Complete interferon-γ receptor 1 (IFNγR1) deficiency is a primary immunodeficiency disease characterized by high susceptibility to recurrent, severe mycobacterial and other intracellular infections. We here report the first successful treatment of the disorder by bone marrow transplantation (BMT). The 8-year-old girl had suffered from recurrent mycobacterial infections in the past and had developed liver cirrhosis with portal hypertension. For conditioning, fractionated total body irradiation (TBI) was used in combination with cyclophosphamide and antithymocyte globulin (ATG). The patient received red cell–depleted bone marrow from her HLA-identical sister. The transplantation course was uneventful and 4 years later, the child remains in excellent clinical condition and free of mycobacterial infections. She has stable mixed lymphohematopoietic chimerism after repeat T-cell transfusions. Liver disease has not further deteriorated. This experience shows that correction of IFNγR1 deficiency is possible by BMT and complications of the disease can be controlled.
Introduction
Complete interferon-γ receptor 1 (IFNγR1) deficiency is an autosomal recessively inherited disorder,1 which has a very poor prognosis due to severe complications from infections caused, in particular, by mycobacteria and other intracellular microorganisms such as Listeria monocytogenes and Salmonella species.2-5In 1996, Newport et al6 identified the gene for IFNγR1 on chromosome 6 (q16-22). Mutations in this gene may lead to a lack of expression or function of IFNγR1. In macrophages this results in a failure to become activated on exposure to IFN-γ, leading to lack of tumor necrosis factor α (TNF-α) production and profound deficiency in intracellular killing of pathogens.6 To date, complete IFNγR1 deficiency has been identified in 9 kindreds (17 patients). Mutations in these patients included nonsense and splice mutations as well as frameshift deletions and insertions. These mutations were located in the segment encoding the extracellular domain of the receptor.7 The resulting premature stop codons upstream of the region encode the transmembrane domain and preclude expression of the receptor. Patients with partial IFNγR1 deficiency have also been described and present with milder clinical phenotypes.1,8Beside abnormalities of IFNγR1, other deficiencies predisposing to mycobacterial infections include deficient expression of IFNγR2 and of interleukin 12 receptor β1 (IL-12Rβ1), deficiency of IL-12, and abnormalities of signal transducer and activator of transcription 1 (STAT1).1,8 9 In patients with complete IFNγR1 deficiency, treatment with IFN-γ is ineffective and the prognosis is extremely poor with a high rate of early deaths in infancy and childhood. We here report on successful treatment of the disease by bone marrow transplantation (BMT).
Study design
The patient was born as the second child of healthy, unrelated parents. There was no family history of hereditary diseases or early deaths. The patient, who had received routine bacille Calmette-Guérin (BCG) vaccination as a newborn, developed BCG histiocytosis at the age of 5 months, responding to antituberculous treatment. At the age of 18 months she presented with atypical mycobacteriosis (Mycobacterium kansasii) involving the lung and multiple sites of the skeletal system. Abnormalities of liver function of unclear etiology were also noted. She was treated with antimycobacterial drugs for 2 years. At the age of 3 years, while still receiving treatment, the child developed progressive hepatomegaly with elevated liver enzymes. A liver biopsy showed chronic active hepatitis with cholangitis and fibrosis. No mycobacteria were detected. Over the following 4 years, liver cirrhosis developed, complicated by portal hypertension and acute bleeding from esophageal varices on 2 occasions. Other complications included Listeria meningitis at the age of 4 years and recurrence of systemic atypical mycobacteriosis (Mycobacterium avium, involving the lung) following discontinuation of antimycobacterial treatment. T-cell immunodeficiencies and chronic granulomatous disease were excluded. In 1997, at the age of 7 years, complete IFNγR1 deficiency was diagnosed as described elsewhere.4 Seven months later, in October 1997, the patient was transferred to our center for BMT.
On admission, the patient was in stable clinical condition with body length and weight at the 25th percentile. She was receiving antibiotic prophylaxis with ciprofloxacin. There was no evidence of active mycobacterial infection. The abdomen was distended with marked hepatosplenomegaly (liver length, 14 cm; spleen length, 17 cm). Liver function was impaired with decreased synthesis of cholinesterases. Computed tomography of the abdomen showed signs of liver cirrhosis and portal hypertension with collateral circulation. Apart from mild icterus, the skin was without any abnormalities. Other organ functions, in particular pulmonary functions, were normal.
The BMT procedure was performed after obtaining written consent from both parents. Conditioning consisted of total body irradiation (TBI) applied in 6 fractions at 2 Gy over 3 days (cumulative dose, 12 Gy) with complete shielding of the lungs, followed by cyclophosphamide at 60 mg/kg/d intravenously in one daily dose on 2 days (total dose, 120 mg/kg) and antithymocyte globulin (ATG; Merieux, Leimen, Germany) at 10 mg/kg/d daily for 3 days (total dose, 30 mg/kg). Bone marrow from the HLA-identical 12-year-old sister was infused, following depletion of red cells from the graft because of blood group incompatibility. The number of nucleated cells was 3.87 × 108/kg.
Results and discussion
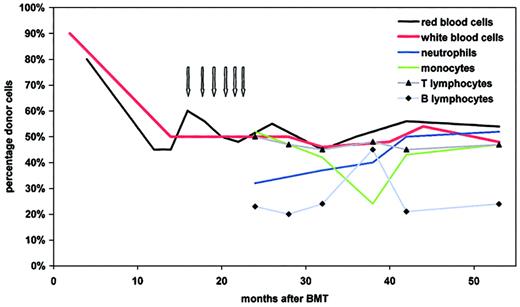
Hematologic reconstitution was characterized by appearance of leukocytes on day +18 after BMT. Granulocyte colony-stimulating factor (G-CSF) at 5 μg/kg/d was used from day +18 until day +24. Reticulocytes more than 20% were reached on day +35 and platelet transfusions were required until day +71. Prophylaxis for graft-versus-host disease (GVHD) prophylaxis consisted of anti–IL-2 receptor antibodies (Leukotac, Biotest, Dreieich, Germany) at a dose of 0.2 mg/kg/d from day 0 until day +17, and at 0.1 mg/kg/d from day +18 until day +39. On day +9, cyclosporin A was started at a dose of 2 mg/kg/d and was continued until day +57. The patient remained free of infections and of complications from GVHD and there was no evidence of liver toxicity. Antimycobacterial prophylaxis was discontinued at 6 weeks after BMT. At 4 months after BMT, the blood group was more than 80% donor type, and molecular studies, performed 9 months after BMT, revealed normal expression of IFNγR1. Repeated chimerism analysis at 15 months showed mixed chimerism with 50% of blood cells of recipient type. In an attempt to prevent further autologous reconstitution, donor leukocytes were infused monthly at escalating doses (total number of infused T cells, 4.3 × 107/kg).Chimerism remained stable with approximately 50% of red and white blood cells of donor origin. This mixed chimerism, which persists until now, is also observed within leukocyte subpopulations, with stable proportions of lymphocytes, neutrophils, and monocytes being donor derived (Figure1). Analysis of IFNγR1 expression by monocytes revealed 2 populations, one with normal expression and another, presumably host derived, with absent expression (data not shown).
Blood cell chimerism as determined at various time points after BMT.
Red blood cell chimerism was analyzed by quantitative blood group determination. Mixed chimerism of unseparated and of fluorescence-activated cell-sorted subpopulations of leukocytes was determined quantitatively, using multiplex amplification of short tandem repeat markers and fluorescence detection.10 The arrows depict the dates of donor T-cell infusions (first dose: 1 × 106/kg; second dose 2 × 106/kg; subsequent doses 1 × 107/kg).
Blood cell chimerism as determined at various time points after BMT.
Red blood cell chimerism was analyzed by quantitative blood group determination. Mixed chimerism of unseparated and of fluorescence-activated cell-sorted subpopulations of leukocytes was determined quantitatively, using multiplex amplification of short tandem repeat markers and fluorescence detection.10 The arrows depict the dates of donor T-cell infusions (first dose: 1 × 106/kg; second dose 2 × 106/kg; subsequent doses 1 × 107/kg).
Immune functions in the patient have normalized, with regular antibody responses after vaccination and normal T-cell immune functions. Except persistent mild thrombocytopenia with platelet counts at 50 000 to 70 000/μL, likely resulting from hypersplenism, hematologic functions are normal. No transplantation-related delayed complications have developed. Growth parameters are at the 30th percentile and the patient has entered puberty. She remains in excellent clinical condition more than 4 years after BMT (Karnofsky index 100%).
Deficiency of IFNγR1 in the patient was complicated by advanced liver disease presumably caused by the recurrent atypical mycobacterial infections. This complication had deteriorated to a point that liver transplantation was considered, which, however, was postponed until successful reconstitution after BMT. In the conditioning regimen the use of busulfan was avoided, which is known for its liver toxicity, and TBI was used instead. In addition, the use of cyclosporin A, another hepatotoxic drug, was avoided during the early phase after BMT. Following BMT, liver functions did not deteriorate further and, in fact, the hepatic disease in the patient has remained stable until now.
The uncomplicated treatment course and beneficial outcome of BMT observed in this child with complete IFNγR1 deficiency may be related to the stable clinical status at the time of BMT, in particular the control of mycobacterial infections and the absence of significant organ damage except advanced liver disease. The future will show whether the attained immunologic reconstitution after BMT is stable and sufficient to prevent further life-threatening infections. At present, more than 4 years after BMT, the patient is leading a normal life, but may need liver transplantation in case of further deterioration of liver function.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002- 02-0433.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Wilhelm Friedrich, University Children's Hospital Ulm, Prittwitzstr 43, D-89073 Ulm, Germany; e-mail:wilhelm.friedrich@medizin.uni-ulm.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal