Acute graft-versus-host disease (GVHD) is thought to derive from direct T-cell injury of target tissues through perforin/granzyme, Fas/FasL interactions, and the effects of inflammatory cytokines. Animal models and some clinical trials support the notion that inhibition of inflammatory mediators such as interleukin-1 (IL-1), tumor necrosis factor α, and interferon γ may ameliorate or prevent GVHD. We hypothesized that blockade of IL-1 during the period of initial T-cell activation would reduce the risk of severe GVHD. We tested this hypothesis in a double-blind, placebo-controlled randomized trial of recombinant human IL-1 receptor antagonist (IL-1Ra) in 186 patients undergoing allogeneic stem cell transplantation. Randomization was stratified by degree of histocompatibility and stem cell source. All patients were conditioned with cyclophosphamide and total body irradiation. GVHD prevention consisted of cyclosporine and methotrexate in all patients. Recombinant human IL-1Ra or saline placebo was given from day −4 to day +10. Randomization was stratified according to GVHD risk. The 2 groups were well-matched for pretreatment characteristics. Moderate to severe GVHD (grades B-D) developed in 57 (61%) of 94 patients receiving IL-1Ra and in 51 (59%) of 86 patients on placebo (P = .88). There was no difference in hematologic recovery, transplantation-related toxicity, event-free survival, or overall survival. We conclude that blockade of IL-1 using IL-1Ra during conditioning and 10 days immediately after transplantation is not sufficient to reduce GVHD or toxicity or to improve survival.
Introduction
Acute graft-versus-host disease (GVHD) results from a complex interaction of donor T cells and recipient organs that involves recognition of minor histocompatibility antigens in an inflammatory milieu. Tissue damage from conditioning regimens, infections, and the underlying illness may provide a milieu that fosters T-cell recognition of susceptible tissues. Clinical injury is thought to derive from direct T-cell injury through perforin/granzyme, Fas/FasL interactions, and the effects of inflammatory cytokines. Because of their central role in inflammation, inhibitors of interleukin-1 (IL-1) and tumor necrosis factor α (TNFα) were developed and studied in animal models of inflammation and in human diseases. Animal models suggested that specific inhibition of single cytokines could have a major impact on GVHD and survival. We were particularly interested in IL-1 receptor antagonist (IL-1Ra), because it is a pure, competitive inhibitor of IL-1α and IL-1β, and it has no intrinsic effects on hematopoiesis. Murine modeling demonstrated that administration of IL-1Ra either immediately after marrow infusion or immediately prior to the projected time of onset of acute GVHD was effective in preventing GVHD-related mortality,1 although not in all models.
The association of excess IL-1 with clinical GVHD is somewhat controversial. Peripheral blood mononuclear cells (PBMCs) containing IL-1, IL-1β, and TNFα have been associated with acute GVHD, although plasma concentrations of the same cytokines correlated less well with disease.2-4 In another study, increased IL-1 was noted in the skin of patients with GVHD, but IL-1 levels were nonspecifically elevated in PBMCs regardless of GVHD severity.5 By contrast, a Dutch study found that increased serum levels of IL-10, but not IL-1, correlated with acute GVHD.6 Clinical interest in IL-1Ra was further supported by evidence of clinical efficacy in a phase 1/2 trial of IL-1Ra in the therapy of steroid-resistant GVHD, as well as a similar trial of soluble IL-1 receptor.7,8 In addition, a variable number of tandem repeats polymorphism associated with increased IL-1Ra production in donor cells has been associated with milder, acute GVHD in recipients of human leukocyte antigen (HLA)–identical bone marrow transplant (BMT), suggesting a productive role for IL-1Ra.9 Finally, experimental evidence suggested that increases in cytokine production due to conditioning regimen effects were associated with a higher risk of acute GVHD.10 One possibility is that the early production of inflammatory mediators primed and activated the donor T cells and increased their effectiveness as GVHD mediators. IL-1 amplifies T-cell proliferation in a mixed lymphocyte reaction (MLR) by enhancing the function of dendritic cell stimulators.11 Addition of IL-1ra to MLR does not diminish T-cell proliferation,12 but does suppress production of chemokines that are important for the trafficking of leukocytes in vitro.13
We hypothesized that blockade of IL-1 during the period of initial T-cell activation would reduce the risk of severe GVHD and perhaps of transplantation-related toxicity. We tested this hypothesis in a double-blind, placebo-controlled randomized trial of specific IL-1 inhibition, with IL-1Ra as a supplement to conventional GVHD prophylaxis during allogeneic stem cell transplantation.
Patients, materials, and methods
Study design
The trial was a double-blind, stratified, randomized, placebo-controlled comparison of patients with hematologic disorders who received either IL-1Ra or placebo. Randomization was stratified by hospital, the source of stem cells (ie, bone marrow versus peripheral blood), and donor type (histocompatible family member versus partially matched family donors and unrelated donors). The primary end-point was the development of grades B-D acute GVHD14 within 100 days of stem cell infusion. Secondary end-points were survival at day 100, incidence of transplantation-related toxicities (eg, hepatic veno-occlusive disease, interstitial pneumonitis), and time to engraftment (defined as the first of 3 consecutive days of an absolute neutrophil count of at least 500 cells/μL). An early stopping rule was incorporated into the study based on the development of severe adverse events within 35 days of stem cell infusion. A data safety monitoring board, consisting of transplantation experts who were uninvolved with the trial, performed 2 interim analyses. The institutional review boards of each of the participating centers approved the study.
Treatment protocol
All patients received cyclophosphamide 1800 mg/m2 on 2 consecutive days, followed by total body irradiation, 13.6 Gy in 8 fractions. Cyclosporine was administered beginning on day −3 at 2.5 mg/kg intravenously every 12 hours. Methotrexate was given at a dose of 15 mg/m2 on day +1 and at a dose of 10 mg/m2 on days +3, +6, and +11. Study drug consisted of either recombinant human IL-1Ra (provided by Amgen, Thousand Oaks, CA) administered at 0.5 mg/kg per hour by continuous intravenous infusion from day −4 through +10 or saline placebo. This dose and schedule were adapted from a phase 1/2 trial, suggesting efficacy in steroid-resistant GVHD.7 The IL-1Ra is a clear, colorless solution with no infusional toxicities that was indistinguishable from placebo. All patients were treated in reverse isolation using oral antibiotics for bacterial and fungal prophylaxis. Patients who were seropositive forHerpes simplex virus received acyclovir prophylaxis. Upon engraftment, all patients received Pneumocystis cariniiprophylaxis and cytomegalovirus prophylaxis if clinically indicated. Acute GVHD was graded by 2 independent reviewers at each institution and scored according to the International Bone Marrow Transplant Registry grading scale.14
Measurement of IL-1 receptor antagonist and soluble TNF receptor levels
Blood was collected in preservative-free heparin. Plasma was separated from mononuclear cells by centrifugation over a Ficoll density gradient. Plasma samples were then divided into 1-mL aliquots, frozen, and stored at −80°C. Samples were thawed and assayed in batches for levels of soluble TNF receptor (p55) and IL-1ra by enzyme-linked immunosorbent assay (R & D Systems, Minneapolis, MN). Assays were performed according to the manufacturer's protocol.
Patients
Enrolled were 185 patients from the allogeneic transplantation programs of Dana-Farber Cancer Institute/Brigham and Women's Hospital, the Children's Hospital, Boston, MA, and University of Minnesota, Minneapolis, MN, between March 1997 and February 1999. Randomization was stratified according to GVHD risk. Most patients (118) had histocompatible family member donors. Unrelated donors were obtained from the National Marrow Donor Program or international registries. Histocompatibility was determined by serology for major histocompatibility class (MHC) I and by molecular techniques for MHC class II. Using these criteria, 68 patients had matched or single-antigen mismatched unrelated donors or single-antigen mismatched family member donors. Peripheral blood stem cells (PBSCs) were administered to 28 patients, and the rest received bone marrow. The 2 groups were well-matched for pretreatment characteristics (Table1).
Patient characteristics
| . | IL-1RA . | Placebo . |
|---|---|---|
| No. | 96 | 90 |
| Median age (range), years | 41 (3-59) | 44 (8-64) |
| Sex | 49M/47F | 52M/39F |
| Stem cell source | ||
| Bone marrow | 82 | 76 |
| PBSC | 14 | 14 |
| Histocompatible family member | 61 | 57 |
| 5/6 matched family member or unrelated donor | 35 | 33 |
| Diagnosis* | ||
| CML | 48 | 38 |
| ALL | 5 | 8 |
| AML | 17 | 23 |
| MDS | 12 | 10 |
| Myeloma | 1 | 1 |
| NHL | 7 | 7 |
| PNH | 2 | 0 |
| CLL | 3 | 2 |
| Other | 2 | 0 |
| . | IL-1RA . | Placebo . |
|---|---|---|
| No. | 96 | 90 |
| Median age (range), years | 41 (3-59) | 44 (8-64) |
| Sex | 49M/47F | 52M/39F |
| Stem cell source | ||
| Bone marrow | 82 | 76 |
| PBSC | 14 | 14 |
| Histocompatible family member | 61 | 57 |
| 5/6 matched family member or unrelated donor | 35 | 33 |
| Diagnosis* | ||
| CML | 48 | 38 |
| ALL | 5 | 8 |
| AML | 17 | 23 |
| MDS | 12 | 10 |
| Myeloma | 1 | 1 |
| NHL | 7 | 7 |
| PNH | 2 | 0 |
| CLL | 3 | 2 |
| Other | 2 | 0 |
CML (chronic myeloid leukemia) includes stable phase, accelerated phase, or blast transformation. ALL/AML (acute lymphocytic leukemia/acute myeloid leukemia) includes patients in first, second, or subsequent remission. MDS (myelodysplastic syndrome) includes patients with RA/RARS (refractory anemia/refractory anemia with ringed sideroblasts), RAEB (refractory anemia with excess of blasts), CML (chronic myeloid leukemia), and RAEB-T (refractory anemia with excess of blasts in transformation). NHL indicates non-Hodgkin lymphoma; PNH, paroxysmal nocturnal hemoglobinuria; CLL, chronic lymphocytic leukemia; and PBSC, peripheral blood stem cell.
Statistics
This was a randomized, double-blind, placebo-controlled trial. Treatment was assigned centrally by the Brigham and Women's Research Pharmacy (Boston, MA) in a 1:1 ratio within institution, donor category (6 of 6 matched sibling or not), and source of stem cells (bone marrow or peripheral blood). Incidence of grade B to D acute GVHD was compared by the Fisher exact test, as were other categorical outcome variables. Continuous variables were compared using the 2-sample ttest. Overall survival was calculated using the method of Kaplan and Meier and compared by the log-rank test. The rates of grades B-D and grades C-D acute GVHD as well as relapse were calculated using a cumulative incidence function approach, so that the competing risk of death within the first 100 days without the development of GVHD could be addressed. Time to neutrophil recovery was compared by the Wilcoxon test. The accrual goal of 176 randomized patients for whom bone marrow provided the source of stem cells was chosen to provide approximately 80% power to detect a 20% reduction in the rate of moderate to severe acute GVHD, from 55% to 35%, with IL-1Ra, testing at the .05 2-sided significance level. The sequential design used an O'Brien-Fleming boundary. Statistical comparisons of cytokine levels between groups of patients were performed using the Student t test.
A data safety and monitoring board (DSMB) was established for the study. Data on primary and secondary efficacy endpoints and on all safety end-points were to be presented to the DSMB twice during the conduct of the trial, as well as at the conclusion of the study. Additional safety oversight was provided for pediatric patients. DSMB reviews took place at 45% and 72% of full information. Interim data were assessed using the repeated confidence intervals method of Jennison and Turnbull. At the time of the second interim analysis, the confidence interval estimate of the difference between IL-1Ra and placebo excluded a 20% improvement in the rate of moderate to severe acute GVHD with IL-1Ra, suggesting that it was unlikely that IL-1Ra would be able to demonstrate the anticipated magnitude of improvement if the study were to continue to 176 randomized bone marrow transplant patients. The DSMB recommended immediate closure of the study at that time. This report is based on data on all patients randomized on the study.
Results
There were 186 patients randomized. IL-1Ra was assigned to 96 while 90 were to receive placebo. However, 8 patients subsequently withdrew consent and did not receive treatment on study. In 6 of these cases, limited data were available to the study team. Therefore, the analyses presented here include all patients with data. The 2 cohorts shared similar demographics (Table 1). The Data Safety Monitoring Committee planned 2 interim analyses. The study was stopped after the second interim analysis was performed in February 1999, when the DSMB determined that the study would not reach statistical significance.
Cyclosporine and methotrexate
To be sure cyclosporine dosing was equivalent, we examined the lowest weekly cyclosporine level for the first 5 weeks after marrow infusion. The mean and standard deviation of these levels are shown in Table2. Similarly, the number of methotrexate doses received (of 4 prescribed doses) and the total dose of methotrexate was comparable (Table2).
Cyclosporine and methotrexate levels
| Time after transplantation . | IL-1Ra . | Placebo . | P . |
|---|---|---|---|
| Cyclosporine trough (mean ± SD μg/L) | |||
| Week 1 | 225 ± 78 (n = 90) | 235 ± 81 (n = 89) | .37 |
| Week 2 | 273 ± 82 (n = 90) | 268 ± 87 (n = 89) | .66 |
| Week 3 | 259 ± 74 (n = 91) | 283 ± 94 (n = 86) | .07 |
| Week 4 | 258 ± 90 (n = 85) | 265 ± 101 (n = 86) | .64 |
| Week 5 | 256 ± 96 (n = 82) | 270 ± 129 (n = 79) | .44 |
| Prescribed dose administered, % | |||
| Day 1 | 99 | 99 | .17 |
| Day 3 | 100 | 98 | .17 |
| Day 6 | 99 | 98 | .40 |
| Day 11 | 86 | 77 | .09 |
| Time after transplantation . | IL-1Ra . | Placebo . | P . |
|---|---|---|---|
| Cyclosporine trough (mean ± SD μg/L) | |||
| Week 1 | 225 ± 78 (n = 90) | 235 ± 81 (n = 89) | .37 |
| Week 2 | 273 ± 82 (n = 90) | 268 ± 87 (n = 89) | .66 |
| Week 3 | 259 ± 74 (n = 91) | 283 ± 94 (n = 86) | .07 |
| Week 4 | 258 ± 90 (n = 85) | 265 ± 101 (n = 86) | .64 |
| Week 5 | 256 ± 96 (n = 82) | 270 ± 129 (n = 79) | .44 |
| Prescribed dose administered, % | |||
| Day 1 | 99 | 99 | .17 |
| Day 3 | 100 | 98 | .17 |
| Day 6 | 99 | 98 | .40 |
| Day 11 | 86 | 77 | .09 |
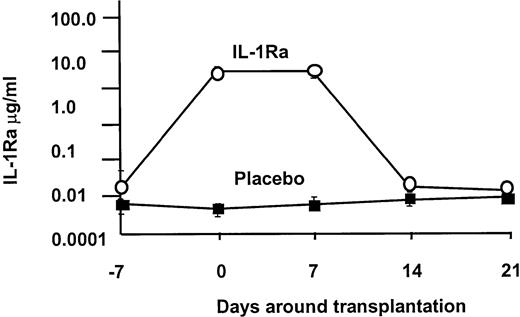
IL-1ra levels
Based on our phase 1/2 trial, we projected that this dose of IL-1Ra would establish a plasma level of approximately 2 μg/mL. As shown in Figure1, the 2 groups began therapy with equivalent IL-1Ra levels (1289 ± 544 vs 91 pg/mL), and on day 0 the levels were significantly higher in the IL-1Ra arm (3 643 430 ± 231 989 vs 488 ± 61 pg/mL), where they remained throughout the infusion period. In both groups, the drug levels returned to baseline by day 21.
IL-1 receptor antagonist levels.
IL-1Ra levels were equivalent at the onset of the infusion, but in the IL-1Ra group, serum levels rapidly rose 1000-fold over control. Drug level was sustained at this high level until the discontinuation of the infusion, when it returned to baseline.
IL-1 receptor antagonist levels.
IL-1Ra levels were equivalent at the onset of the infusion, but in the IL-1Ra group, serum levels rapidly rose 1000-fold over control. Drug level was sustained at this high level until the discontinuation of the infusion, when it returned to baseline.
Graft-versus-host disease
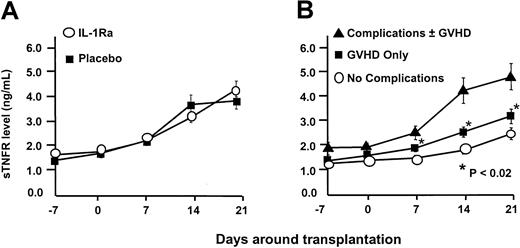
The primary efficacy end-point was the development of grades B-D acute GVHD within 100 days of marrow infusion. Using the cumulative incidence method, the rate of moderate to severe GVHD (grades B-D) developed in 57 (61%) of 94 patients receiving IL-1Ra and in 51 (59%) of 86 patients on placebo (P = .88). There was also no difference in the rate of grades C-D GVHD: 19% versus 20% (P = .85). The probability of developing grades B-D GVHD was not influenced by the source of stem cells. Similar results were observed when we looked at grades B-D GVHD in patients receiving matched family member transplants: 54% versus 52% (P = .85); and alternative donor transplants: 71% versus 72% (P > .99). Marrow and peripheral blood stem cells resulted in similar outcomes in all categories (data not shown). Too few pediatric patients were studied to assess whether young patients might have responded differently. The drug was unable to prevent GVHD despite our ability to increase circulating IL-1Ra levels in this group by more than 103-fold (Figure 1). TNF is another inflammatory cytokine that has been implicated as a contributing factor in the development of GVHD. However, soluble TNF receptor (sTNFR) levels were equivalent in both IL-1Ra and placebo arms (Figure2A). Yet despite high IL1-Ra levels early in the course of the transplantation, patients with acute GVHD or complications of transplantation developed significantly higher levels of sTNFR (P < .02; Figure 2B).
Soluble TNF receptor levels.
(A) TNF levels rose to an identical degree in patients while receiving IL-1Ra and placebo and for 11 days after the infusion was complete. (B) Despite the presence of high circulating levels of IL-1Ra, the development of acute GVHD and/or the development of transplantation-related toxicity resulted in elevations in TNF levels.
Soluble TNF receptor levels.
(A) TNF levels rose to an identical degree in patients while receiving IL-1Ra and placebo and for 11 days after the infusion was complete. (B) Despite the presence of high circulating levels of IL-1Ra, the development of acute GVHD and/or the development of transplantation-related toxicity resulted in elevations in TNF levels.
Engraftment and toxicity
Secondary end-points were time to neutrophil engraftment, incidence of transplantation-related toxicities, and survival at day 100. As shown in Table 2, neutrophil recovery was similar in both groups, 24 days versus 27 days (P = .74). We observed no difference in the rate of respiratory complications, hepatic veno-occlusive disease, or mucositis.
Relapse and survival
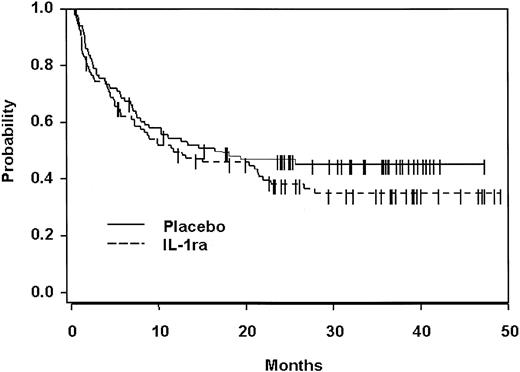
The relapse rates calculated using the cumulative incidence method were 29% for the IL-1Ra group and 27% for the placebo group (P = .66). The median event-free survival on IL-1Ra is 226 days, compared to 382 on placebo (P = .17). As shown in Figure3, there was no difference between the 2 groups in overall survival. Patients receiving IL-1Ra have a median survival of 357 days corresponding to 35% (90% CI, 26%-44%) at 3 years. The placebo patients have a median survival of 500 days and a 3-year survival of 45% (90% CI, 36%-54%;P = .23).
Overall survival.
No difference in survival was detectable in either arm. Moreover, there was no difference between the groups in the use of PBSCs versus bone marrow or matched family member donors versus unrelated and partially matched donors.
Overall survival.
No difference in survival was detectable in either arm. Moreover, there was no difference between the groups in the use of PBSCs versus bone marrow or matched family member donors versus unrelated and partially matched donors.
Secondary study end-points
| . | IL-1Ra . | Placebo . | P . |
|---|---|---|---|
| Hepatic veno-occlusive disease (%) | 29 | 27 | .74 |
| Median time to neutrophil recovery (d) | 24 | 23.5 | .25 |
| 100-day survival (all patients) | 74% | 76% | > .99 |
| (95% CI, 66%-83%) | (95% CI, 66%-85%) |
| . | IL-1Ra . | Placebo . | P . |
|---|---|---|---|
| Hepatic veno-occlusive disease (%) | 29 | 27 | .74 |
| Median time to neutrophil recovery (d) | 24 | 23.5 | .25 |
| 100-day survival (all patients) | 74% | 76% | > .99 |
| (95% CI, 66%-83%) | (95% CI, 66%-85%) |
Discussion
The successful application of hematopoietic stem cell transplantation in the treatment of a spectrum of diseases of the blood and immune system, disorders of metabolism, and malignancies prompted efforts to increase the applicability of the procedure as well as increase its safety. A critical hurdle in the accomplishment of these goals has been GVHD. Although GVHD has been reasonably well-prevented and treated in young patients with histocompatible donors, it remains an impressive hurdle in older hematopoietic stem cell transplantation (HSCT) recipients and in patients receiving grafts from alternative donors. A rational approach to the prevention of GVHD depends on an understanding of the pathophysiology of this inflammatory disorder. While the role of T cells is no longer in question, limitations of T-cell depletion as well as calcineurin blockers in HSCT have prompted investigations of alternative approaches. The problems of T-cell depletion and the subsequent risk of relapse, poor immune reconstitution, lymphoproliferative disorders, and graft failure are well-described. We adopted an alternative approach based on the hypothesis that T cells inherently at rest at the time of infusion of the stem cell product would be subjected to a milieu that supports inflammation. The milieu reflects the presence of major and minor histocompatibility antigens in the context of inflammatory cytokines, adhesion molecules, growth factors, and all of the other cellular and humor mediators of inflammation. The infused T cells then may respond to the inflamed, injured host by proliferation, cytokine secretion, recruitment of other inflammatory mediators, and direct T-cell–mediated attack on host tissues. Thus, if this cycle of tissue injury, followed by T-cell recruitment and activation, followed by a sustained inflammatory response, could be interrupted, GVHD might be prevented. Extensive animal studies as well as prior clinical studies of cytokine inhibition with monoclonal antibodies to TNF, soluble IL-1 receptor, and IL-1Ra suggested that specific inhibition of IL-1 might be effective in GVHD prophylaxis.1,7,8,15 On the other hand, the beneficial effect was not seen in all models.16
A double-blind, randomized trial was performed to evaluate the role of IL-1 in the development of acute GVHD as well as other transplantation-related toxicities that might be due to dysregulated inflammatory cytokines. We chose IL-1Ra because of evidence both in murine models and a human phase 1/2 trial that it had efficacy in the therapy of GVHD, as well as efficacy in another inflammatory disorder, rheumatoid arthritis, where IL-1 has been implicated in the pathophysiology. In addition, it has the advantage of being a pure, competitive antagonist of IL-1α and IL-1β. It has no infusional toxicity, making a double-blind trial feasible.
There was no discernible benefit in any of the outcome measures evaluated in this study. All of the transplants engrafted in a similar time frame, and all of the patients developed similar degrees of transplantation-related toxicity, including mucositis, respiratory failure, veno-occlusive disease, and, most important, acute GVHD. There was no effect on survival. The lack of effect of IL-1Ra on engraftment of the marrow (24 days vs 23.5 days in the IL-1Ra and placebo groups, respectively) is important, because experimental data suggest that IL-1Ra is one of several cytokines that contribute to the control of hematopoiesis.8 9
We cannot definitively conclude that IL-1Ra cannot influence transplantation outcomes because it is possible that different schedules or dosing would give different results. Furthermore, the increase in TNFα levels despite the IL-1 blockade suggests that either IL-1 inhibition was insufficient or that combined therapy would be necessary to prevent transplantation complications. Because we do not have independent confirmation of a biologic effect of IL-1Ra, it is possible that the drug was discontinued too early and that additional length of treatment might have been of benefit.
Neutralization of TNFα has been shown to prevent acute GVHD in preclinical studies,10 and this approach is currently under evaluation in phase 2 trials. Administration of IL-1Ra did not modulate TNFα levels as it had in an earlier clinical trial,2 and it is possible that TNFα is the dominant of the 2 cytokines with respect to mediating acute GVHD. However, the absence of activity at a dose that produces drug levels during the transplantation period when cytokines appear to be dysregulated strongly suggests that blockade of IL-1 using IL-1Ra during conditioning and 10 days immediately after transplantation is not sufficient to reduce GVHD or toxicity, or to improve survival.
We thank Scott Bressler and Madeline Munoz for excellent technical assistance, as well as the numerous house staff and nurses who contributed to the care of these patients.
Prepublished online as Blood First Edition Paper, July 18, 2002; DOI 10.1182/blood-2002-03-0985.
Supported in part by a grant from the Food and Drug Administration (FD-R-001704).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joseph H. Antin, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail:jantin@partners.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal