Oral anticoagulant therapy, which is used for prophylaxis and management of thrombotic disorders, causes similar reductions in plasma levels of vitamin K–dependent procoagulant and anticoagulant clotting factor zymogens. When we measured levels of circulating activated protein C, a physiologically important anticoagulant and anti-inflammatory agent, in patients on oral anticoagulant therapy, the results unexpectedly showed that such therapy decreases levels of activated protein C substantially less than levels of protein C, prothrombin, and factor X, especially at lower levels of prothrombin and factor X. Thus, we suggest that oral anticoagulant therapy results in a relatively increased expression of the protein C pathway compared with procoagulant pathways not only because there is less prothrombin to inhibit activated protein C anticoagulant activity, but also because there is a disproportionately higher level of circulating activated protein C.
Introduction
Although unfractionated or low-molecular-weight heparins are often used for immediate management of thrombotic risks or manifestations, oral anticoagulants (OACs) are generally used for prolonged therapy.1 OACs act as antagonists of the normal biosynthesis of vitamin K–dependent proteins. Vitamin K is required for the synthesis of biologically active procoagulant factors II, VII, IX, and X, as well as the anticoagulant factors protein C, protein S, and protein Z. These plasma proteins participate in calcium-dependent interactions with negatively charged phospholipids through γ-carboxyglutamyl (Gla) residues in their amino-terminal region. Vitamin K is required for the postribosomal conversion of glutamyl residues in precursor proteins to Gla residues in the mature plasma proteins. OACs block Gla formation, resulting in dysfunctional proteins that lack 8 to 11 Gla residues per protein molecule. Partially carboxylated clotting factors are dysfunctional because of impaired interactions with phospholipid membranes.2 During sustained oral anticoagulation, procoagulant activity levels of vitamin K–dependent clotting factors average 15% to 25%, whereas antigen levels average approximately 40% to 60%, and the various vitamin K–dependent proteins decrease in parallel to each other.3-7
Activated protein C (APC) is a normal circulating component of plasma8 whose formation from zymogen protein C is thought to be effected by the thrombin/thrombomodulin complex in a reaction that is enhanced by the endothelial protein C receptor.9,10 Oral anticoagulant therapy causes a decrease in circulating soluble endothelial protein C receptor.11APC is a physiologic anticoagulant and anti-inflammatory agent that was recently approved by the Food and Drug Administration (FDA) for therapy of severe sepsis because it substantially reduces sepsis-related mortality.12 Low levels of circulating APC were recently suggested to be a risk factor for venous thrombosis.13Smirnov et al14 reported that prothrombin inhibits APC anticoagulant activity and hypothesized that during anticoagulant therapy the reduction of prothrombin levels would result in an increased effectiveness of APC. To shed light on the physiology of APC, we have an interest to quantitate APC in various human subjects. Recently, we measured circulating APC in plasma of patients with systemic lupus erythematosus (SLE) with antiphospholipid antibodies who did not use OACs.15 We extended our studies to a group of SLE patients with antiphospholipid antibodies who used OACs and also to a group of patients who used OACs for cardiac diseases. Here we report the unexpected finding that OACs reduce levels of circulating APC less than those of protein C, prothrombin (FII), and factor X (FX).
Study design
Blood samples were collected by venipuncture using plastic tubes containing 3.8% trisodium citrate (0.129 M; 9:1 vol/vol) from 84 patients with SLE (28 of these were using OACs) and from 31 cardiac patients on OAC therapy. Protein C, FII, and FX levels were measured in platelet-poor plasma. Protein C levels were measured by a coagulometric assay (Protein C Reagent, coagulometric; Dade Behring Marburg GmbH, Germany) and by a chromogenic assay (Berichrom protein C; Dade Behring) that measures all forms of protein C in plasma. Levels of factors II and X were measured with a coagulometric method that determines levels of procoagulantly active factors (Thromborel S, Dade Behring). Protein C, FII, and FX levels were expressed as the percentage of the level in pooled normal plasma (40 healthy blood donors). Circulating APC levels were measured by an enzyme capture assay that measures all forms of APC.8
Results and discussion
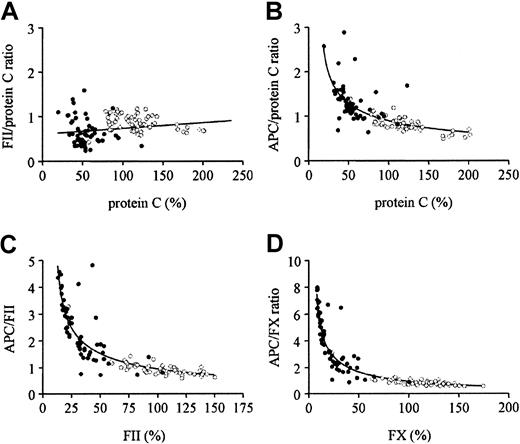
The mean values for APC, protein C, FII, and FX in subjects on OACs were 69%, 22% (coagulometric protein C), 54% (chromogenic protein C), 35%, and 24%, respectively, whereas the values for SLE patients not receiving OACs were 94%, 123% (coagulometric protein C), 119% (chromogenic protein C), 102%, and 111%, respectively. For all subjects on OACs, levels of FII, FX, and protein C similarly and proportionally decreased during OAC treatment (data not shown) as previously reported3-7 and as illustrated in Figure1A for FII and protein C (chromogenic) where the ratio of FII/protein C did not vary much over the range of protein C observed. In contrast, when the ratio of APC to protein C (chromogenic), FII, and FX were calculated and graphed as a function of protein C (chromogenic), FII, and FX, respectively, each of these ratios increased as the concentration of level of each zymogen factor decreased because of OACs (Figure 1B-D). Compared with data for subjects not taking OACs (Figure 1B-D), there was a strong and inverse relationship between the APC/protein C ratio and protein C (B), the APC/FII ratio and FII (C), and the APC/FX ratio and FX (D). This finding indicates that during OAC treatment the decrease in APC levels is much less than the respective decreases in protein C, FII, and FX. Notably for FII and FX levels below 25%, the APC/FII and APC/FX ratios increase from 2 to 5 and 2 to 8, respectively.
Comparisons of effects of oral anticoagulant therapy on circulating APC, protein C, FII, and FX.
● represents patients using oral anticoagulants. ○ indicates SLE patients without oral anticoagulant treatment. (A) Correlation between FII/protein C ratio and protein C level (chromogenic). (B) Correlation between APC/protein C ratio and protein C level (chromogenic). (C) Correlation between APC/FII ratio and FII level. (D) Correlation between APC/FX ratio and FX level.
Comparisons of effects of oral anticoagulant therapy on circulating APC, protein C, FII, and FX.
● represents patients using oral anticoagulants. ○ indicates SLE patients without oral anticoagulant treatment. (A) Correlation between FII/protein C ratio and protein C level (chromogenic). (B) Correlation between APC/protein C ratio and protein C level (chromogenic). (C) Correlation between APC/FII ratio and FII level. (D) Correlation between APC/FX ratio and FX level.
The APC immunocapture assay used in our studies captures both normally carboxylated and partially carboxylated forms of APC. The protein C chromogenic assay used here also reflects both normally and partially carboxylated forms of protein C. Thus, the increase of the ratio of APC/protein C at low concentrations of protein C indicates that all forms of APC are disproportionately higher than all forms of protein C zymogen. This finding is consistent with the increased ratios of APC/FII and APC/FX at low FII and FX levels.
Elevated plasma prothrombin levels, often because of the 20210A prothrombin gene polymorphism, are a significant risk factor for venous thrombosis.16 Because prothrombin inhibits APC anticoagulant activity in vitro and because this inhibition is reduced at low concentrations of prothrombin, it was hypothesized that during anticoagulant therapy the reduction of prothrombin levels would result in an increased effectiveness of whatever APC is available.14 Furthermore, it was assumed, without experimental data, that the level of APC would be proportional to the level of protein C in patients using OACs.14 On the basis of the results here, we suggest that OAC therapy results in a relatively increased expression of the protein C pathway compared with procoagulant pathways not only because there is less prothrombin to inhibit activated protein C anticoagulant activity but also because there is a disproportionately higher level of circulating APC. As both plasmin and factor Xa are capable of activating protein C to give APC,17 18 it can be speculated that the contribution of these (or other) proteases in the generation of basal levels of APC during OAC therapy explains why APC levels are not reduced proportionally to prothrombin.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002-01-0329.
Supported by grants from The Dutch League against Rheumatism (NR 97.1.401) and the National Institutes of Health (R37HL52246).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
M. J. A. Simmelink, Thrombosis and Haemostasis Laboratory, Department of Haematology (G03.647), University Medical Center Utrecht, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands; e-mail:m.simmelink@lab.azu.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal