peripheral blood samples of STI571-resistant patients with CML. Moreover, SCH66336 enhances STI571-induced apoptosis in STI571-sensitive cells and, in patients with STI571 resistance from gene amplification, cooperates with STI571 to induce apoptosis. Our data provide a rationale for combination clinical trials of STI571 and SCH66336 in CML patients and suggest that combination therapy may be effective in patients with STI571 resistance.
Introduction
STI571 (imatinib mesylate; Gleevec) represents a promising therapy for BCR-ABL–positive leukemia, but clinical resistance to STI571 can confound disease treatment. Multiple mechanisms account for clinical resistance, some involving alterations in BCR-ABL itself. Reactivation of BCR-ABL signaling either through point mutation or gene amplification of BCR-ABL has been observed in STI571-resistant patients1-3 and in a number of STI571-resistant BCR-ABL–positive cell lines.4-6 Some mechanisms of clinical STI571 resistance may be BCR-ABL independent, arising from altered drug uptake or metabolism, as demonstrated in a nude mouse model.7 Secondary genetic alterations in leukemic cells, which typically accompany chronic myeloid leukemia (CML) progression,8,9 may also confer drug resistance because the survival of late-stage CML leukemic cells may be less dependent on BCR-ABL tyrosine kinase activity.10 Indeed, STI571-induced hematologic responses occur less frequently and are less durable in patients in blast crisis.11 12 For these reasons it is likely that combination therapies will be most effective at eradicating BCR-ABL–positive leukemia.
Farnesyl transferase inhibitors (FTI) represent a novel class of chemotherapeutic agents originally developed to antagonize oncogenic Ras, but they have been shown to have activity against a wide range of transformed cells, regardless of Ras mutation. The clinical candidate FTI SCH66336 (lonafarnib) inhibits the proliferation of several human cancer cell lines and is active against human tumor xenografts in nude mice.13,14 We and others15,16 have shown the antileukemic activity of SCH66336 in cell culture models of BCR-ABL transformation and in mouse models of BCR-ABL–positive leukemia. Phase 1 clinical data have established that SCH66336 inhibits protein farnesylation in vivo and is generally well tolerated.17Additionally, clinical data with the FTI R115777 (Janssen) showed responses in 29% of patients with advanced and refractory leukemia.18 In the current study, we determined the effectiveness of SCH66336 alone and in combination with STI571 on STI571-resistant cell lines and patient samples.
Study design
Cell lines
Baf/BCR-ABL-r, AR230-r, and LAMA-84-r cell lines are resistant to STI571 because of amplification of the BCR-ABL gene and have been described previously.5 STI571-resistant K562 cells6 were a kind gift from James Griffin (Dana Farber Cancer Institute, Boston, MA). The STI571-resistant mutant of BCR-ABL, T315I, was generated using the Quikchange XL kit (Stratagene, La Jolla, CA) and was introduced into Baf3 cells using retroviral transduction. Parental and STI571-resistant cell lines were maintained in RPMI 1640 supplemented with 10% inactivated fetal bovine serum. For the STI571-resistant cell lines, media were supplemented with 500 nM STI571.
Compounds
The farnesyl transferase inhibitor (FTI) SCH66336 was a gift from Schering-Plough Research Institute (Kenilworth, NJ) and STI571 was a gift from Novartis (Basel, Switzerland). Both compounds were stored as 10 mM stocks in dimethyl sulfoxide (DMSO).
Measurement of cell viability and apoptosis
Cell viability was measured at daily intervals using trypan blue dye exclusion. Apoptosis was measured in cells after incubation with drugs by staining for annexin-positive cells using the ApoAlert Annexin V kit (Clontech, Palo Alto, CA). Immunoblotting was performed as described previously,16 and caspase-3 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Patient material and methylcellulose colony assays
Low-density mononuclear cells were isolated from either fresh or cryopreserved peripheral blood leukocytes using Lymphoprep (Nycomed, Oslo, Norway). Cells from STI571-resistant patients (refractory or relapsed disease despite adequate dose and duration of STI571 therapy) were plated in Iscoves methylcellulose medium (Methocult H4330; Stemcell Technologies, Vancouver, British Columbia, Canada) supplemented with 20 ng/mL recombinant human interleukin IL-3 (hIL-3), hG-CSF, hGM-CSF, hIL-6 (Amgen, Thousand Oaks, CA), and 100 ng/mL Flt3 ligand (R&D Systems Abingdon, Oxon, United Kingdom). Cells from STI571-naive patients were plated as described previously.16
Results and discussion
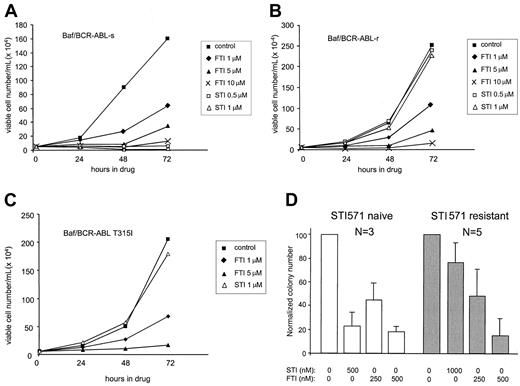
To determine whether resistance to STI571 correlates with resistance to SCH66336, parental Baf/BCR-ABL cells (s) and STI571-resistant (r) cell lines were placed in liquid culture containing DMSO (control), 0.5 or 1.0 μM STI571, or increasing concentrations of SCH66336. Although STI571 has no effect on the growth of STI571-resistant Baf/BCR-ABL cells, SCH66336 induces a dose-dependent inhibition of proliferation (Figure1). The antiproliferative effects of SCH66336 on Baf/BCR-ABL cells are primarily the consequence of G2/M blockade and not the induction of apoptosis.16 SCH66336 inhibits the growth of Baf/BCR-ABL-r, K562-r, AR230-r, and LAMA-84-r cells in a manner similar to that for the respective parental STI571-sensitive (s) cell lines (Figure 1B and data not shown). STI571 resistance in Baf/BCR-ABL-r, LAMA-84-r, and AR230-r cells is caused by gene amplification and enhanced expression of the BCR-ABLgene,5 a phenomenon that corresponds to STI571 resistance in patients.1 Similarly, SCH66336 inhibits the proliferation of Baf3 cells expressing the STI571-resistant T315I mutant1 of BCR-ABL (Figure 1C). Thus, SCH66336 is effective on BCR-ABL–positive leukemic cells despite STI571 resistance because of the amplification or mutation of BCR-ABL.
STI571-resistant cells remain sensitive to SCH66336.
Parental (A) and STI571-resistant Baf/BCR-ABL cells (B, C) were seeded at 5 × 104 cells/mL in cytokine-free RPMI + 10% inactivated fetal bovine serum in the presence of DMSO (control), SCH66336 (FTI), or STI571 (STI). BaF/BCR-ABL-r and BaF/BCR-ABL T315I are resistant to STI571 because of the amplification and mutation of BCR-ABL, respectively. Viable cells were assessed at daily intervals by dye exclusion, and results are representative of at least 3 experiments. (D) Hematopoietic progenitor cells were derived from STI571-naive patients (white bars, n = 3) or from patients clinically resistant to STI571 (gray bars, n = 5) and were grown in methylcellulose containing the indicated concentration of SCH66336 or STI571. Numbers are normalized to control (DMSO) and are presented as means ± SD of duplicate plates.
STI571-resistant cells remain sensitive to SCH66336.
Parental (A) and STI571-resistant Baf/BCR-ABL cells (B, C) were seeded at 5 × 104 cells/mL in cytokine-free RPMI + 10% inactivated fetal bovine serum in the presence of DMSO (control), SCH66336 (FTI), or STI571 (STI). BaF/BCR-ABL-r and BaF/BCR-ABL T315I are resistant to STI571 because of the amplification and mutation of BCR-ABL, respectively. Viable cells were assessed at daily intervals by dye exclusion, and results are representative of at least 3 experiments. (D) Hematopoietic progenitor cells were derived from STI571-naive patients (white bars, n = 3) or from patients clinically resistant to STI571 (gray bars, n = 5) and were grown in methylcellulose containing the indicated concentration of SCH66336 or STI571. Numbers are normalized to control (DMSO) and are presented as means ± SD of duplicate plates.
SCH66336 was also effective against BCR-ABL–positive leukemia cells from patients with clinical resistance to STI571. Primary hematopoietic cells from 5 CML patients who had STI571-resistant disease were cultured in methylcellulose in the presence of increasing concentrations of SCH66336. The precise mechanism of clinical resistance to STI571 for these 5 patients is unknown, but none harbored mutations in the BCR-ABL tyrosine kinase domain (F.-X.M., unpublished observations, June 2001). Cellular resistance to STI571 was indicated by robust hematopoietic colony formation in the presence of 1 μM STI571, more than double the IC50 for hematopoietic cells from CML patients naive to STI571 treatment (Figure 1D). Colony formation from STI571-resistant hematopoietic progenitors was significantly inhibited by SCH66336, with an IC50 between 250 and 500 nM, a value similar to that for hematopoietic cells from STI571-naive CML patients. These results indicate that the growth of CML cells from patients with STI571-resistant disease is inhibited by SCH66336.
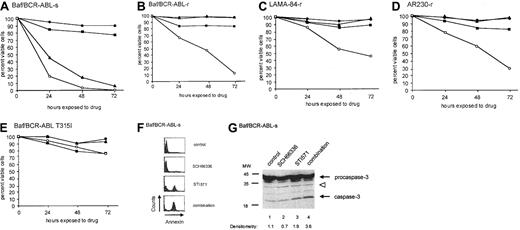
Although SCH66336 inhibits cell proliferation, as a single agent it has little effect on cell viability at concentrations up to 5 μM on either STI571-sensitive or -resistant cell lines (Figure2). Although cells resistant to STI571 because of BCR-ABL amplification remain viable in the presence of SCH66336 or STI571, they undergo a marked decrease in cell viability following exposure to both drugs (Figure 2B-D). When treated with a combination of SCH66336 and STI571, these cells are nonviable by 96 to 120 hours following drug treatment and do not recover (not shown). This reflects the fact that SCH66336 restores STI571-induced apoptosis in otherwise resistant cells. In contrast, there was no synergistic increase in apoptosis for Baf3 cells expressing the BCR-ABL mutant T315I (Figure 2E), which does not bind STI571, suggesting that some degree of ABL tyrosine kinase blockade is necessary for the combination effect.
SCH66336 sensitizes parental and STI571-resistant cells to STI571-induced apoptosis.
(A-E) Parental Baf/BCR-ABL-s (A) and STI571-resistant Baf/BCR-ABL-r (B), LAMA-84-r (C), AR230-r (D) cells, and Baf3 cells expressing the T315I mutant of BCR-ABL (E) were seeded at 5 × 104cells/mL in cytokine-free RPMI + 10% fetal bovine serum in the presence of DMSO (●), 1 μM STI571 (▴), 5 μM SCH66 336 (▪), or a combination of both drugs (○). Viability of cells was assessed at daily intervals by dye exclusion. Results are representative of at least 3 experiments. (F, G) Parental Baf/BCR-ABL-s cells were pretreated with DMSO or 1 μM SCH66336 for 48 hours, after which cells were split into 2 groups—no further treatment (control and SCH66336 cells) or treatment for an additional 8 hours with 1 μM STI571 (STI571 and combination cells). Cells were analyzed for either annexin V staining (F) by fluorescence-activated cell sorter analysis using the Apo-Alert kit (Clontech), or they were lysed and immunoblotted with antibody against caspase-3 (G). Densitometric analysis was performed on active caspase-3 and was normalized to levels of the indicated background band (open arrowhead).
SCH66336 sensitizes parental and STI571-resistant cells to STI571-induced apoptosis.
(A-E) Parental Baf/BCR-ABL-s (A) and STI571-resistant Baf/BCR-ABL-r (B), LAMA-84-r (C), AR230-r (D) cells, and Baf3 cells expressing the T315I mutant of BCR-ABL (E) were seeded at 5 × 104cells/mL in cytokine-free RPMI + 10% fetal bovine serum in the presence of DMSO (●), 1 μM STI571 (▴), 5 μM SCH66 336 (▪), or a combination of both drugs (○). Viability of cells was assessed at daily intervals by dye exclusion. Results are representative of at least 3 experiments. (F, G) Parental Baf/BCR-ABL-s cells were pretreated with DMSO or 1 μM SCH66336 for 48 hours, after which cells were split into 2 groups—no further treatment (control and SCH66336 cells) or treatment for an additional 8 hours with 1 μM STI571 (STI571 and combination cells). Cells were analyzed for either annexin V staining (F) by fluorescence-activated cell sorter analysis using the Apo-Alert kit (Clontech), or they were lysed and immunoblotted with antibody against caspase-3 (G). Densitometric analysis was performed on active caspase-3 and was normalized to levels of the indicated background band (open arrowhead).
We tested whether the drug combination showed enhanced killing of STI571-sensitive (s) cells relative to either drug alone. Indeed, a combination of SCH66336 and STI571 inhibited the viability of Baf/BCR-ABL-s cells to a greater degree than STI571 alone (Figure 2A; analysis of variance, P = .019 at 24 hours following drug exposure). Annexin V staining of STI571-sensitive Baf/BCR-ABL-s (Figure2F) and Baf/BCR-ABL-r (not shown) cells further demonstrates a synergistic increase in apoptosis associated with the drug combination. Treatment of Baf/BCR-ABL-s cells with STI571 and SCH66336 induced modestly higher levels of active caspase-3 than treatment with either drug alone (compare 3.6 and 1.9, Figure 2G), but it is unknown whether the synergistic action on cell death can be accounted for by enhanced caspase activation alone. Cell cycle blockade by SCH66336 cannot account for the decrease in cell viability in the presence of both drugs because another cell cycle inhibitor, LY294002 (Sigma), does not cooperate with STI571 to induce apoptosis in Baf/BCR-ABL-r cells (not shown).
Previously we demonstrated that SCH66336 sensitizes Baf/BCR-ABL cells to apoptotic stimuli such as serum starvation and γ-irradiation.16 Here we show that SCH66336 sensitizes Baf/BCR-ABL cells to STI571-induced apoptosis as well. However, in patients with STI571 resistance, STI571 is not an apoptotic stimulus, begging the question of how SCH66336 restores sensitivity to STI571 in these cells. Baf/BCR-ABL-r cells have increased BCR-ABL tyrosine kinase activity compared to parental Baf/BCR-ABL cells,5 and STI571 reduces tyrosine kinase activity, but not below the threshold needed to induce apoptosis. Because SCH66336 blocks BCR-ABL signaling by inhibiting Ras16 and other downstream effector molecules, the combination of SCH66336 and STI571 likely inhibits BCR-ABL signaling below a critical threshold required for survival. This mechanism implies that combination blockade of the BCR-ABL signaling pathway remains a viable strategy in STI571 resistance. Our data demonstrate that SCH66336 and STI571 represent a potent combination for patients in whom STI571 resistance results from the amplification of BCR-ABL, as frequently occurs.2 3 We speculate that the enhanced activity of STI571 in combination with SCH66336 would also apply when mutations of the BCR-ABL kinase domain reduce, but do not eliminate, STI571 binding. In these patients, combination therapy with SCH66336 and STI571 may be more effective and better tolerated than dose escalation of STI571 alone. However, in patients in whom the BCR-ABL kinase mutation significantly reduces STI571 binding (as in the T315I mutation), it is possible that combination therapy would be no better than single-agent SCH66336 therapy. Experiments on cells from a large number of relapsed patients, in which the mechanism of STI571 resistance has been fully characterized, are required to test our hypotheses. Nonetheless, as a well-tolerated oral agent that enhances STI571-induced apoptosis of BCR-ABL–transformed leukemic cells, SCH66336 is an appealing candidate for clinical testing in combination with STI571 in patients with newly diagnosed or STI571-resistant CML.
Supported by a grant from the Schering-Plough Research Institute and by grants CA76418 and CA86991 from the National Cancer Institute. G.Q.D. is the Birnbaum Scholar of the Leukemia and Lymphoma Society of America.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
George Q. Daley, Whitehead Institute for Biomedical Research, 9 Cambridge Center, Cambridge, MA 02142; e-mail: daley@wi.mit.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal