CD9, a 24-kDa member of the tetraspanin family, influences cellular growth and development, activation, adhesion, and motility. Our investigation focuses on the hypothesis that the CD9 second extracellular loop (EC2) is important in modulating cell adhesive events. Using a Chinese hamster ovary (CHO) cell expression system, we previously reported that CD9 expression inhibited cell adhesion to fibronectin and fibronectin matrix assembly. For the first time, a functional epitope on CD9 EC2 that regulates these processes is described. Binding of mAb7, an EC2-specific anti-CD9 monoclonal antibody, reversed the CD9 inhibitory activity on CHO cell adhesion and fibronectin matrix assembly. This reversal of cell phenotype also was observed in CHO cells expressing CD9 EC2 truncations. Furthermore, our data showed that the EC2 sequence173LETFTVKSCPDAIKEVFDNK192 was largely responsible for the CD9-mediated CHO cell phenotype. Two peptides, 135K-V172 (peptide 5b) and168P-I185 (peptide 6a), selectively blocked mAb7 binding to soluble CD9 and to CD9 on intact cells. These active peptides reversed the influence of CD9 expression on CHO cell adhesion to fibronectin. In addition, confocal microscopy revealed that CD9 colocalized with the integrin α5β1 and cytoskeletal F-actin in punctate clusters on the cell surface, particularly at the cell margins. Immunoprecipitation studies confirmed CD9 association with β1 integrin. The cellular distribution and colocalization of focal adhesion kinase and α-actinin with cytoskeletal actin was also influenced by CD9 expression. Thus, CD9 may exhibit its effect by modulating the composition of adhesive complexes important in facilitating cell adhesion and matrix assembly.
Introduction
CD9, a member of the tetraspanin or transmembrane 4 super family (TM4SF) of proteins,1 is widely distributed on the surface of normal and malignant cells as well as on a variety of cell lines.2-4 Several members of the tetraspanin family, including CD9, form noncovalent associations with integrins, particularly β1 integrins.5-10 Tetraspanins may have a direct role in integrin activity via their physical association with integrins5 and participate in signaling pathways regulating integrin function.11-14
Anti-CD9 mAb perturbation studies have explored the role of CD9 on human platelets and other cells.15-21 Platelets are activated upon treatment with anti-CD9 mAbs, such as mAb7,17 a response initially thought to be due solely to cross-linking of CD9 with FcγRII (CD32).18 Subsequently, a measurable platelet activation response via CD9 and independent of CD32 was demonstrated.7 In addition, anti-CD9 mAbs have been shown to mediate the proliferation, adhesion, and motility of neural cells.19-21
Expression of CD9 and other tetraspanins alters the activation, adhesion, and motility of cells in response to extracellular matrix proteins.5 For example, purified CD922 and CD9 expressing Chinese hamster ovary (CHO) cells23preferentially bind to the extracellular matrix (ECM) protein fibronectin (FN), in particular to a 40-kDa segment containing the HEP2/IIICS binding domain. CD9 surface expression facilitates CHO cell spreading on FN, inhibits extracellular FN matrix assembly and cell adhesion to FN,23 yet increases CHO cell haptotactic motility to FN.24 In addition, the transfection of CD9 into poorly motile CD9-negative pre-B cells (Raji) up-regulates the motility of these cells across FN and laminin (LN).25Conversely, transfection of CD9 into nonlymphoid, motile cell lines down-regulates their motility to FN and LN.26 Thus, CD9 influence on adhesive activity can vary between cell types and may be related to its specific linkage to other surface proteins that form large molecular complexes and facilitate clustering of signaling molecules.
Previous investigations have shown that EC2, the fourth transmembrane (TM4) segment, and the cytoplasmic carboxyl tail of tetraspanins affect cell function. For example, Lagaudriére-Gesbert et al27 demonstrated that a chimeric CD81/CD9 containing CD81 TM1-3 and extracellular loop 1 (EC1) domains combined with CD9 EC2 TM4 regions mimicked intact CD9 by increasing the sensitivity of LHBEGF cells to diphtheria toxin (DT). Additionally, the interaction between CD9 EC2 and the EC domain of proHB-EGF was important for up-regulation of both mitogenic and DT-binding activities of proHB-EGF.28 29
Our present investigation examined the importance of the CD9 EC2 region on the adhesion and pericellular FN matrix assembly of CD9 CHO cells. Using peptides that correspond to amino acid sequences of EC2 and CD9 mutants lacking specific regions of EC2, we identified EC2 domains important in the regulation of CHO cell adhesive functions. Confocal microscopy and immunoprecipitation (IP) studies demonstrated that CD9 but not mutant EC2 CD9 is in association with β1. CD9 expression in CHO cells also influenced the colocalization of α5β1 and actin as well as the distribution of α-actinin and focal adhesion kinase (FAK), key components of cell adhesion complexes.
Materials and methods
Materials
The expression vectors pRc/CMV and pBluescriptII/SK+ were obtained from Invitrogen (Carlsbad, CA), and from Stratagene (La Jolla, CA), respectively. Transblot and DC protein assay kit were from Bio-Rad (Hercules, CA). AmpliTaq DNA polymerase was acquired from Perkins-Elmer Cetius (Foster City, CA). RPMI 1640, LipofectAMINE, Opti-MEM I Reduced Serum Medium, l-glutamine, Geneticin, and human plasma FN were purchased from Gibco BRL (Gaithersburg, MD). Fetal bovine serum was from Hyclone Laboratories (Logan, UT). EZ-Link Sulfo-NHS-LC biotinylation kit, NeutrAvidin, and SuperSignal were purchased from Pierce (Rockford, IL). Protein G PLUS/Protein A agarose beads were acquired from Oncogene Research Products (Boston, MA). Anti-CD9 monoclonal antibody mAb7 has been described previously.17 Anti-CD9 RAP2, a polyclonal antibody specific for the EC1 region of CD9, was developed in our laboratory. Anti-α5 antibody PB1 and anti-β1 antibody 7E2 were from Developmental Studies Hybridoma Bank (Iowa City, IA). AK1, an antiplatelet GPIb antibody, was provided by Dr M. Berndt (Melbourne, Australia). Alexa Fluor 488–conjugated goat anti–mouse antibody and Alexa Fluor 594–conjugated goat anti–mouse antibody were obtained from Molecular Probes (Eugene, OR). Mouse anti–FAK (clone 4-4A) antibody and mouse anti–α-actinin (clone AT6.172) were acquired from Chemicon (Temecula, CA). Anti–mouse IgG antibody, goat anti–mouse fluorescein isothiocyanate (FITC), and phalloidin-tetramethylrhodamine-5(and 6)-isothiocyanate (TRITC) were purchased from Sigma Chemical (St Louis, MO). Rabbit anti–bovine FN antibody was from Accurate Chemical (Westbury, NY).
Methods
Generation of CD9 deletion mutants.
The isolation and cloning of CD9 cDNA into the mammalian expression vector pRc/CMV (pRc/CMVCD9) has been described previously.1 A CHO cell clone transfected with pRc/CMVCD9 was designated CD9-CHO-N3. A second CD9 CHO cell clone CD9-CHO-A6 was generated for this study, using the pRc/CMVCD9 plasmid as described previously. The strategy for the deletion of the CD9 EC2 and TM4 regions (Δ113-228) has been described elsewhere.23
The oligonucleotide primers (Table 1) used to construct the CD9 EC2 truncation cDNAs were designed according to the reported CD9 nucleotide sequence.1 These oligonucleotide primers were used in polymerase chain reaction (PCR) amplifications using full-length CD9 cDNA as a template to generate mutant CD9 cDNAs. To construct CD9 EC2 deletions, 3 PCR amplifications were used. The first PCR was done using full-length CD9 cDNA as a template with a CD9 SphI 5′ primer (5′-CAGTGCATGCTGGGACTGTTCTTCGGCTTC-3′) containing the SphI site at position +416 in the CD9 open reading frame with either 3′ Δ133-192 (5′-TGCGCCGATGATGTGGAACAGCTTGTTGTAGGT-3′), 3′ Δ152-192 (5′-GCCGA TGATGTGGAAGTTCAACGCATAGTG-3′), or 3′ Δ173-192 (5′-GCCGATGATG TGGAATACGTCCTTCTTGGG-3′) primers generating 154-bp, 208-bp, and 271-bp fragments, respectively.
PCR primers for CD9 deletions
| Oligonucleotide primers . | Nucleotide sequence . |
|---|---|
| 5′CD9 Sph1 | 5′-CAGTGCATGCTGGGACTGTTCTTCGGCTTC-3′ |
| 5′ Δ133-192 | 5′-ACCTACAACAAGCTGTTCCACATCATCGGCGCA-3′ |
| 3′Δ133-192 | 5′-TGCGCCGATGATGTGGAACAGCTTGTTGTAGGT-3′ |
| 5′ Δ152-192 | 5′-CACTATGCGTTGAACTTCCACATCATCGGC-3′ |
| 3′Δ152-192 | 5′-GCCGATGATGTGGAAGTTCAACGCATAGTG-3′ |
| 5′ Δ173-192 | 5′-CCCAAGAAGGACGTATTCCACATCATCGGC-3′ |
| 3′Δ173-192 | 5′-GCCGATGATGTGGAATACGTCCTTCTTGGG-3′ |
| 3′Apal | 5′-GATCGGGCCCTCTAGATCGAATTCC-3′ |
| Oligonucleotide primers . | Nucleotide sequence . |
|---|---|
| 5′CD9 Sph1 | 5′-CAGTGCATGCTGGGACTGTTCTTCGGCTTC-3′ |
| 5′ Δ133-192 | 5′-ACCTACAACAAGCTGTTCCACATCATCGGCGCA-3′ |
| 3′Δ133-192 | 5′-TGCGCCGATGATGTGGAACAGCTTGTTGTAGGT-3′ |
| 5′ Δ152-192 | 5′-CACTATGCGTTGAACTTCCACATCATCGGC-3′ |
| 3′Δ152-192 | 5′-GCCGATGATGTGGAAGTTCAACGCATAGTG-3′ |
| 5′ Δ173-192 | 5′-CCCAAGAAGGACGTATTCCACATCATCGGC-3′ |
| 3′Δ173-192 | 5′-GCCGATGATGTGGAATACGTCCTTCTTGGG-3′ |
| 3′Apal | 5′-GATCGGGCCCTCTAGATCGAATTCC-3′ |
The second PCR was performed using full-length CD9 cDNA as a template with a 3′ ApaI primer (homologous to the pRc/CMV vector backbone sequence) with either 5′ Δ133-192 (5′-ACCTACAACAAGCTGTTCCACATCATCGGCGCA-3′), 5′ Δ152-192 (5′-CACTATGCGTTGAACTTCCACATCATCGGC-3′), or 5′ Δ173-192 (5′-CCCAAGAAGGACGTATTCCACATCATCGGC-3′) primers, respectively, generating a 611-bp fragment in each case.
For the third PCR amplification, corresponding overlapping PCR products were used as templates and extended for 15 cycles, after which CD9 SphI and ApaI primers were used for an additional 30 cycles to generate CD9 EC2 internal deletion products of 765 bp for Δ133-192, 819 bp for Δ152-192, and 882 bp for Δ173-192. These PCR products were cleaved with SphI andApaI and subcloned into the pBSSKCD9 vector backbone from which the SphI/ApaI portion of the CD9 cDNA/vector sequence had been removed, generating complete CD9 cDNAs with the targeted regions in CD9 EC2 missing. The CD9 EC2 truncation cDNAs were subcloned into the original pRc/CMVCD9 construct from which the full-length CD9 cDNA had been removed. The fmole DNA sequencing system was used to obtain and confirm CD9 EC2 truncation cDNA sequences. In summary, Δ133-192, Δ152-192, and Δ173-192 CD9 cDNAs had truncations of 180 bp (60 aa), 123 bp (41 aa), and 60 bp (20 aa) in CD9 EC2, respectively.
Cell transfections.
Wild-type CHO cells were grown in 6-well tissue culture plates at 3 × 105/well to 50% to 70% confluency. Cells were rinsed once with serum-free RPMI 1640 and transfected with 2 μg of plasmid DNA using LipofectAMINE according to manufacturer's protocol. At 72 hours after transfection, cells were passed 1:10 in selective growth media supplemented with 0.75 mg/mL Geneticin G418, and stable transfectants were selected. Mock control transfections were performed and designated as CHO MOCK.
Cell culture.
Mock- and CD9-transfected CHO cells1 30 were routinely grown in growth media (RPMI 1640 with 25 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] and l-glutamine supplemented with 10% fetal bovine serum and 0.75 mg/mL Geneticin). For cell-cycle synchronization, cells were grown to 100% confluency for 48 hours, washed twice with phosphate-buffered saline (PBS), and harvested by a 2-minute exposure to 0.05% Trypsin-0.53 mM EDTA (ethylenediaminetetraacetic acid) at 37°C. The collected cells were washed twice in growth media, transferred to 75-cm2 culture flasks (2 × 106 cells/flask), and cultured overnight, yielding a monolayer enriched in cells at the G0/G1stage. Cell cycle synchronized cells were used in all CHO cell experiments.
Determination of mutant CD9 surface expression.
CHO cells expressing intact CD9 or CD9 mutants were harvested as described above. 250 000 cells in labeling media (RPMI, 5% goat serum) were labeled with 4 μg mAb7, RAP5a, RAP2, or MOPC21 (MIgG) for one hour at 4°C. The cells were then washed with PBS, resuspended in labeling media, and labeled with a species-specific FITC-conjugated antibody for 1 hour at 4°C. After washing, the cells were analyzed by flow cytometry using a FACSCalibur Flow Cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA).
Identification of the mAb7 binding region by competitive immunoprecipitation of CD9 using CD9 peptides and flow cytometric analysis of CHO cells.
Immunoprecipitations.
Venous blood from healthy donors was collected using the anticoagulant acid citrate dextrose, ACD (85 mM sodium citrate, 100 mM dextrose, and 70 mM citric acid), at a ratio of 8.6:1.4 and centrifuged at 135g to obtain platelet-rich plasma. Platelets were pelleted by centrifugation at 850g, washed with CGS (10 mM sodium citrate, 30 mM dextrose, and 120 mM NaCl, pH 6.5), and resuspended at 2.5 × 108 platelets/mL in Tyrode buffer (138 mM NaCl, 2.9 mM KCl, 12 mM NaHCO3, 0.4 mM MgCl2, 55 mM dextrose, 0.36 mM NaH2PO4 H2O, and 1.8 mM CaCl2, pH 7.4). Platelets were lysed in an equal volume of ice-cold 2x lysis buffer (2% Triton X-100, 1% NP-40, 300 mM NaCl, 5 mM EDTA, and 20 mM Tris [Tris(hydroxymethyl)aminomethane], pH 7.5 supplemented with EDTA-free protease inhibitor tablets) for 20 minutes at 4°C. The lysate was clarified for 15 minutes at 21 000g, and 1-mL aliquots were added to either 2 μg mAb7, control mouse IgG1, κ (MOPC 21), or mAb7 that had been preincubated for 30 minutes at room temperature with 200 μg of peptide 5a, 5, 5b, or 6a (Table 2). Lysate/mAb/peptide mixtures were incubated with agitation for 1 hour at 4°C, followed by addition of Protein A/G PLUS-Agarose and incubation at 4°C for 1 hour. The collected immunoprecipitates were washed with 1x lysis buffer, eluted with nonreduced sample buffer (20% glycerol, 4% sodium dodecyl sulfate [SDS], 0.01% bromophenol blue, and 0.125 M Tris-HCl, pH 6.8), and fractionated on a 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel. Separated proteins were transferred to polyvinylidene fluoride (PVDF) membranes and probed using 1:10 000 mAb7 (1 mg/mL), followed by 1:20 000 horseradish peroxidase–conjugated goat anti–mouse IgG. Blocking and incubation steps were performed in 5% nonfat dried milk, TBS/T (10 mM Tris, pH 7.4, 150 mM NaCl, and 0.1% Tween-20); the wash steps were performed in TBS/T alone. Blots were developed by chemiluminescence using SuperSignal West Pico and exposed to CL-XPosure Film.
Synthetic peptides corresponding to portions of CD9 EC2
| Peptide designation . | Amino acid sequence . | CD9 residues* . |
|---|---|---|
| 5a | YSHKDEVIKEVQEFYKDTYNKLKT | 111-134 |
| 5 | YKDTYNKLKTKDEPQRETLKAI | 125-146 |
| 5b | KDEPQRETLKAIHYALNCCGLAGGVEQFISDICPKKDV | 135-172 |
| 6a | PKKDVLETFTVKSCPDAI | 168-185 |
| Peptide designation . | Amino acid sequence . | CD9 residues* . |
|---|---|---|
| 5a | YSHKDEVIKEVQEFYKDTYNKLKT | 111-134 |
| 5 | YKDTYNKLKTKDEPQRETLKAI | 125-146 |
| 5b | KDEPQRETLKAIHYALNCCGLAGGVEQFISDICPKKDV | 135-172 |
| 6a | PKKDVLETFTVKSCPDAI | 168-185 |
Peptide sequence and residue location based upon platelet CD9 cDNA by Lanza et al.1
Flow cytometric analysis.100 μg of peptide 5a, 5, 5b, or 6a was incubated with 1 μg mAb7 for 30 minutes at room temperature. CD9 CHO cells (clone A6) were harvested, washed twice with PBS, and resuspended in labeling media (2.5 × 105 cells/mL); 4 μL mAb7-peptide solution was added to 2.5 × 105 cells and incubated for 1 hour at 4°C. MOPC21 and mAb7 alone were used as negative and positive controls, respectively. Cells were washed with PBS, resuspended in labeling media, and bound mAb7 was detected with a species-specific FITC-conjugated second antibody, followed by flow cytometry as described above.
Basic adhesion assay.
24-well culture plate wells were coated with human plasma FN (10 μg/mL FN in PBS for 3 hours at 37°C) and blocked with PBS, 3% bovine serum albumin (BSA) for 1 hour at 37°C. Mock- and CD9-CHO cells were harvested as described previously. After a 30-minute rest period at 37°C, 105 cells/well were seeded in FN-coated 24-well plates and incubated for 3 hours at 37°C. For some experiments, cells were incubated in the presence of 0.5 μM of peptides corresponding to amino acid sequences of CD9 EC2. Wells were washed 4 times with adhesion media, stained with modified Wright Giemsa, and adherent cells were counted in 5 randomly selected high-power fields of view per well using an inverted phase contrast microscope, Olympus IMT-2 (Olympus, Lake Success, NY). All assays were run in triplicate, and the cell counts were reported as the number of adherent cells/mm2 of well surface area. The mean number of adherent cells/mm2 ± SE of 3 independent assays were reported (n = 45).
Immunofluorescent imaging of pericellular FN matrix.
Mock- and CD9-transfected CHO cells were grown to 100% confluency, as previously described,23 on human plasma FN-coated dual-chamber Lab-Tek chamber slides (1 × 105cells/chamber) in the presence of 50 μg/mL bovine plasma FN. After washing with PBS, the cells were incubated 1 hour at 4°C with 4 μg/mL goat IgG to block nonspecific binding sites. The cells were washed with PBS and incubated with 4 μg/mL polyclonal rabbit anti–bovine FN primary antibody for 1 hour at 4°C. After washing, the cells were labeled using 5 μg/mL FITC-conjugated goat anti–rabbit antibody (1 hour at 4°C), fixed for 15 minutes with 4% paraformaldehyde, and coverslips were applied with Fluoromount-G. Epifluorescent digital images of the pericellular FN matrix were captured using a Zeiss Axiophot microscope. The analysis was carried out with 3 independent preparations.
Colocalization analysis using laser scanning confocal microscopy.
Mock- and CD9-transfected CHO cells were grown for 3 hours on human plasma FN-coated dual-chamber Lab-Tek chamber slides (1 × 104 cells/chamber). Adherent cells were washed with PBS and blocked with goat IgG (4 μg/mL) in labeling media. After washing, cells were incubated with mAb7 for 30 minutes at 4°C, washed, and labeled with 5 μg/mL Alexa Fluor 488–conjugated goat anti–mouse antibody for 30 minutes at 4°C. The cells were then washed and incubated with either 4 μg/mL PB1 (anti-α5β1) or 7E2 (anti-β1) at 4°C for 30 minutes. After washing, bound mAb was detected by incubation with 5 μg/mL Alexa Fluor 594–conjugated goat anti–mouse antibody for 30 minutes at 4°C. Finally, cells were washed, fixed for 15 minutes with 4% paraformaldehyde, and coverslips were applied with Fluoromount-G. The stained cells were examined using a Zeiss LSM 510 laser scanning confocal microscope system, and images of labeled cells were digitally captured. As mAb7, PB1, and 7E2 all were mouse IgG isotype, CD9 always was labeled first. Alexa Fluor 488–conjugated goat anti–mouse antibody was used at a concentration to ensure that all mAb7 sites were saturated prior to the addition of Alexa Fluor 594–conjugated goat anti–mouse antibody used to detect bound anti-α5 PB1 or anti-β1 7E2. The saturating concentrations of Alexa Fluor conjugates were determined as follows. Cells were washed with PBS and incubated with increasing concentrations of Alexa Fluor 488–conjugated goat anti–mouse antibody for 30 minutes at 4°C, followed by washing and addition of Alexa Fluor 594–conjugated goat anti–mouse antibody. Saturation binding of mAb7 by Alexa Fluor 488–conjugated goat anti–mouse antibody was accomplished when no Alexa Fluor 594 binding was detected. The saturating concentration for Alexa Fluor 594 goat anti–mouse as a secondary antibody for labeling bound PB1 or 7E2 also was determined in an identical manner. Spatial location of the intracellular proteins F-actin, FAK, and α-actinin was determined using a modified immunostaining protocol of Bell and Safiejko-Mroczka31that stabilized protein location, especially cytoskeleton F-actin. The adherent cells on Lab-Tek chamber slides were cross-linked with 1 mM freshly prepared dithiobis (succinimiddyl proprionate) (DSP) in Hanks balanced salt solution (HBSS) for 10 minutes at 37°C. The cells were gently extracted with 0.5% Triton X-100 in stabilizing buffer (1 mM EGTA [ethyleneglycoltetraacetic acid], 4% polyethylene glycol 8000, 0.0015% phenol red, and 100 mM piperazine diethanesulfonic acid [PIPES], pH 6.9) containing 1 mM DSP for 10 minutes at 37°C. The cells were then rinsed with 0.5% Triton X-100 in stabilizing buffer for 5 minutes at 37°C. Following washes with PBS, nonspecific protein binding sites were blocked with 0.1 M glycine in PBS for 5 minutes at room temperature, followed by washing in PBS. F-actin was labeled using 1 mL of 2 μM Phalloidin-TRITC, and adhesion complex components α5β1, FAK, and α-actinin were labeled using 4 μg/mL PB1, anti-FAK (clone 4-4A), or anti–α-actinin (clone AT6.172), respectively, for 1 hour at 4°C. The cells were washed with PBS and labeled with 5 μg/mL FITC-conjugated anti-mouse antibody. After washing and fixing in 4% paraformaldehyde for 15 minutes at room temperature, coverslips were applied using Fluoromount-G. The stained cells were examined using a Zeiss LSM 510 laser scanning confocal microscope system in sequential mode, and images of labeled cells were digitally captured.
Immunoprecipitation and Western blotting.
CHO cells were harvested by trypsinization as previously described and washed twice with PBS, 10 mM EDTA. Cell surface proteins were biotinylated using an EZ-Link Sulfo-NHS-LC biotinylation kit according to the manufacturer's protocol. After washing 3 times with PBS, cells (4 × 106 cells/mL) were lysed for 1 hour at 4°C using a nondenaturing lysis buffer (1% CHAPS [3-[(3-cholamidopropyl)dimethylamonio]-1-propyl sulfonate], HEPES, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 2 mM NaF). Cytoskeletal debris was pelleted at 10 000g for 10 minutes, and the lysate was precleared overnight at 4°C using Protein G PLUS/Protein A. Lysates were immunoprecipitated with anti-CD9 mAb7 or RAP2, mouse IgG, or 7E2 (10 μg/mL) and Protein G PLUS/Protein A agarose for 6 hours at 4°C with agitation. Captured immune complexes were washed 8 times with lysis buffer containing 0.1% CHAPS, eluted using nonreducing Laemmeli sample buffer, resolved by 5% to 20% SDS-PAGE, and transferred to Transblot. Blots were blocked with immune stain buffer (10 mM Tris, pH 7.4, 0.9% NaCl, 5% BSA, 0.05% Tween-20) overnight at 4°C. The blots were hybridized with NeutrAvidin for 1 hour at room temperature and washed 5 times with 10 mM Tris, pH 7.4, 100 mM NaCl, 0.05% Tween-20, followed by development with SuperSignal. For reimmunoprecipitation, the eluate from the mAb7 or RAP2 immunoprecipitate was diluted 3-fold with lysis buffer and precleared, as described above. After addition of anti-β1 7E2, immune complexes were captured with Protein G PLUS/Protein A agarose and eluted in lysis buffer supplemented with 0.5% SDS and 2.5 mM EDTA at 70°C for 10 minutes.
Results
Surface expression of CD9 deletion mutants
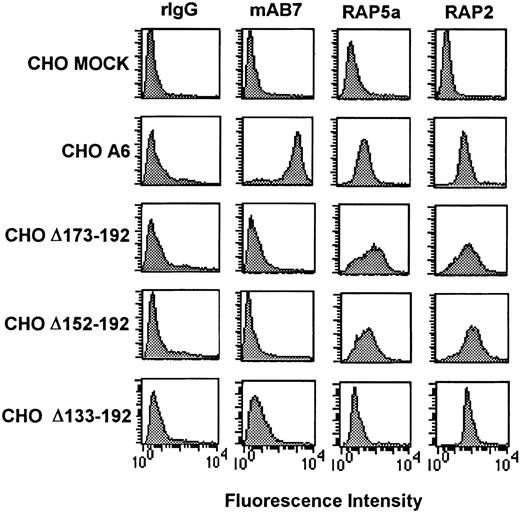
To characterize the regions within CD9 EC2 that are important for the adhesive phenotype of CHO cells, a series of internally truncated CD9 cDNA were cloned into the mammalian expression vector pRcCMV and transfected into CHO cells. Surface expression of wild-type or mutant CD9 proteins was confirmed by flow cytometric analysis using RAP2, an anti-CD9 EC1 antibody, and anti-CD9 EC2 antibodies mAb7 and RAP5a. CHO cells with equivalent surface expression of either full-length CD9 or CD9 truncated proteins were selected for further analysis (Figure1). Equivalent CD9 mRNA expression was verified by Northern blot analysis (data not shown).
Flow cytometry analysis of CD9 cDNA-transfected CHO cells using anti-CD9 EC1 and EC2 antibodies.
Cell suspensions were incubated with rabbit IgG (rIgG), anti-CD9 EC2 antibodies mAb7 or RAP5a, or anti-CD9 EC1 RAP2. Bound antibody was detected by a species-specific FITC-conjugated antibody. The measured mean fluorescence intensity of anti-CD9 EC1 RAP2 suggested that each CD9 clone had equivalent CD9 surface density. The lack of anti-CD9 mAb7 binding on all CD9 EC2 deletion mutants suggests that the mAb7 epitope is located on CD9 EC2.
Flow cytometry analysis of CD9 cDNA-transfected CHO cells using anti-CD9 EC1 and EC2 antibodies.
Cell suspensions were incubated with rabbit IgG (rIgG), anti-CD9 EC2 antibodies mAb7 or RAP5a, or anti-CD9 EC1 RAP2. Bound antibody was detected by a species-specific FITC-conjugated antibody. The measured mean fluorescence intensity of anti-CD9 EC1 RAP2 suggested that each CD9 clone had equivalent CD9 surface density. The lack of anti-CD9 mAb7 binding on all CD9 EC2 deletion mutants suggests that the mAb7 epitope is located on CD9 EC2.
EC2 of CD9 contains functional domains important in CHO cell adhesion and pericellular FN matrix assembly
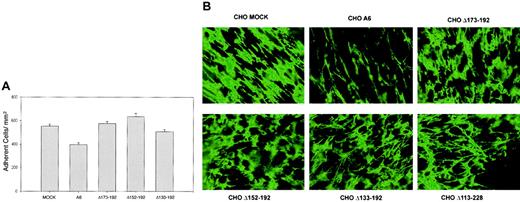
Our previous investigations showed that CD9 CHO A6 cells were approximately 30% less adherent to FN and assembled approximately 50% less pericellular FN matrix than MOCK CHO cells.23 This phenotype was reversed by treatment of CHO A6 cells with anti-CD9 mAb7 or the deletion of CD9 amino acids 113-228 (EC2, TM4, and the C-terminal cytoplasmic tail deletion), suggesting that CD9 EC2 was important for the CD9 CHO cell adhesive phenotype.
To delineate the functional EC2 domain for cell adhesion, adhesion assays were performed using the EC2 deletion mutant clones Δ133-192, Δ152-192, and Δ173-192 (Table 3). As shown in Figure 2A, each mutant clone was significantly more adhesive to FN than cells expressing full-length CD9 (P < .001). These assay results suggest that the 20 amino acid sequence, 173LETFTVKSCPDAIKEVFDNK,192absent in all 3 mutant CD9 clones, was at least partly responsible for the influence of CD9 on FN adhesion. These data, when compared with results using the Δ113-228 mutant,23 confirmed that the TM4 and C-terminus segments did not play a significant role in the observed adhesion phenotype. We also examined the influence of the first CD9 extracellular loop (EC1) on CHO cell adhesion.23The adhesion level of the mutant EC1 expressing CHO cells (CHO Δ35-57) was significantly greater than wild-type CD9 expressing CHO cells (P < .01), but not significantly different from MOCK-transfected cells (P = .35). These results suggest that there is coordination between the 2 EC loops in mediating the adhesion phenotype.
Amino acid sequence of EC2
| Clone . | EC2 amino acid sequence . | |||
|---|---|---|---|---|
| 113 | 133 | 152 | 173192 | |
| CHO A6 | HKDEVIKEVQEFYKDTYNKL | KTKDEPQRETLKAIHYALN | CCGLAGGVEQFISDICPKKDV | LETFTVKSCPDAIKEVFDNK |
| Δ133-192 | HKDEVIKEVQEFYKDTYNKL | |||
| Δ152-192 | HKDEVIKEVQEFYKDTYNKL | KTKDEPQRETLKAIHYALN | ||
| Δ173-192 | HKDEVIKEVQEFYKDTYNKL | KTKDEPQRETLKAIHYALN | CCGLAGGVEQFISDICPKKDV | |
| Clone . | EC2 amino acid sequence . | |||
|---|---|---|---|---|
| 113 | 133 | 152 | 173192 | |
| CHO A6 | HKDEVIKEVQEFYKDTYNKL | KTKDEPQRETLKAIHYALN | CCGLAGGVEQFISDICPKKDV | LETFTVKSCPDAIKEVFDNK |
| Δ133-192 | HKDEVIKEVQEFYKDTYNKL | |||
| Δ152-192 | HKDEVIKEVQEFYKDTYNKL | KTKDEPQRETLKAIHYALN | ||
| Δ173-192 | HKDEVIKEVQEFYKDTYNKL | KTKDEPQRETLKAIHYALN | CCGLAGGVEQFISDICPKKDV | |
CHO A6 contains the complete amino acid sequence of CD9 EC2. Clones Δ133-192, Δ152-192, and Δ173-192 have deleted sequences in EC2.
CD9 influence on CHO cell adhesion to FN and pericellular FN matrix assembly is reversed by CD9 EC2 deletion mutant Δ173-192, Δ152-192, and Δ133-192 expression.
(A) MOCK, A6, and CD9 deletion mutants Δ173-192, Δ152-192, and Δ133-192 CHO cells were allowed to adhere to FN, as described in “Materials and methods.” After stringent washing, adherent cells were counted in 5 high-power (× 40) fields of view/well from 3 wells per assay and reported as the number of adherent cells/mm2. Data are expressed as the means ± SEs of 3 independent assays. All deletion mutants had adhesive phenotypes comparable to the CHO MOCK cells. (B) MOCK, A6, and CD9 EC2 deletion mutants Δ173-192, Δ152-192, Δ133-192, and Δ113-228 CHO cells were grown to 100% confluency in the presence of bovine plasma FN. Immunofluorescent images revealed that partial (Δ173-192, Δ152-192, and Δ133-192) or complete (Δ113-228) EC2 deletions restored the pericellular FN matrix assembly as effectively to that observed with MOCK CHO cells. Original magnification × 25.
CD9 influence on CHO cell adhesion to FN and pericellular FN matrix assembly is reversed by CD9 EC2 deletion mutant Δ173-192, Δ152-192, and Δ133-192 expression.
(A) MOCK, A6, and CD9 deletion mutants Δ173-192, Δ152-192, and Δ133-192 CHO cells were allowed to adhere to FN, as described in “Materials and methods.” After stringent washing, adherent cells were counted in 5 high-power (× 40) fields of view/well from 3 wells per assay and reported as the number of adherent cells/mm2. Data are expressed as the means ± SEs of 3 independent assays. All deletion mutants had adhesive phenotypes comparable to the CHO MOCK cells. (B) MOCK, A6, and CD9 EC2 deletion mutants Δ173-192, Δ152-192, Δ133-192, and Δ113-228 CHO cells were grown to 100% confluency in the presence of bovine plasma FN. Immunofluorescent images revealed that partial (Δ173-192, Δ152-192, and Δ133-192) or complete (Δ113-228) EC2 deletions restored the pericellular FN matrix assembly as effectively to that observed with MOCK CHO cells. Original magnification × 25.
Next, we examined pericellular FN matrix production by the EC2 mutant clones. As seen in Figure 2B, immunofluorescent labeling of the pericellular FN matrix revealed that each of the mutant clones was capable of assembling an extracellular FN matrix comparable to that assembled by CHO MOCK cells. The levels of FN matrix produced by these mutant CHO cell lines suggests that the sequence173L-K192 involved in CD9 CHO cell adhesion may also regulate the extent of pericellular FN matrix assembly in CHO A6 cells.
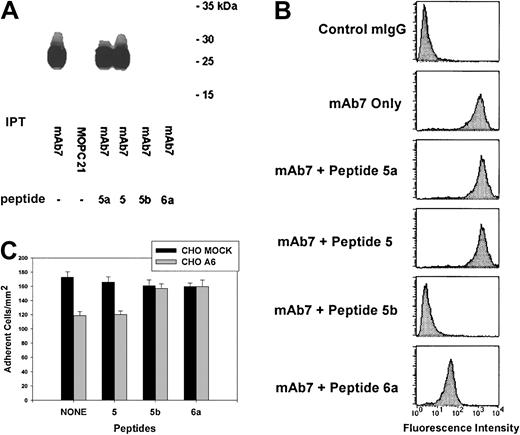
A key epitope for anti-CD9 EC2 mAb7 is within EC2135–185
The effect of peptides corresponding to regions of EC2 (Table 2) on mAb7-mediated immunoprecipitation of CD9 was investigated. As shown in Figure 3A, mAb7 alone immunoprecipitated CD9. While peptides 5a and 5 did not block CD9-mAb7 binding, peptides 5b and 6a inhibited the immunoprecipitation of CD9 by mAb7. The common sequence PKKDV of peptides 5b and 6a may be essential for mAb7/CD9 binding.
CD9 EC2 peptides 5b (135K-V172) and 6a (168P-I185) competitively block anti-CD9 mAb7 binding to soluble and cell surface CD9 as well as reverse the CD9 inhibitory influence on CHO cell adhesion to fibronectin.
(A) Platelet lysate was added to either anti-CD9 mAb7, control mouse IgG1, κ (MOPC 21), or mAb7 incubated with either peptides 5a (111Y-T134), 5 (125Y-I146), 5b (135K-V172), or 6a (168P-I185). The immunoprecipitates (IPTs) were fractionated by SDS-PAGE and transferred to PVDF membrane. CD9 was detected using mAb7. Peptides 5b and 6a block immunoprecipitation of CD9 by antibody mAb7 from human platelet lysate. (B) The peptides 5a (111Y-T134), 5 (125Y-I146), 5b (135K-V172), or 6a (168P-I185) were incubated for 30 minutes in the presence of anti-CD9 antibody mAb7. A species-specific IgG and antibody mAb7 alone were used as the negative and positive controls, respectively. Flow cytometry analysis revealed peptides 5b and 6a blocked mAb7 binding as indicated by the left shift in fluorescence intensity. (C) MOCK and A6 CHO cells were allowed to adhere to FN in the presence of peptides corresponding to segments of CD9 EC2 as described in “Materials and methods.” After stringent washing, adherent cells were counted in 5 high-power (× 40) fields of view/well from 3 wells per assay and reported as the number of adherent cells/mm2. Data are expressed as the means ± SEs of 3 independent assays. The presence of peptides 5b and 6a reversed the inhibitory influence of CD9 on A6 CHO cell adhesion to fibronectin.
CD9 EC2 peptides 5b (135K-V172) and 6a (168P-I185) competitively block anti-CD9 mAb7 binding to soluble and cell surface CD9 as well as reverse the CD9 inhibitory influence on CHO cell adhesion to fibronectin.
(A) Platelet lysate was added to either anti-CD9 mAb7, control mouse IgG1, κ (MOPC 21), or mAb7 incubated with either peptides 5a (111Y-T134), 5 (125Y-I146), 5b (135K-V172), or 6a (168P-I185). The immunoprecipitates (IPTs) were fractionated by SDS-PAGE and transferred to PVDF membrane. CD9 was detected using mAb7. Peptides 5b and 6a block immunoprecipitation of CD9 by antibody mAb7 from human platelet lysate. (B) The peptides 5a (111Y-T134), 5 (125Y-I146), 5b (135K-V172), or 6a (168P-I185) were incubated for 30 minutes in the presence of anti-CD9 antibody mAb7. A species-specific IgG and antibody mAb7 alone were used as the negative and positive controls, respectively. Flow cytometry analysis revealed peptides 5b and 6a blocked mAb7 binding as indicated by the left shift in fluorescence intensity. (C) MOCK and A6 CHO cells were allowed to adhere to FN in the presence of peptides corresponding to segments of CD9 EC2 as described in “Materials and methods.” After stringent washing, adherent cells were counted in 5 high-power (× 40) fields of view/well from 3 wells per assay and reported as the number of adherent cells/mm2. Data are expressed as the means ± SEs of 3 independent assays. The presence of peptides 5b and 6a reversed the inhibitory influence of CD9 on A6 CHO cell adhesion to fibronectin.
We also examined the ability of these EC2 peptides to block mAb7 binding to CD9 expressed on CHO A6 cells. As seen in Figure 3B, neither peptide 5a nor 5 blocked mAb7 binding to CD9. However, the reduction in mean fluorescence intensity from that of CHO A6 cells exposed to mAb7 alone indicated both peptides 5b and 6a blocked mAb7 binding to CD9.
Next, the ability of peptides 5b and 6a to alter the CD9 influence on CHO cell adhesion was tested. As shown in Figure 3C, the adhesive phenotype of CHO A6 cells exposed to peptides 5b and 6a was equivalent to that of CHO MOCK cells, while peptide 5 had no effect.
These results suggest that the mAb7 epitope is contained, at least in part, within the CD9 EC2 sequence corresponding to peptides 5b and 6a (amino acids 135-185), which partially overlap173L-K192, the sequence identified to have a role in modulating CD9 CHO cell adhesion events.
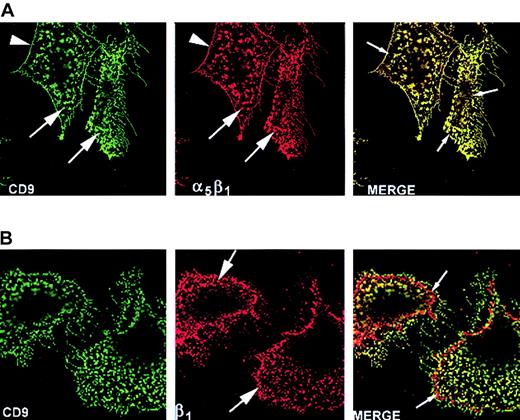
CD9 colocalizes with integrin α5β1 and actin cytoskeleton
The adhesive functions of CHO cells are dependent on interactions involving the cell and extracellular matrix as well as the cytoskeleton and integrins.32-34 The spatial relationship between CD9, α5β1, and the actin cytoskeleton was investigated using laser scanning confocal microscopy. We examined whether CD9 was colocalized with integrin α5β1 or F-actin of the CHO cell cytoskeleton. Having established the specificity of our labeling system (see “Materials and methods”), subconfluent CHO A6 cells were examined following mAb7 labeling of CD9 and PB1 labeling of α5β1. An optical image of the basal region of adherent cells (Figure 4A) revealed that CD9 (green) and α5β1 (red) were each located in punctate patches (large arrows) across the basal surface and along filipodia, yet appeared concentrated at the cell margin (arrowhead). A zone deficient of CD9 and α5β1 labeling (small arrows) was noted just inside the cell margin. Virtually all α5β1was colocalized with CD9 (yellow).
Laser scanning confocal microscopy analysis revealed CD9 colocalized with integrin α5β1, but not all integrin subunit β1, on the basal surface of subconfluent FN-adherent CHO A6 cells.
Cell cycle–synchronized CHO A6 cells were grown on FN-coated slides for 3 hours, as described in “Materials and methods.” (A) mAb7-labeled CD9 (green) and PB1-labeled α5β1 (red) were located in punctate clusters (large arrows), particularly at the cell margin (arrowheads) and along filipodia. Colocalization of CD9 and α5β1 (yellow) was nearly total. Note the CD9-integrin α5β1–deficient zone just inside the cell margin (small arrows). (B) mAb7-labeled CD9 (green) and 7E2-labeled integrin subunit β1(red) were found in punctate patches, particularly at the cell margin (large arrows) on the basal surface. Most integrin β1colocalized (yellow) with CD9. However, the zone just inside the cell margin previously described as CD9 and integrin subunit α5β1-free contained integrin subunit β1 not colocalized with CD9. Original magnification × 100.
Laser scanning confocal microscopy analysis revealed CD9 colocalized with integrin α5β1, but not all integrin subunit β1, on the basal surface of subconfluent FN-adherent CHO A6 cells.
Cell cycle–synchronized CHO A6 cells were grown on FN-coated slides for 3 hours, as described in “Materials and methods.” (A) mAb7-labeled CD9 (green) and PB1-labeled α5β1 (red) were located in punctate clusters (large arrows), particularly at the cell margin (arrowheads) and along filipodia. Colocalization of CD9 and α5β1 (yellow) was nearly total. Note the CD9-integrin α5β1–deficient zone just inside the cell margin (small arrows). (B) mAb7-labeled CD9 (green) and 7E2-labeled integrin subunit β1(red) were found in punctate patches, particularly at the cell margin (large arrows) on the basal surface. Most integrin β1colocalized (yellow) with CD9. However, the zone just inside the cell margin previously described as CD9 and integrin subunit α5β1-free contained integrin subunit β1 not colocalized with CD9. Original magnification × 100.
The spatial relationship between CD9 and integrin subunit β1 also was examined. As shown in Figure 4B, antibody 7E2 labeled integrin β1 (red) was located in punctate patches (large arrows) across the lower cell surface in a similar manner as CD9 (green) and α5β1. However, β1also occupied the α5β1-free zone previously described (small arrows). CD9 (green) was colocalized with β1 (yellow in merge) in most of the punctate patches across the lower cell surface, cell margin, and filipodia. However, CD9 was not colocalized with β1 in the α5β1-free zone.
IP experiments confirmed a direct association of CD9 with β1 in CHO cell lysates by first immunoprecipitating CD9 complexes with mAb7 (Figure 5A), dissociating the isolated complexes, and then reimmunoprecipitating β1 from the isolated proteins using the 7E2 antibody (Figure 5B). In addition, wild-type and mutant CD9 were immunoprecipitated with anti-EC1 RAP2, and then the dissociated isolated complexes immunoprecipitated with 7E2. As expected, β1 was found in association with CD9 in the wild-type CD9 lysate; however, β1 was not in complex with Δ133-192 CD9 (Figure 5C). These results suggest the CD9-α5β1 association through EC2 was important for CD9 influence on CHO cell adhesive function.
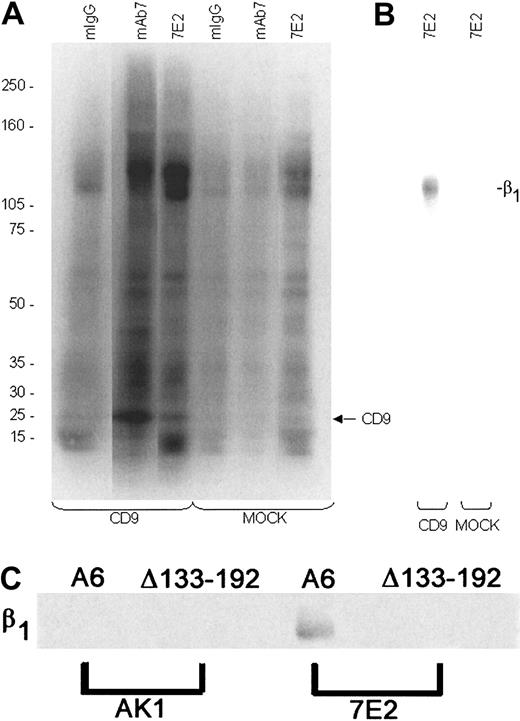
CD9 coimmunoprecipitates with integrin subunit β1 from CHO A6 but not CHO Δ133-192 cell lysates.
Biotinylated surface proteins from CHO cell lysates were immunoprecipitated with anti-CD9 mAb7 or RAP2, anti-β1 7E2, nonspecific binding mouse IgG, or anti-GPIb AK1, and the immune complexes captured by Protein A/Protein G agarose. (A) Western blots of the immunoprecipitates (IPTs) were probed with NeutrAvidin and developed using SuperSignal. A protein with the apparent molecular weight of CD9 was identified in the mAb7 and 7E2 IPTs from CHO CD9 (A6) cell lysates. (B) mAb7 IPTs from MOCK and CHO A6 cells were dissociated, reimmunoprecipitated using 7E2, and reprobed. A protein corresponding to the apparent molecular weight of β1 was identified from the CD9 mAb7 IPTs but not from the MOCK mAb7 IPTs. (C) RAP2 IPTs from CHO A6 and CHO Δ133-192 cells were dissociated, reimmunoprecipitated using 7E2, and reprobed. A protein corresponding to the apparent molecular weight of β1 was identified from the CHO A6 7E2 IPTs of RAP2 eluate but not from the CHO Δ133-192 7E2 IPTs or the AK1 IPTs from CHO A6 or Δ133-192 cells.
CD9 coimmunoprecipitates with integrin subunit β1 from CHO A6 but not CHO Δ133-192 cell lysates.
Biotinylated surface proteins from CHO cell lysates were immunoprecipitated with anti-CD9 mAb7 or RAP2, anti-β1 7E2, nonspecific binding mouse IgG, or anti-GPIb AK1, and the immune complexes captured by Protein A/Protein G agarose. (A) Western blots of the immunoprecipitates (IPTs) were probed with NeutrAvidin and developed using SuperSignal. A protein with the apparent molecular weight of CD9 was identified in the mAb7 and 7E2 IPTs from CHO CD9 (A6) cell lysates. (B) mAb7 IPTs from MOCK and CHO A6 cells were dissociated, reimmunoprecipitated using 7E2, and reprobed. A protein corresponding to the apparent molecular weight of β1 was identified from the CD9 mAb7 IPTs but not from the MOCK mAb7 IPTs. (C) RAP2 IPTs from CHO A6 and CHO Δ133-192 cells were dissociated, reimmunoprecipitated using 7E2, and reprobed. A protein corresponding to the apparent molecular weight of β1 was identified from the CHO A6 7E2 IPTs of RAP2 eluate but not from the CHO Δ133-192 7E2 IPTs or the AK1 IPTs from CHO A6 or Δ133-192 cells.
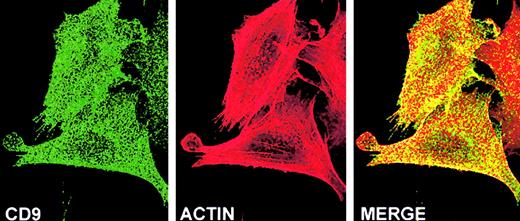
Finally, the CD9–F-actin spatial relationship was investigated (Figure6). F-actin (red) was diffusely located at the basal region of the adherent CHO cells and was also seen in stress fibers that extend into numerous filipodia. F-actin colocalized (yellow in merge) with CD9 (green) in the main body of the cell, particularly at the cell periphery. These results suggested that CD9 colocalization with α5β1 and/or the cytoskeleton might be partly responsible for the altered adhesive functions of CHO A6 cells.
CD9 and cytoskeletal F-actin are colocalized on the basal surface of subconfluent FN-adherent CHO A6 cells.
Cell cycle synchronized CHO A6 cells were grown on FN-coated slides for 3 hours, as described in “Materials and methods.” Laser scanning confocal microscopy analysis revealed that many of the punctate patches of mAb7-labeled CD9 (green) and phalloidin-TRITC labeled F-actin (red) were colocalized (yellow), particularly at the periphery of the cell body. Original magnification × 100.
CD9 and cytoskeletal F-actin are colocalized on the basal surface of subconfluent FN-adherent CHO A6 cells.
Cell cycle synchronized CHO A6 cells were grown on FN-coated slides for 3 hours, as described in “Materials and methods.” Laser scanning confocal microscopy analysis revealed that many of the punctate patches of mAb7-labeled CD9 (green) and phalloidin-TRITC labeled F-actin (red) were colocalized (yellow), particularly at the periphery of the cell body. Original magnification × 100.
CD9 expression alters adhesion complex composition
To further investigate the effect of CD9 expression on adhesive cell functions, we examined the localization of proteins typically incorporated into adhesion complexes. In the CHO cell, the integrin α5β1 is predominately responsible for cell-matrix and membrane-cytoskeleton interaction.35-37 As previously described,23 flow cytometric analysis of MOCK-, CD9-, and mutant CHO cells shows transfection of CD9 cDNA did not alter the surface expression of integrin α5β1. Laser scanning confocal microscopy images confirmed the equivalent staining (Figure 7) of α5β1 (green) on these clones. Equivalent amounts of F-actin (red) also appeared to be present in these cells. However, CD9 expression reduced α5β1colocalization with F-actin (yellow in merge). CHO Δ133-192 cells expressing a truncated EC2 had equivalent colocalization of α5β1/F-actin, as seen in MOCK CHO cells. These data suggest that CD9 EC2 down-regulates α5β1/F-actin interactions.
Truncation of CD9 EC2 reverses CD9-induced reduction of integrin α5β1 and cytoskeletal F-actin colocalization in fibronectin-adherent CHO cells.
CHO MOCK, A6, and Δ133-192 cells were grown on FN for 3 hours, followed by integrin α5β1 and cytoskeleton F-actin labeling as described in “Materials and methods.” Images of the basal surface of the cells by laser scanning confocal microscopy revealed that PB1-labeled α5β1 (green) and phalloidin-TRITC–labeled F-actin (red) were found in each cell type. Colocalization of α5β1 and F-actin (yellow) was reduced in CHO A6 cells compared to CHO MOCK and CHO Δ133-192 cells, suggesting that CD9 EC2 influences α5β1–F-actin colocalization. As described earlier,23 it is evident that CD9 CHO and CHO Δ133-192 cells have a polygonal spread morphology, whereas mock or naive CHO cells exhibit a bipolar fibroblast morphology. Original magnification × 60.
Truncation of CD9 EC2 reverses CD9-induced reduction of integrin α5β1 and cytoskeletal F-actin colocalization in fibronectin-adherent CHO cells.
CHO MOCK, A6, and Δ133-192 cells were grown on FN for 3 hours, followed by integrin α5β1 and cytoskeleton F-actin labeling as described in “Materials and methods.” Images of the basal surface of the cells by laser scanning confocal microscopy revealed that PB1-labeled α5β1 (green) and phalloidin-TRITC–labeled F-actin (red) were found in each cell type. Colocalization of α5β1 and F-actin (yellow) was reduced in CHO A6 cells compared to CHO MOCK and CHO Δ133-192 cells, suggesting that CD9 EC2 influences α5β1–F-actin colocalization. As described earlier,23 it is evident that CD9 CHO and CHO Δ133-192 cells have a polygonal spread morphology, whereas mock or naive CHO cells exhibit a bipolar fibroblast morphology. Original magnification × 60.
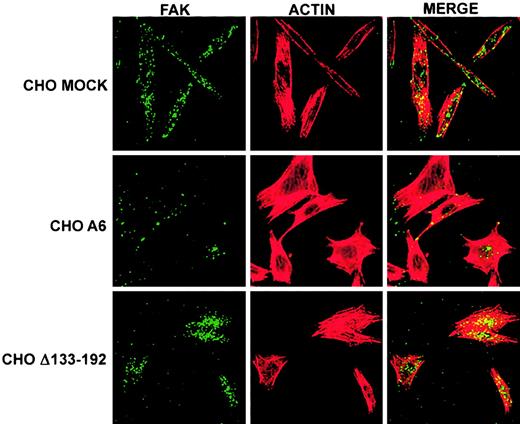
An important signaling molecule typically found in focal adhesion complexes is FAK.38 Immunolabeled FAK and F-actin of basal images of MOCK, A6, and Δ133-192 cells grown on FN showed that CHO A6 cells had significantly less FAK staining (Figure8, green) compared with CHO MOCK or CHOΔ133-192 cells. In addition, a reduction in FAK colocalization with F-actin was also observed in CHO A6 cells compared to CHO MOCK and CHO Δ133-192 cells.
Truncation of CD9 EC2 reverses CD9 influence on spatial distribution of FAK as well as reduction of FAK and cytoskeletal F-actin colocalization in FN-adherent CHO cells.
CHO MOCK, A6, and Δ133-192 cells were grown on FN for 3 hours, followed by FAK and cytoskeleton F-actin labeling as described in “Materials and methods.” Images of the basal surface of the adherent cells using laser scanning confocal microscopy revealed that the spatial distribution of FAK (green) in CD9 expressing CHO A6 cells appeared to be altered compared with that of CHO MOCK and CHO Δ133-192 cells, while the spatial distribution of F-actin (red) appeared to be equivalent in each cell type. CHO A6 cells also had less FAK and F-actin colocalization (yellow). The reversal of FAK distribution and F-actin colocalization in CHO Δ133-192 cells indicates that CD9 EC2 influences these phenomena. Original magnification × 40.
Truncation of CD9 EC2 reverses CD9 influence on spatial distribution of FAK as well as reduction of FAK and cytoskeletal F-actin colocalization in FN-adherent CHO cells.
CHO MOCK, A6, and Δ133-192 cells were grown on FN for 3 hours, followed by FAK and cytoskeleton F-actin labeling as described in “Materials and methods.” Images of the basal surface of the adherent cells using laser scanning confocal microscopy revealed that the spatial distribution of FAK (green) in CD9 expressing CHO A6 cells appeared to be altered compared with that of CHO MOCK and CHO Δ133-192 cells, while the spatial distribution of F-actin (red) appeared to be equivalent in each cell type. CHO A6 cells also had less FAK and F-actin colocalization (yellow). The reversal of FAK distribution and F-actin colocalization in CHO Δ133-192 cells indicates that CD9 EC2 influences these phenomena. Original magnification × 40.
Finally, we examined the effect of CD9 expression on the cytoskeletal associated protein α-actinin, a constituent of adhesion complexes important in cytoskeleton anchorage to integrin complexes and filament cross-linking. Immunolabeling in basal images revealed severely reduced staining of α-actinin (Figure 9, green) in CHO A6 cells and reduced F-actin colocalization (yellow). Conversely, CHO Δ133-192 cells expressed equivalent levels of α-actinin and actin colocalization seen in MOCK CHO cells. In summary, reduced amounts of FAK and α-actinin staining, reduced colocalization of these proteins with F-actin, and reduced colocalization of integrin α5β1 with cytoskeletal F-actin suggested that the pronounced effect of CD9 on CHO cell adhesion phenotypes was due in part to modulation of cytoskeletal-integrin complexes by CD9 through EC2.
Truncation of CD9 EC2 reverses CD9 influence on level of α-actinin expression and cytoskeletal F-actin colocalization in FN-adherent CHO cells.
CHO MOCK, A6, and Δ133-228 cells were grown on FN for 3 hours, followed by α-actinin and cytoskeleton F-actin labeling as described in “Materials and methods.” Images of the basal surface of the adherent cells using laser scanning confocal microscopy revealed less labeled α-actinin (green) in CHO A6 cells than in MOCK or Δ133-192 cells. However, the level of labeled F-actin (red) was equivalent among the cell types. The amount of α-actinin colocalized with F-actin was reduced in CD9 expressing CHO A6 cells compared with CHO MOCK cells. CHO Δ133-192 cells had equivalent α-actinin staining and colocalization with F-actin as seen in CHO MOCK cells. Original magnification × 40.
Truncation of CD9 EC2 reverses CD9 influence on level of α-actinin expression and cytoskeletal F-actin colocalization in FN-adherent CHO cells.
CHO MOCK, A6, and Δ133-228 cells were grown on FN for 3 hours, followed by α-actinin and cytoskeleton F-actin labeling as described in “Materials and methods.” Images of the basal surface of the adherent cells using laser scanning confocal microscopy revealed less labeled α-actinin (green) in CHO A6 cells than in MOCK or Δ133-192 cells. However, the level of labeled F-actin (red) was equivalent among the cell types. The amount of α-actinin colocalized with F-actin was reduced in CD9 expressing CHO A6 cells compared with CHO MOCK cells. CHO Δ133-192 cells had equivalent α-actinin staining and colocalization with F-actin as seen in CHO MOCK cells. Original magnification × 40.
Discussion
In this study, we demonstrate that the second extracellular loop (EC2) of CD9 modulates cell adhesion and pericellular FN matrix assembly of transfected CHO cells. For the first time, a functional epitope on CD9 is described that reverses CD9 modulation of both adhesion and FN matrix assembly. Our investigations also suggest that CD9 associates with the integrin α5β1 in punctate clusters on the cell surface, particularly at the cell margins. In addition, CD9 can colocalize with cytoskeletal F-actin. This colocalization appears to modulate the composition of adhesive complexes as evidenced by the cellular distribution of FAK and α-actinin and the level of colocalization of these 2 proteins with cytoskeletal actin. Thus, our results show that portions of the second extracellular loop of CD9 directly or indirectly regulate integrin-matrix activity as well as proteins involved in cellular signaling.
In previous studies, we found that anti-CD9 mAb7 binding or the expression of a CD9 mutant Δ113-228 reversed the adhesive phenotype of CD9-CHO cells on FN.23 The potential importance of this CD9 region has also been shown by others using CD9/CD81 chimeras.27-29 Our studies extended these findings by identifying a region within this CD9 segment that was functionally important. In our investigation, EC2 mutants Δ173-192, Δ152-192, and Δ133-192 had an adhesive and FN matrix assembly phenotype comparable to that of CHO MOCK cells. These results suggest that the 20 amino acid sequence 173L-K192 could be all or partly responsible for the influence of CD9 on CHO cell FN adhesion and pericellular FN matrix assembly.
We have reported a novel antibody-binding region on CD9 that is identified by mAb7. This is the first identification of an epitope region that has functional activity. This region was identified using peptides composed of the amino acid sequences135K-V172 and 168P-I185(peptides 5b and 6, respectively). These 2 peptides individually blocked mAb7 binding to soluble CD9 and to CD9 on intact cells as well as reversed the adhesive phenotype of CD9-CHO cells. These data infer that the common amino acid sequence PKKDV may be an essential part of the mAb7 epitope. Since mAb7 is a conformation-sensitive antibody and binds to CD9 only under nonreduced conditions (data not shown), our results suggest that the solubilized CD9 and peptides 5b and 6a assumed an epitope-competent conformation similar to that of CD9 expressed on the intact cell surface. Interestingly, peptide 6a contains part of the 20 amino acid sequence 173L-K192 that is critical for CHO cell adhesion and pericellular fibronectin matrix assembly.
The association of tetraspanins with other cell surface proteins, particularly integrins, has been described for various cell lines. Digital images acquired by laser scanning confocal microscopy of CD9-CHO cells revealed CD9 colocalized with integrin α5β1 in punctate clusters. However, a significant amount of the β1 subunit near the cell periphery was not colocalized with CD9, and this integrin remains to be identified. We previously demonstrated that solubilized CD9 and CD9 expressing CHO cells bind to FN.23 Immunoprecipitation of cell lysates using anti-CD9 mAb7 followed by reimmunoprecipitation using anti-β1 7E2 confirmed that wild-type but not EC2 mutant CD9 was associated with integrin β1. These results and the colocalization results together strongly suggest CD9-α5β1 association through EC2 was important for the adhesive phenotype of CD9-transfected CHO cells.
Other studies confirm the importance of the EC2 region in regulating CD9-mediated events. Sakuma et al39 proposed that aa119-138 contain the CD9 binding site for HB-EGF and may play an essential role in the up-regulation of juxtacrine activity of proHB-EGF. Shaw et al,25 through functional readouts and amino acid sequence comparison of human and feline CD9, proposed that aa169-180 have a regulatory role in cell motility on extracellular matrix proteins. Furthermore, studies by our own laboratory40 demonstrated that a CD9 region within aa168-185 contains an FN-binding site. Similarly, Higginbottom et al41 identified the TM4SF CD81 EC2 sequence aa179-193 as the minimal epitope for binding the E2 envelope glycoprotein of the hepatitis C virus. Finally, studies by Rubinstein et al42suggested that the EC2/TM4 region is important in CD9 association with the mature β1 integrin. These latter findings are supported by Yauch et al,43 who showed that the CD151 COOH-terminal portion of EC2 (aa182-217) is involved in extracellular contact between integrin α3β1 and CD151. Using these data and the recent report of Kitadokoro et al,44 we propose a working model for CD9 that maps proposed functional regions (Figure10). Interestingly, the amino acid region identified by peptide inhibition assays, mAb7 epitope mapping, and the CD9 deletion mutants corresponds to a loop region that may be projected from the EC2 backbone. Kitadokoro et al44proposed in recent structural studies a CD81 EC2 head subdomain that is stabilized by the 2 TM4SF invariant juxtaposed cysteines where, through disulfide bridging, the EC2 region extends in opposite directions. Based on these data, it is probable that Cys152 forms a disulfide bond with Cys183 and Cys153 with Cys167, forming opposing loops as shown in the proposed model. This working model provides accessible head subdomains exposing regions that have been identified as having critical roles in receptor function, cell adhesion events, and the mAb7 monoclonal antibody epitope.
Schematic model of CD9 and proposed functional domains.
Amino acid sequence determination and antibody-binding studies suggest that CD9 consists of 2 EC loops, EC1 and EC2, and 4 TM domains (TM1-TM4), with the N and C termini located intracellularly.1 EC2 aa119-138 has been identified as the binding site for HB-EGF39; aa168-185 as a key region for CD9-FN binding; aa144-185 competes for mAb7 binding to intact CD9; aa169-180 has been identified to play a role in regulating cell motility25; and aa 173-192 affects CHO cell adhesion and pericellular FN matrix assembly and encompasses a corresponding region on CD151 that has been reported to facilitate TM4SF-integrin association (aa182-217).42 Preliminary data suggest that EC135–58 in conjunction with EC2113–133modulates cell spreading. EC1 appears critical for CD9 effects on cell proliferation and cell survival (Bao et al, manuscript submitted).
Schematic model of CD9 and proposed functional domains.
Amino acid sequence determination and antibody-binding studies suggest that CD9 consists of 2 EC loops, EC1 and EC2, and 4 TM domains (TM1-TM4), with the N and C termini located intracellularly.1 EC2 aa119-138 has been identified as the binding site for HB-EGF39; aa168-185 as a key region for CD9-FN binding; aa144-185 competes for mAb7 binding to intact CD9; aa169-180 has been identified to play a role in regulating cell motility25; and aa 173-192 affects CHO cell adhesion and pericellular FN matrix assembly and encompasses a corresponding region on CD151 that has been reported to facilitate TM4SF-integrin association (aa182-217).42 Preliminary data suggest that EC135–58 in conjunction with EC2113–133modulates cell spreading. EC1 appears critical for CD9 effects on cell proliferation and cell survival (Bao et al, manuscript submitted).
Evidence of the potential role of CD9 on cell signaling was also provided by the apparent differences in the localization of components typically incorporated into adhesion complexes, such as FAK. Our results suggest that the distribution of FAK and its colocalization with cytoskeletal actin are altered by the expression of CD9 in CHO cells. The partial restoration of the spatial distribution and colocalization with actin upon the expression of the CD9 Δ133-192 may be directly related to the affected EC2. Therefore, we surmise that the differences in adhesive functions of transfected CHO cells are linked to differences in adhesive complex composition resulting in altered cell signaling and cell-substrate interaction. This hypothesis is bolstered also by the unique distribution and degree of cytoskeletal colocalization of α-actinin, a protein important in integrin complex attachment to the cytoskeleton.
Supported by the Vascular Biology Center of Excellence, the National Heart, Lung and Blood Institute of the National Institutes of Health (HL53514); and the American Heart Association southeastern affiliate.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Lisa K. Jennings, Vascular Biology Center of Excellence, The University of Tennessee Health Science Center, 956 Court Ave, Coleman Bldg, H300, Memphis, TN 38163; e-mail:ljennings@utmem.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal