Activin receptor–like kinase 1 (ALK-1) is an orphan type I receptor of the transforming growth factor beta (TGF-β) receptor family. In vivo studies have demonstrated that this endothelial-specific receptor is implicated in angiogenesis. In this study, we addressed the cellular function of ALK-1 in cultured human microvascular endothelial cells from the dermis (HMVEC-d's) using adenoviral expression of a constitutively active form of ALK-1 (ALK-1QD). We observed that ALK-1QD expression inhibits cell proliferation through an arrest in the G1 phase in the cell cycle. ALK-1QD expression also inhibited migration. This inhibition was also observed in other endothelial cells (human microvascular endothelial cells [HMEC-1's], HMVECs from the lung, and human umbilical vein endothelial cells [HUVECs]). Finally, ALK-1QD expression decreased readhesion and spreading to different matrices. This led us to examine the dynamic formation of adhesion complexes. We demonstrated that while β-gal–infected cells reorganized actin stress fibers and focal adhesion complexes at the edge of a wound, ALK-1QD–infected cells did not. To identify downstream genes implicated in ALK-1 cellular responses, we next performed a cDNA array analysis of the expressed genes. There were 13 genes found to be significantly induced or suppressed by ALK-1QD. Among them, 2 genes encoded cell cycle–related proteins (c-myc and p21/waf1), 3 encoded components of the cytoskeleton-focal adhesion complex (β-actin, paxillin, and zyxin), and 2 encoded members of the TGF-β family (BMPRII and GDF-15). Taken together, our results suggest that ALK-1 is implicated in the maturation phase of angiogenesis. Disruption of this latter phase of angiogenesis may be an important step in the development of hereditary hemorrhagic telangiectasia.
Introduction
Angiogenesis, the formation of new blood capillaries from a preexisting capillary network, is a multistep process that can be roughly divided into 2 phases. In the activation phase, endothelial cells degrade the perivascular basement membrane, migrate into the extracellular space, proliferate, and form capillary sprouts and tubular structures. In the maturation phase, endothelial cells cease migration and proliferation, reconstitute a basement membrane, and recruit smooth muscle cells permitting the maintenance of vessel wall integrity. A balance of proangiogenic and antiangiogenic factors very tightly regulates angiogenesis. Important angiogenic factors include basic fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and transforming growth factor beta 1 (TGF-β1).
Activin receptor–like kinase 1 (ALK-1) is an orphan type I receptor of the TGF-β receptor family. In contrast to other receptors of the same family, ALK-1 expression is restricted to a few cell types. It is expressed in endothelial cells of highly vascularized tissues (lung and placenta) and at lower levels in other tissues and vascular smooth muscle cells.1 This receptor has been implicated in angiogenesis by in vivo studies: (1) mutations in ALK-1 are implicated in hereditary hemorrhagic telangiectasia (HHT),2 an autosomal dominant vascular dysplasia, associated with epistaxis and telangiectases and in more severe cases with pulmonary and cerebral arteriovenous shunts3; and (2) mouse embryos lacking ALK-1 die by midgestation with profound defects in vascularization, with fusion of capillary plexi into cavernous vessels, hyperdilation of large vessels, and large shunts between arteries and veins,4,5 and the zebrafish mutant ALK-1 has an abnormal circulation pattern.6 Together, these studies clearly demonstrate that ALK-1 is required for angiogenesis. However, no cellular function related to angiogenesis has been ascribed to ALK-1 to date.
The physiologic ligand for ALK-1 is not known and the type II receptor(s) it interacts with is not clearly identified. In experiments where type II TGF-β receptor (TβRII) or type II activin receptors (ActRII and ActRIIB) are overexpressed, ALK-1 can bind TGF-β1 or activin respectively.7-10 However, these complexes do not signal, as determined by growth responses, alterations in fibronectin expression or the ability to activate a plasminogen activator inhibitor 1 gene promoter construct.7-9 Scatchard analysis of equilibrium binding data showed that the affinity of TβRII for125I-TGF-β1 was relatively low (dissociation constant [Kd] = 800 pM) when ALK-1 was coexpressed in COS1 cells whereas it was high (Kd = 10 pM) when ALK-5 was coexpressed.7 In nontransfected cells (mink lung epithelial cells and porcine aortic endothelial cells), ALK-1 does not bind TGF-β1.9 In human umbilical vascular endothelial cells (HUVECs), contradictory results have been reported: One study demonstrated that antisera to ALK-1 could immunoprecipitate endogenous ALK-1/TβRII crosslinked complexes4 while in another study these antibodies failed to immunoprecipitate TGF-β1 or TGF-β3 receptor complexes.11 Finally, it was recently described that a ligand for ALK-1, distinct from TGF-β1 and -β3, might be present in the serum.12 Although the endogenous ligand for ALK-1 is not known, the intracellular signal transduction pathway indicates that ALK-1 transduces BMP-like signals. Using a constitutively active form of ALK-1, it was demonstrated that ALK-1 phosphorylates Smad1 and activates luciferase expression driven by Xvent2 or TLx2 promoters which contain a BMP-responsive region.13 14
The aim of our study was to determine the biologic functions and molecular targets of ALK-1 in capillary endothelial cells. Since the natural ligand for ALK-1 has not been conclusively identified, we used a constitutively active form of ALK-1 (ALK-1QD), containing a Gln to Asp mutation in the penultimate residue of the regulatory domain (GS domain), which results in constitutive ligand-independent receptor activation. This study was performed in human microvascular endothelial cells from the dermis (HMVEC-d's) since in HHT, numerous telangiectases can be observed in the skin. We observed that ALK-1QD inhibited proliferation of HMVEC-d's by stopping the cells in the G1 phase of the cell cycle. ALK-1QD expression also inhibited migration, and decreased readhesion and spreading of HMVEC-d's to different matrices. This led us to examine the dynamic formation of adhesion complexes. We observed that ALK-1QD–expressing cells were not able to reorganize these focal complexes at the edge of a wound. Finally, a microarray analysis performed 15 hours after infection revealed 13 genes that were either positively or negatively regulated by ALK-1QD. Among the genes, 2 were related to the cell cycle (p21/waf1 and c-myc), 3 to focal adhesion plaque and the cytoskeleton (β-actin, paxillin, and zyxin) and 2 belonged to the TGF-β/TGF-β receptor family (BMPRII and GDF-15).
Materials and methods
Cell culture, recombinant adenoviruses, and infection
HMVEC-d's and human lung microvascular endothelial cells (HMVEC-l's; Clonetics, Walkersville, MD) were maintained in endothelial growth medium (EGM-2-MV; Clonetics) supplemented with 5% fetal bovine serum (FBS; Clonetics). HMVEC-d's express both ALK-1 and ALK-5, as detected by reverse transcriptase–polymerase chain reaction (RT-PCR). Human microvascular endothelial cells (HMEC-1's; generous gift from Dr F. Candal, Centers for Disease Control, Atlanta, GA) were maintained in MCDB 131 medium (Life Technologies, Cergy Pontoise, France) supplemented with 15% FBS, 10 ng/mL EGF (Peprotech, Rocky Hill, NJ) and 1 μg/mL hydrocortisone (Sigma, St Quentin Fallavier, France). HUVECs (generous gift from Dr D. Gulino, Institute of Structural Biology, Grenoble, France), isolated according to the method of Jaffé and colleagues,15 were maintained in EGM-2-MV (Clonetics) supplemented with 10% FBS. Recombinant adenoviral vectors expressing the constitutively active form of ALK-1 (AdALK-1QD, mutation of glutamine 201 in aspartic acid) or ALK-5 (AdALK-5TD, mutation of threonine 204 in aspartic acid) tagged with an influenza virus hemagglutinin (HA) epitope (generated by Dr M. Fujii and kindly given to us by Dr K. Miyazono, University of Tokyo, Japan)16 were used for infection. A recombinant adenovirus expressing bacterial β-galactosidase (Adβ-gal) was used as control. Endothelial cells were infected for 15 hours in complete medium with Adβ-gal or AdALK-1QD at a multiplicity of infection (MOI) of 1, 5, 10, or 50 as indicated.
Western blot analysis
HMVEC-d's were lysed in RIPA buffer. Proteins in the lysates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by immunoblotting using a rat monoclonal antibody against the HA epitope (clone 3F10; Roche, Meylan, France) and anti–phosphoSmad1/5 and anti–phosphoSmad2/3 antibodies given to us by Dr P. ten Dijke (The Netherlands Cancer Institute, Amsterdam).
Cell number
At the indicated time of culture, the number of viable cells was determined using either a particle counter (Z1; Beckman Coulter, Roissy, France) or the WST-1 colorimetric assay (Roche).
Cell cycle analysis
At 15 hours after infection, the cells were rinsed and incubated for another 9 hours. Cells were then trypsinized and resuspended in phosphate buffered saline (PBS) containing 1 g/L glucose, 4% paraformaldehyde, 1% Triton X-100, and 2 μg/mL Hoechst no. 33258 (Sigma). We collected 20 000 events on a flow cytometer (FACScan; Becton Dickinson, Pont de Claix, France) and the percentage of cells in the G1, S, and G2/M phases of the cell cycle was determined.
Apoptosis assay
At 15, 24, and 48 hours after infection, the cells were trypsinized, fixed for 1 hour with 2% paraformaldehyde, and permeabilized in 0.1% triton X-100, 0.1% sodium citrate for 2 minutes on ice. Apoptotic cells were measured with TdT-mediated dUTP nick end labeling (TUNEL) in presence of fluorescein dUTP (Roche). The number of fluorescent apoptotic cells was determined by flow cytometry (FACScalibur; Becton Dickinson).
Cell migration in the wound assay
At 15 hours after infection, confluent HMVEC-d, HMEC-1, HMVEC-l, and HUVEC monolayers were wounded with a plastic pipet tip, placed back at 37°C in a CO2 incubator, and photographed at regular intervals (0, 24, 32, and 48 hours). Closure of the wound was followed by time-lapse microscopy with pictures taken every 30 minutes for 48 hours. Video images were collected with MetaVue software (Universal Imaging Corporation, Downingtown, PA) and analyzed using Track Image Bio processing software (Orme, Toulouse, France).
Cell migration in the transwell assay
Confluent monolayers of HMVEC-d's were infected with Adβ-gal or AdALK-1QD (MOI = 10) and labeled with the fluorophore DiI (5 μg/mL, Molecular Probes) for 15 hours. Cells were then trypsinized and suspended at a final concentration of 5 × 105/mL in EGM-2-MV with 0.5% FBS. A 100 μL aliquot of the cell suspension was added to transwell inserts (8 μm; Falcon HTSF Fluoroblok inserts; Becton Dickinson). The inserts were held in 24-well companion plates (Becton Dickinson) containing 500 μL EGM-2-MV with either 0.5% or 5% FBS and incubated for 24 hours at 37°C. Migration of cells through the optically opaque insert membrane to the lower culture plate was assessed by measurement of DiI fluorescence on a fluorimeter (BMG LabTechnologies, Offenburg, Germany).
Adhesion measurement
At 15 hours after infection, the cells were trypsinized and seeded onto uncoated (culture-treated plastic; Falcon, Becton Dickinson) or coated (Greiner Bio-One, Frickenhausen, Germany) multiwells. Coating was performed with either 0.2 mg/mL fibronectin (Sigma), 1 mg/mL gelatin (Sigma), 0.1 mg/mL collagen I (Becton Dickinson), or 0.1 mg/mL collagen IV (Sigma). Cells were photographed 3 hours after readhesion. The number of attached cells was determined after trypsinization with a particle counter (Z1; Beckman Coulter).
Cell spreading measurement
At 15 hours after infection, the cells were trypsinized and seeded onto fibronectin-coated glass slides (10 μg/mL) for 30 minutes. Cells were then fixed and stained with Coomassie blue. The numbers of fully spread (> 1000 μm2), partially spread (between 100 and 1000 μm2), and round (< 100 μm2) cells were determined. Cell area was determined using National Institutes of Health Image software.
Immunofluorescence
For immunofluorescence staining, cells were cultured on vitronectin-coated (10 μg/mL vitronectin for 2 hours at 37°C) Lab-Tek 4-well chamber slides (NUNC; Polylabo, Strasbourg, France) and infected with adenovirus for 15 hours. Then, the monolayer was wounded as described above or left unwounded. The monolayers were fixed and permeabilized (4% paraformaldehyde, 0.2% Triton X-100) 3 hours after wounding. Cells were stained with mouse monoclonal antibody against paxillin (Upstate Biotechnology, Lake Placid, NY) or with phalloidin–fluorescein isothiocyanate (FITC; Sigma) to visualize actin microfilaments, and counterstained with Hoechst no. 33258 (Sigma).
Differential hybridization of Atlas human cDNA expression arrays
Total RNA from HMVEC-d's infected for 15 hours with Adβ-gal, AdALK-1QD, or AdALK-5TD were isolated (Rneasy; Qiagen, Courtaboeuf, France) and treated with Dnase I. 33P-radiolabeled cDNA synthesis was carried out by RT-PCR as described in the Atlas cDNA expression arrays user manual (Clontech, Palo Alto, CA). Microarray analysis was performed in 2 independent experiments. Radiolabeled cDNAs were hybridized to nylon membranes carrying 1176 cancer-related cDNAs (Atlas Human Cancer 1.2 Arrays; Clontech, 7851-1). Hybridization signals were quantitated with a β-imager (FujifilmBAS-5000, Fuji) using Image Reader and Image Gauge softwares (Fuji). A 1.5-fold change in gene expression was arbitrarily deemed biologically significant.
Quantitative reverse transcriptase–real time PCR (RT-rtPCR)
Quantitative RT-rtPCR analysis of ALK-1QD–controlled gene expression of β-actin, paxillin, zyxin, c-myc, MIC-1/GDF15, and p21/waf1 was performed using the LightCycler apparatus (Roche) with sequence-specific primer pairs. The FastStart DNA Master SYBR Green I system (Roche) was used for real-time monitoring of amplification. Quantitation was performed using the LightCycler software (Roche).
Statistics
Statistical analysis was performed using Studentt test. (*P < .05; **P < .01.)
Results
Infection of HMVEC-d's
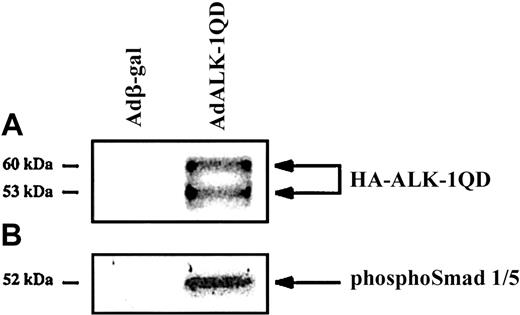
HMVEC-d's were infected for 15 hours with either AdALK-1QD or Adβ-gal, as a control, at an MOI of 10. Under these conditions, more than 95% of Adβ-gal–infected HMVEC-d's were β-gal–positive. Expression of HA-tagged ALK-1QD was detected by Western blotting with an anti-HA antibody (Figure 1A). This antibody detected 2 specific bands, which probably represent differentially N-glycosylated forms of ALK-1, as previously noted.17 Constitutive ALK-1 activity was verified using a specific anti–phosphoSmad1/5 antibody (Figure 1B). ALK-1QD appeared to specifically induce the phosphorylation of Smad1/5 since no signal was detected with an anti–phosphoSmad2/3 antibody (data not shown).
Expression and constitutive activity of ALK-1QD in HMVEC-d's.
HMVEC-d's were infected for 15 hours with AdALK-1QD or Adβ-gal at an MOI = 10. Cell lysates (20 μg) were immunoblotted with antibodies against HA (A) and phosphoSmad1/5 (B).
Expression and constitutive activity of ALK-1QD in HMVEC-d's.
HMVEC-d's were infected for 15 hours with AdALK-1QD or Adβ-gal at an MOI = 10. Cell lysates (20 μg) were immunoblotted with antibodies against HA (A) and phosphoSmad1/5 (B).
ALK-1QD inhibits HMVEC-d proliferation
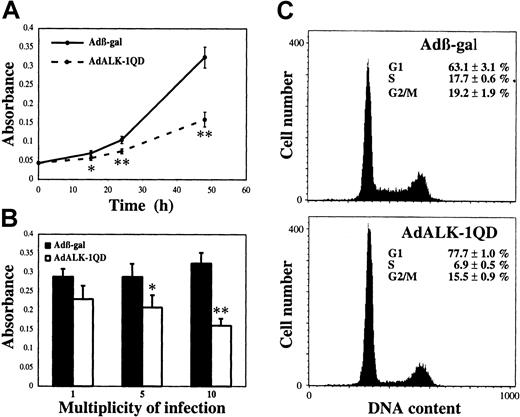
To determine the potential role of ALK-1 in proliferation, we measured the number of viable cells after AdALK-1QD infection in comparison to Adβ-gal infection. Figure2A shows that ALK-1QD expression significantly decreased the number of viable HMVEC-d's as soon as 15 hours after infection. This effect was dependent on the concentration of adenovirus, being significative at an MOI of 5 (Figure 2B). This decrease may have resulted from an inhibition of either cell proliferation or cell survival. We therefore measured the number of apoptotic cells following infection with the TUNEL method. We found that ALK-1QD expression did not induce apoptosis of HMVEC-d's at the times studied (15 hours: 0.08% ± 0.01% versus 0.06% ± 0.01%; 24 hours: 1.72% ± 0.04% versus 0.71% ± 0.435%; 48 hours: 0.74% ± 0.08% versus 0.65% ± 0.22%, Adβ-gal and AdALK-1QD, respectively). We then analyzed the effect of ALK-1QD expression on the distribution of cells in the cell cycle by flow cytometry. AdALK-1QD treatment led to an accumulation of cells in the G1 phase (77.7% versus 63.1%, AdALK-1QD and Adβ-gal) and a decrease in S phase (6.9% versus 17.7%, AdALK-1QD and Adβ-gal; Figure2C). Again, no apoptosis could be detected as visualized by levels of DNA lower than that seen in G1 phase.
ALK-1QD inhibits HMVEC-d proliferation.
HMVEC-d's were infected for 15 hours with AdALK-1QD or Adβ-gal. (A) The quantity of viable cells was determined 15, 24, and 48 hours after infection using the WST-1 assay. (B) MOI-dependent effect (1, 5, 10) of AdALK-1QD versus Adβ-gal on viable cell number measured 48 hours after infection. (C) G1 arrest following AdALK-1QD infection. At 24 hours after infection, cells were labeled with Hoechst and analyzed for cell cycle distribution by flow cytometry. **P < .01 and *P < .05.
ALK-1QD inhibits HMVEC-d proliferation.
HMVEC-d's were infected for 15 hours with AdALK-1QD or Adβ-gal. (A) The quantity of viable cells was determined 15, 24, and 48 hours after infection using the WST-1 assay. (B) MOI-dependent effect (1, 5, 10) of AdALK-1QD versus Adβ-gal on viable cell number measured 48 hours after infection. (C) G1 arrest following AdALK-1QD infection. At 24 hours after infection, cells were labeled with Hoechst and analyzed for cell cycle distribution by flow cytometry. **P < .01 and *P < .05.
ALK-1QD decreases HMVEC-d migration
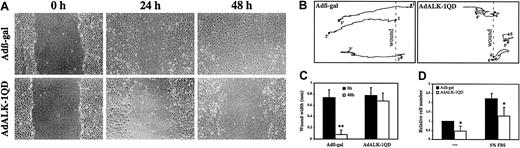
Endothelial cell migration is another important step of angiogenesis. Therefore, we examined the effect of ALK-1QD expression versus β-gal expression on migration of endothelial cells using the monolayer wound assay. At 15 hours after infection, the monolayers were wounded with a pipet tip and the closing of the wound was photographed at different time intervals and analyzed by time-lapse microscopy. Videos depicting this may be found on the Blood website; see the Supplemental Videos link at the top of the online article. In Adβ-gal–infected cells, the wound was half-closed after 24 hours and completely closed by 48 hours (Figure3A,C; β-gal video). In contrast, in ALK-1QD–expressing cells, the wound was still open after 48 hours and there was a decrease in cell density (Figure 3A,C; ALK-1 video). Cellular movements were recorded and measured using time-lapse video microscopy. Comparison of the paths of migration of ALK-1QD–expressing cells and β-gal–infected cells showed that while β-gal–infected cells have a relatively direct trajectory toward the center of the wound, ALK-1QD–expressing cells move in an unorientated manner and many of them detach shortly after wounding. We also measured ALK-1QD effect on migration using transwell chambers and we found a similar effect. ALK-1QD expression resulted in a 2-fold decrease in motility (no chemoattractant) and in migration (5% FBS in the lower chamber; Figure 3D).
ALK-1QD inhibits HMVEC-d migration in a wound assay.
(A) At 15 hours after infection, the cell monolayer was scratched to create a wound. At time 0, 24, and 48 hours after wounding, the cells were observed by phase contrast microscopy and photographed. Original magnification, × 50. (B) Comparison of the path of migration of AdALK-1QD and Adβ-gal HMVEC-d infected cells at their initial positions (1-6, time 0, wounding) and at their final positions (1′-6′, 48 hours after wounding). (C) The mean wound width was measured at time 0 (wounding) and 48 hours after wounding (n = 10). D: Infected and labeled cells were placed in transwell filter chambers in 0.5% FBS. The inserts were then placed in 24-well plate in 0.5% or 5% FBS. Fluorescence measurements to assess migration of DiI-labeled cells through the transwell membrane were taken. The mean fluorescence of Adβ-gal–infected cells in 0.5% FBS was set at 1 (mean of 3 experiments performed in triplicates). **P < .01 and *P < .05.
ALK-1QD inhibits HMVEC-d migration in a wound assay.
(A) At 15 hours after infection, the cell monolayer was scratched to create a wound. At time 0, 24, and 48 hours after wounding, the cells were observed by phase contrast microscopy and photographed. Original magnification, × 50. (B) Comparison of the path of migration of AdALK-1QD and Adβ-gal HMVEC-d infected cells at their initial positions (1-6, time 0, wounding) and at their final positions (1′-6′, 48 hours after wounding). (C) The mean wound width was measured at time 0 (wounding) and 48 hours after wounding (n = 10). D: Infected and labeled cells were placed in transwell filter chambers in 0.5% FBS. The inserts were then placed in 24-well plate in 0.5% or 5% FBS. Fluorescence measurements to assess migration of DiI-labeled cells through the transwell membrane were taken. The mean fluorescence of Adβ-gal–infected cells in 0.5% FBS was set at 1 (mean of 3 experiments performed in triplicates). **P < .01 and *P < .05.
ALK-1QD inhibits migration of HMEC-1's, HMVEC-l's, and HUVECs
We next wanted to know if ALK-1QD inhibition of migration could also be observed in other endothelial cell types. We therefore performed a similar experiment with HMEC-1's, HMVEC-l's, and HUVECs. Expression and constitutive activity of ALK-1QD were verified by Western blot analysis in these 3 different cell types (data not shown). These different endothelial cells were infected for 15 hours with either AdALK-1QD or Adβ-gal (MOI = 50) and then a wound was created. We observed that ALK-1QD expression inhibited the closing of the wound of these 3 different endothelial cell types (Figure4).
ALK-1QD inhibits HMEC-1, HMVEC-l, and HUVEC migration in a wound assay.
These endothelial cells were infected for 15 hours with AdALK-1QD or Adβ-gal (MOI = 50). At 15 hours after infection, the cell monolayer was scratched to create a wound. The cells were observed by phase contrast microscopy and photographed at time 0 (wounding) and when the wound was closed in Adβ-gal–infected cells (24 hours for HMEC-1's, 48 hours for HMVEC-l's, and 32 hours for HUVECs). Original magnifications, × 50.
ALK-1QD inhibits HMEC-1, HMVEC-l, and HUVEC migration in a wound assay.
These endothelial cells were infected for 15 hours with AdALK-1QD or Adβ-gal (MOI = 50). At 15 hours after infection, the cell monolayer was scratched to create a wound. The cells were observed by phase contrast microscopy and photographed at time 0 (wounding) and when the wound was closed in Adβ-gal–infected cells (24 hours for HMEC-1's, 48 hours for HMVEC-l's, and 32 hours for HUVECs). Original magnifications, × 50.
ALK-1QD decreases HMVEC-d readhesion and spreading
Migration involves discrete phases of adhesion and disadhesion. Therefore, we also determined whether ALK-1QD induced changes in this aspect of cellular behavior. To this end, HMVEC-d's were infected with either AdALK-1QD or Adβ-gal. At 15 hours after infection, the cells were trypsinized and seeded back onto plastic. Less adherent and less spread cells were observed in ALK-1QD–expressing cells (Figure5A). The number of adherent cells was determined 30 minutes after reseeding (Figure 5B). We found that ALK-1QD expression significantly decreased the number of adherent cells on plastic (28%). We also observed a decrease in cell reattachment when the cells were seeded on plates coated with different extracellular matrix proteins (fibronectin, gelatin, collagen I, and collagen IV; Figure 5B). We also looked at the effect of ALK-1QD on spreading. The numbers of fully spread versus partially spread and round cells were determined 30 minutes after reseeding. ALK-1QD expression decreased the number of fully spread cells (65% versus 36%, Adβ-gal and AdALK-1QD, Figure 5C). The average cell area was also significantly reduced by ALK-1QD expression (1012 μm2 versus 1362 μm2, AdALK-1 and Adβ-gal; P < .01).
ALK-1QD inhibits HMVEC-d readhesion and spreading.
HMVEC-d's were infected (MOI = 10) with AdALK-1QD or Adβ-gal. At 15 hours after infection, cells were trypsinized and reseeded onto uncoated or coated (fibronectin, gelatin, collagen I, and collagen IV) plastic multiwell dishes. (A) Adherent cells were photographed 3 hours after reseeding under a phase contrast microscope (original magnification, × 100). (B) The number of adherent cells was determined with a particle counter 30 minutes after reseeding. (C) The number of fully spread versus partially spread and round cells were determined 30 minutes after reseeding on fibronectin.
ALK-1QD inhibits HMVEC-d readhesion and spreading.
HMVEC-d's were infected (MOI = 10) with AdALK-1QD or Adβ-gal. At 15 hours after infection, cells were trypsinized and reseeded onto uncoated or coated (fibronectin, gelatin, collagen I, and collagen IV) plastic multiwell dishes. (A) Adherent cells were photographed 3 hours after reseeding under a phase contrast microscope (original magnification, × 100). (B) The number of adherent cells was determined with a particle counter 30 minutes after reseeding. (C) The number of fully spread versus partially spread and round cells were determined 30 minutes after reseeding on fibronectin.
ALK-1QD inhibits the relocalization of focal complexes
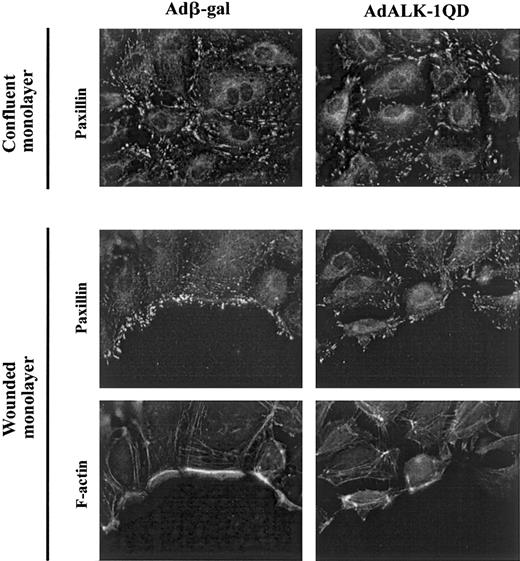
Since ALK-1QD inhibited migration and readhesion, we postulated that ALK-1 might have an effect on the formation of focal adhesion. We therefore examined the effect of AdALK-1QD on these complexes in migrating cells and in confluent monolayers. To do this, at 15 hours after infection, some adherent cells were wounded and fixed 3 hours later. Staining of the control confluent monolayer of AdALK-1QD–infected HMVEC-d's for paxillin, a good marker of focal adhesions, did not reveal any significant difference with the staining of Adβ-gal–infected cells (Figure 6, top). Staining for paxillin in wounded monolayers of Adβ-gal–infected cells showed discrete focal adhesions at the extreme leading edge of the wound and at the base of lamellipodia (Figure 6, bottom). In contrast, in ALK-1QD–infected cells, the staining remained in focal adhesions within the body of the cells (Figure 6, bottom), suggesting a defect in dynamic formation of focal adhesions in ALK-1QD–expressing cells. Phalloidin labeling of the actin fibers also indicated a defect in their formation near the wound in ALK-1QD–infected cells (Figure 6, bottom).
ALK-1QD inhibits relocalization of paxillin and β-actin at the edge of the wound.
At 15 hours after infection, the monolayer was wounded with a pipet tip (bottom panel) or left unwounded (top panel). After 3 hours, the monolayers were fixed and stained for paxillin and F-actin as described in “Materials and methods.” Original magnification, × 1000.
ALK-1QD inhibits relocalization of paxillin and β-actin at the edge of the wound.
At 15 hours after infection, the monolayer was wounded with a pipet tip (bottom panel) or left unwounded (top panel). After 3 hours, the monolayers were fixed and stained for paxillin and F-actin as described in “Materials and methods.” Original magnification, × 1000.
ALK-1QD–regulated genes in HMVEC-d's
To further explore the molecular mechanisms behind ALK-1–induced changes in HMVEC-d behavior, we examined the changes in cellular gene expression, which occurred in response to ALK-1QD, by microarray analysis using a membrane spotted with 1176 cancer-related cDNAs. This experiment was performed twice and yielded similar results. As shown in Table 1, we found 13 genes that were either positively regulated (8 genes) or negatively regulated (5 genes) 15 hours after AdALK-1QD infection. Among these genes, 2 encoded proteins related to the cell cycle; the Cdk inhibitor p21/WAF1, which was up-regulated, and the product of the proto-oncogene c-myc, which was down-regulated. There were 3 genes that encode proteins that are related to the cytoskeleton and more precisely to focal adhesion plaques: β-actin, paxillin, and zyxin, which were down-regulated. The regulation of these 5 genes was confirmed by quantitative RT-rtPCR (Table 1). There were 2 other genes that encoded proteins that belong to the TGF-β family, the growth and differentiation factor 15 (GDF-15) and the BMP type II receptor (BMPRII), which were up-regulated. A similar experiment was performed with the constitutive active form of ALK-5 (ALK-5TD). Out of the 13 genes found to be regulated by ALK-1QD, only BMPRII and GRP78/BiP were also found to be up-regulated by ALK-5TD (Table 1).
ALK-1QD– and ALK-5TD–regulated genes in HMVEC-d's 15 hours after infection
| Gene/protein name (accession number) . | ALK-1QD/β-gal* . | ALK-5TD/β-gal* . | |
|---|---|---|---|
| cDNA array . | rtPCR . | cDNA array . | |
| Up-regulated genes | |||
| MIC-1/GDF-15 (Q99988) | 7.95 | 5.01 | 1.06 |
| GRP78/BiP (P11021) | 4.46 | ND | 2.23 |
| Ubiquitin (Q9BWD6) | 3.92 | ND | 1.01 |
| BMPRII (Q13873) | 2.41 | ND | 1.97 |
| SODI (P00441) | 2.07 | ND | 0.89 |
| Glycyl tRNA synthetase (P41250) | 1.98 | ND | 0.80 |
| MAP1B (P46821) | 1.91 | ND | 1.07 |
| p21/waf1 (P38936) | 1.75 | 1.75 | 0.70 |
| Down-regulated genes | |||
| Zyxin (Q15942) | 0.46 | 0.62 | 0.84 |
| β-actin (Q9UE89) | 0.47 | 0.52 | 0.93 |
| Paxillin (P49023) | 0.51 | 0.53 | 0.80 |
| c-myc (P01106) | 0.51 | 0.48 | 1.12 |
| HMG-I (P17096) | 0.59 | ND | ND |
| Gene/protein name (accession number) . | ALK-1QD/β-gal* . | ALK-5TD/β-gal* . | |
|---|---|---|---|
| cDNA array . | rtPCR . | cDNA array . | |
| Up-regulated genes | |||
| MIC-1/GDF-15 (Q99988) | 7.95 | 5.01 | 1.06 |
| GRP78/BiP (P11021) | 4.46 | ND | 2.23 |
| Ubiquitin (Q9BWD6) | 3.92 | ND | 1.01 |
| BMPRII (Q13873) | 2.41 | ND | 1.97 |
| SODI (P00441) | 2.07 | ND | 0.89 |
| Glycyl tRNA synthetase (P41250) | 1.98 | ND | 0.80 |
| MAP1B (P46821) | 1.91 | ND | 1.07 |
| p21/waf1 (P38936) | 1.75 | 1.75 | 0.70 |
| Down-regulated genes | |||
| Zyxin (Q15942) | 0.46 | 0.62 | 0.84 |
| β-actin (Q9UE89) | 0.47 | 0.52 | 0.93 |
| Paxillin (P49023) | 0.51 | 0.53 | 0.80 |
| c-myc (P01106) | 0.51 | 0.48 | 1.12 |
| HMG-I (P17096) | 0.59 | ND | ND |
ND indicates not determined.
Mean of 2 experiments.
Discussion
ALK-1 is an orphan endothelial-specific type I receptor of the TGF-β receptor family that has been implicated in angiogenesis by genetic studies.2 4-6 However, no cellular function or molecular target has been ascribed to ALK-1 yet. In the present study, we show for the first time that activated ALK-1, through Smad1/Smad5 phosphorylation, inhibits proliferation, migration, readhesion, and spreading of HMVEC-d's. This inhibitory effect of ALK-1QD on migration was confirmed in other endothelial cell types (HMEC-1's, HMVEC-l's, and HUVECs), indicating that this is a very general effect that occurs in microvascular and vascular endothelial cells of different tissues.
The most profound effect of ALK-1QD expression that we could observe in HMVEC-d's was the complete absence of migration in the wound assay. This inhibition of migration was also observed in the transwell assay. Time-lapse recording of the wound assay experiment in HMVEC-d's (pictures taken every 30 minutes for 48 hours) showed that ALK-1QD–treated cells start to move in response to wounding in an unorientated manner and many rapidly detached from the surface (Figure3; ALK-1 video). To eliminate the possibility of toxic effects of ALK-1QD expression, we measured apoptosis by the TUNEL technique. If ALK-1QD was to directly elicit apoptosis directly some evidence of TUNEL staining might be observed in adherent cells prior to detachment. This was not the case. We could only visualize apoptotic cells in the population of nonadherent detached cells. Therefore, it is probable that cells detach when they are stimulated to move toward the center of the wound, possibly due to a defect in cytoskeletal reorganization, and then undergo anoı̈kis. Indeed, we observed a defect in the formation of focal adhesion complexes, as visualized by paxillin and actin staining at the edge of the wound. Further, we found that ALK-1QD expression inhibited HMVEC-d readhesion to different matrices (fibronectin, gelatin, collagen I, and collagen IV) and decreased spreading to fibronectin. Interestingly, 3 genes that were found to be down-regulated by ALK-1 are related to the cytoskeleton and more precisely to focal adhesion complexes and stress fibers: β-actin, paxillin, and zyxin, a phosphoprotein that colocalizes with integrins at sites of membrane-substratum adhesion.18 Taken together, these data demonstrate that ALK-1 activation modifies the dynamics of cytoskeletal changes in endothelial cells.
The molecular control of actin filament assembly and disassembly implicates the Rho families of small GTPases. Each has been ascribed to a particular function: Rac1 is essential for the protrusion of lamellipodia and for forward movement; Cdc42 is required to maintain cell polarity and direction of the movement; and Rho is required to maintain cell adhesion during movement.19 Therefore our results suggest that ALK-1 might modify the balance between GTPases. This is in accordance with previous studies that demonstrated that ligands of the TGF-β family could signal through GTPase-dependent mechanisms.20 21
In the present work we also show that activated ALK-1 inhibits HMVEC-d proliferation through an arrest in the G1 phase of the cell cycle. Interestingly, we demonstrate that ALK-1QD regulates the expression of 2 genes that are implicated in cell cycle regulation: the proto-oncogene c-myc and the Cdk inhibitor p21/waf1. These genes represent 2 well-characterized targets in the pathway of TGF-β inhibition of proliferation22 and may point to a mechanism for inhibition of proliferation. Proliferation and adhesion have often been linked. Therefore we can also hypothesize that cells stop proliferating in coordination with a defect in adhesion or migration.
There are 2 other genes regulated by ALK-1 that belong to the TGF-β family: GDF-1523 and BMPRII. It is tempting to speculate that GDF-15, BMPRII, and ALK-1 might form a heteromeric complex and that activation of ALK-1 would induce the synthesis of the different components of its own receptor complex. Such a positive regulatory loop has already been demonstrated for TGF-β1, which stimulates its own synthesis as well as that of its receptors.24,25 The possible existence of such a heteromeric ALK-1/BMPRII complex would be consistent with the recent observation that mutations in either ALK-1 or BMPRII genes predispose to a pulmonary hypertension syndrome characterized by obstruction of precapillary pulmonary arteries.26
The cDNA array analysis also enabled us to identify genes that are not regulated by ALK-1 at the time studied. Specifically, we found that the expression of several genes encoding extracellular matrix components was not altered by ALK-1QD (fibronectin, PAI-1, and SPARC). These genes have been previously shown to be regulated by TGF-β1. This suggests that the profibrotic effects of TGF-β1 are likely independent of ALK-1. Interestingly, out of the 13 genes found to be regulated by ALK-1QD, only GRP78/BiP and BMPRII were found to also be regulated by the constitutive active form of ALK-5 (Table 1). Taken together, these results suggest that ALK-1 and ALK-5 induce different signals in endothelial cells and support our previous statement that TGF-β1 is not a ligand for ALK-1.
The results presented here provide the first evidence implicating ALK-1 in angiogenesis at the cellular level and provide several important clues regarding the cellular signaling mechanisms behind the development of HHT. Using a constitutively active ALK-1 receptor (ALK-1QD), we could clearly demonstrate that ALK-1 inhibits proliferation and migration of HMVEC-d's. These in vitro experiments represent the conceptual opposite of the natural genetic disease HHT, in which mutations in the ALK-1 gene result in haploinsufficiency and reduced levels of ALK-1 receptor at the surface of endothelial cells.3 HHT patients present vessel enlargement and direct arteriovenous connections without intervening capillary beds. This phenotype probably results from an increase in proliferation and migration of capillary endothelial cells that is completely consistent with the “inverse” phenotype observed in ALK-1QD–expressing cells. In further support of these observations, homozygous ALK-1 knockout mice die at midgestation, exhibiting severe vascular abnormalities characterized by excessive fusion of capillaries and hyperdilation of large vessels,4,5 and the zebrafish mutant ALK-1 has an abnormal circulation pattern attributed to an increase in endothelial cell number.6 These findings from HHT patients and ALK-1−/− animals, coupled with our data using a dominant positive ALK-1 receptor, suggest that ALK-1 participates in the negative regulation of endothelial cell proliferation, which is an important step of the maturation phase of angiogenesis. Disruption of this latter phase of angiogenesis may be important in the pathogenesis of human vascular dysplasia.
We thank Dr J. LaMarre (University of Guelph, ON, Canada) for his critical review of the manuscript and for his provocative suggestions. We thank Dr S. Souchelnitskiy (Ludwig Cancer Institute for Cancer Research, Uppsala, Sweden) for helpful discussions. We are grateful to Dr M. Fujii and Dr P. ten Dijke for providing us with ALK-1QD adenovirus and specific anti–phosphoSmad antibodies, respectively. We thank Dr D. Gulino and Dr F. Candal for providing us with HUVECs and HMEC-1's, respectively. We thank M. Keramidas and Dr B. Vailhé (INSERM EMI105, Grenoble, France) for introducing us to endothelial cell migration assays. We acknowledge C. Rosello and X. Ronot (Institut A Boniot, Grenoble, France) for helping us in video tracking analysis of migration in the wound assay. We thank Véronique Collin-Faure and Gwenaëlle LeMoigne for their skillful technical help. We thank the Gene Production Network (GVPN) and the Gene Therapy Laboratory of the CHU in Nantes who amplified the adenoviruses.
Supported by INSERM (EMI 0105), CEA (Direction Sciences du Vivant, Department of Responses and Cellular Dynamics (DRDC)/Angio0105), and a European Union grant (no. QLRT-2000-01302).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sabine Bailly, INSERM EMI 0105 DRDC/Angio, CEA-Grenoble, 17 rue des Martyrs, 38054 Grenoble, France; e-mail:sbailly@cea.fr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal