Abstract
Fludarabine is a nonmyeloablative immunosuppressant increasingly used as a component of alternative conditioning regimens before allogeneic bone marrow transplantation. It is expected to reduce conditioning-related toxicity and proinflammatory activation of the host tissues. However, in our in vitro study, we provide evidence that 2-fluoroadenine 9-β-d-arabinofuranoside (F-Ara) as the active metabolized form of fludarabine damages human microvascular endothelial cells (HMECs) and dermal and alveolar epithelial cell lines after 48 hours of culture when it is used in pharmacologically relevant concentrations (range, 10 μg/mL-1 μg/mL). In addition, flow cytometric analyses revealed a significant up-regulation of intercellular adhesion molecule 1 and major histocompatibility complex (MHC) class I molecules by F-Ara, suggesting a proinflammatory activation of HMECs. Cytotoxicity assays demonstrated that target HMECs pretreated with F-Ara (10 μg/mL) showed increased lysis by allogeneic MHC class I-restricted cytotoxic T lymphocytes from healthy human donors. We conclude that, beside its immunosuppressive activities, F-Ara can be harmful for target tissues of transplantation-related complications and can even stimulate allogeneic immune responses. We identified the pharmaceutical compound defibrotide as protective against F-Ara– induced apoptosis and alloactivation, importantly, without affecting the antileukemic effect of F-Ara. This observation argues for a potential clinical usage of defibrotide in pretransplantation conditioning.
Introduction
Allogeneic stem cell transplantation (SCT) is a well-established method for the treatment of hematologic neoplasias and an increasing variety of other malignant disorders. SCT mainly consists of 2 sequential steps: pretransplantation conditioning, classically consisting of total body irradiation (TBI) and chemotherapy and leading to minimal residual disease and immunosuppression of the recipient, and transfer of allogeneic stem cells that should provide the cure. However, because of disparities in major histocompatibility complex (MHC) and minor histocompatibility antigen (mHAg), severe inflammatory reactions, including acute graft-versus-host disease (GVHD), can occur in different phases after transplantation. Based on studies from our group1 and from several other investigators,2,3 it is widely accepted that conditioning contributes through nonspecific inflammation to these transplantation-related complications. In addition, direct toxicity of TBI especially has been demonstrated.4,5 This has led to the investigation of a variety of alternative conditioning regimens. In addition, new pretransplantation therapies allow the extension of treatment protocols and patient selection. One compound of these novel conditioning concepts is fludarabine, a nonmyeloablative immunosuppressant that had originally been used for the treatment of chronic lymphatic leukemia.6 Fludarabine in combination with, for example, bis-chloro-nitroso urea (BCNU) and melphalan, cyclophosphamide, or other agents can replace TBI or is used together with dose-reduced TBI regimens.7,8 The clinical data obtained thus far argue for comparably low side effects and for hematopoietic and immune cell specificity of fludarabine.9However, the influence of this compound on nonhematopoietic cells, such as endothelial and epithelial cells, has not yet been investigated.
Virtually all transplantation-related complications are associated with endothelial dysfunction and damage.10 We and others11-14 have shown that the endothelium is a target of pretransplantation conditioning in vitro and in vivo. Ionizing radiation induces programmed cell death (apoptosis) in endothelial cells. At the same time, the endothelium is activated in terms of adhesion molecule expression, leading to increased leukocyte–endothelial interactions as a prerequisite for inflammatory processes.15,16 These effects are significantly enhanced by bacterial endotoxins (lipopolysaccharide) that might translocate through damaged mucosal barriers from the gastrointestinal tract.17 In addition, lipopolysaccharide has been shown to increase the antigenicity of endothelial cells toward allogeneic CD8+ cytotoxic T lymphocytes.18These observations prompted us to examine the effects of fludarabine on viability, activation, and allogenicity of human microvascular endothelial cells.
Materials and methods
Cell culture and reagents
The human dermal microvascular endothelial cell line CDC/EU.-HMEC-1 (HMECs) was kindly provided by the Centers for Disease Control and Prevention (Atlanta, GA) and has been established as previously described.19 HMECs were cultured in MCDB131 medium supplemented with 15% fetal calf serum (FCS), 1 μg/mL hydrocortisone (Sigma, Deisenhofen, Germany), 10 ng/mL epidermal growth factor (Collaborative Biochemical Products, Bedford, MA), and antibiotics. All cell culture reagents were purchased from Gibco BRL (Karlsruhe, Germany) unless stated otherwise. 2-Fluoroadenine 9-β-d-arabinofuranoside (F-Ara) was obtained from Sigma. Defibrotide vials were obtained from Prociclide (Crinos, Como, Italy).
Apoptosis assays
An established method for detecting apoptosis in human endothelial cells was performed as previously described20using flow cytometry (FACScan and CellQuest software (Becton Dickinson/Pharmingen, Heidelberg, Germany). Endothelial and epithelial cells were left untreated or were incubated with F-Ara in descending concentrations (range, 10 μg/mL to 0.1 μg/mL) in the presence or absence of defibrotide for 48 hours. Afterward, cells were washed in phosphate-buffered saline (PBS)–10% FCS and were stained with the necrosis-detecting dye propidium iodide (PI; 0.2 μg/mL; Sigma. Apoptotic cells were identified by PI-negative staining and by a characteristic side scatter (SSC) image distinct from that of nonapoptotic cells. At least 3 experiments per cell type were performed.
An alternative method for the detection of apoptosis involved microscopic analysis of DNA fluorescence-labeled cells. Endothelial cells (1 × 105/plate) were seeded in 35-mm Petri dishes (Nunc, Wiesbaden, Germany). These cells were treated as described above and subsequently were fixed with methanol–acetone 1:1 for 2 minutes, washed once in PBS, and stained with 4,6-diamidino-2-phenylindole (DAPI; 0.5 μg/mL; Sigma), and dissolved in 20% glycerin–PBS. Samples were mounted and subjected to microscopic analysis. Nuclear condensation as revealed by DAPI staining in the absence of trypan blue uptake is considered characteristic of apoptosis as opposed to necrosis.21 Quantitative analysis included counting the number of apoptotic cells relative to all identifiable cells from at least 10 microscopic fields, with an average of 70 cells per field. For the sake of the clarity of this paper, DAPI stain results are only displayed for the experiments with endothelial cells and HaCaT and with A 549 cells.
Cell surface analyses
Cell surface expression of intercellular adhesion molecule 1 (ICAM-1; Becton Dickinson/Pharmingen) and MHC class I (w6/32, hybridoma supernatant; American Tissue Culture Collection [ATCC], Manassas, VA) molecules on HMECs was assessed by the indirect immunofluorescence technique and subsequent flow cytometry using the FACScan flow cytometer and the CellQuest analysis program (Becton Dickinson/Pharmingen). Endothelial cells were treated as given and, after incubation, were harvested with trypsin–EDTA (Gibco), washed once in cold PBS–10% FCS, and incubated for 1 hour on ice with 5 μg/mL antiadhesion molecule monoclonal antibodies. Cells were washed again and were incubated with a goat anti-mouse IgG–fluorescein isothiocyanate (FITC)-conjugated antibody F(ab)2 fragment (DAKO, Hamburg, Germany) for 45 minutes on ice. Cells were then washed in PBS–10% FCS and were subjected to analysis. Viability of the cells was determined by concurrent PI (0.2 μg/mL; Sigma) staining. Omitting the first antibody served as a negative control to detect unspecific fluorescence. Using this approach instead of isotype control antibodies was justified by previous observations that endothelial cells lack Fc receptors.22 Therefore, a nonspecific binding of antibodies through their Fc portion could be excluded.
Allostimulation of peripheral blood cells with HMECs
Peripheral blood mononuclear cells (PBMCs) were derived from heparinized (Novo Nordisk, Mainz, Germany) blood of healthy human volunteers or from buffy coats supplied by the Bavarian Red Cross according to a standard protocol using Ficoll-Hypaque (Pharmacia, Freiburg, Germany) density-gradient centrifugation. Cells were then stimulated at a ratio of 1:1 or 2:1 with irradiated (20 Gy) HMECs for 7 days in the presence of interleukin-2 (IL-2; 50 U/mL) and 10% human AB serum (Sigma). Alternatively, PBMCs were selected for CD8+T cells and natural killer (NK) cells using cell isolation kits according to the manufacturer's instructions (Magnetic Cell Sorting [MACS]; Miltenyi Biotech, Bergisch-Gladbach, Germany) based on the deletion of non-CD8+ and non-NK cells, respectively. Stimulation of the selected cells was identical to that of whole PBMC cultures, except for NK cells, which were stimulated for only 3 days.
Cytotoxicity assay
T-cell– or NK-cell–mediated cytotoxicity was assessed according to a well-established protocol,23 using a 4-hour chromium Cr 51 radioisotope assay. HMECs that had been left untreated or were incubated with F-Ara (10 μg/mL) overnight were used as target cells, to be labeled 0.4 mCi (1.48 mBq) Na251CrO4 for 2 hours. After 3 washing steps, target cells were adjusted to 104 cells/mL and were coincubated with PBMCs, CD8+ cells, or NK effector cells at descending effector-to-target ratios for another 4 hours. Supernatants were transferred to dry scintillation plates and were counted in a γ-counter (all from Canberra Packard, Dreieich, Germany). Autologous (effector) B-lymphoblastoid cell lines (B-LCL) and K562 as NK-sensitive cells were taken as additional control targets. Percentage specific lysis was calculated as: [(experimental release − spontaneous release)/(maximal release − spontaneous release)] × 100. Spontaneous release in all experiments was always lower than 20%.
Enzyme-linked immunosorbent assays
Enzyme-linked immunosorbent assay (ELISA) for the detection of IL-4 (Tc2 response) and interferon γ (IFN-γ, Tc1 response), IL-1, and IL-10 in the supernatants of allogeneic effector T cells (see below) were performed exactly according to the manufacturer's instructions (R&D Systems, Minneapolis, MN).
Statistical analysis
The significance of differences between experimental values was assessed by means of the Student t test.
Results
F-Ara induces apoptosis in HMECs
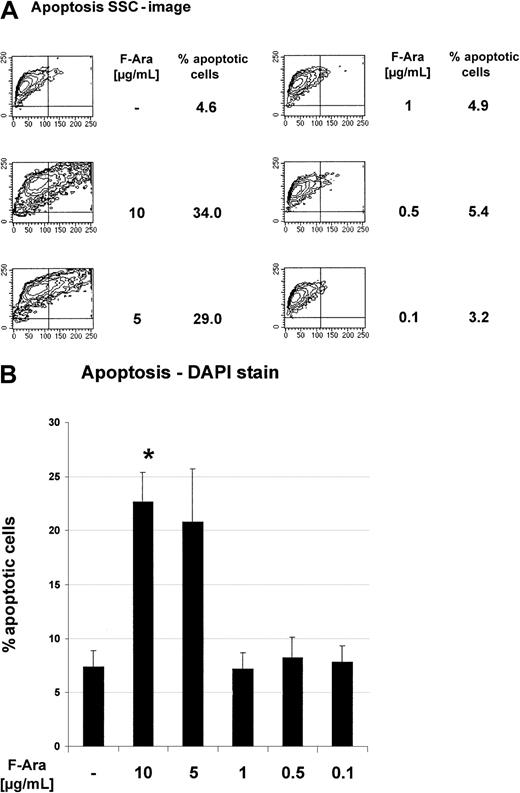
To assess the influence of F-Ara on the viability of cultured human endothelial cells, HMECs were incubated with descending pharmacologically relevant concentrations (10 μg/mL-0.1 μg/mL) of F-Ara as the metabolized form of fludarabine. The median intracellular level of the active (cytotoxic) fludarabine triphosphate in target cells is 20 μM, representing a concentration 5.8 μg/mL (Medac Schering, Hamburg, Germany; manufacturer's instructions). After 48 hours of incubation, HMECs were subjected to apoptosis assays using the detection of cellular granularity of PI-negative cells (SSC image in flow cytometry) and microscopic analyses of DAPI-stained cells, respectively. Independent of the assay system, Figure1A-B clearly demonstrates that F-Ara caused apoptosis in HMECs in concentrations of 10 and 5 μg/mL, whereas 1 μg/mL was no longer effective. The critical threshold of the cytotoxicity of F-Ara was between 2 and 3 μg/mL. Apoptosis by F-Ara was already detectable after 24 hours, although to a lesser extent (data not shown).
Fludarabine induces programmed cell death in HMECs.
HMECs were left untreated or were incubated with F-Ara in descending concentrations for 48 hours and were subjected to flow cytometric analysis (A) or microscopic DAPI stain analysis. (A) Contour plots of the SSC image (x-axis) of PI-negative cells plotted against the forward scatter image (y-axis) as a parameter for cellular granularity versus cell size. (B) Quantitative fluorescence microscopy analysis of DAPI-stained endothelial cells. Results are given in percentage apoptotic HMECs (% apoptotic cells) ± SD (out of n = 10 microscopic fields with an average of 70 cells per field). Representatives of at least 5 independent experiments are shown. *P < .001 of untreated control versus F-Ara (10 μg/mL)–treated cells.
Fludarabine induces programmed cell death in HMECs.
HMECs were left untreated or were incubated with F-Ara in descending concentrations for 48 hours and were subjected to flow cytometric analysis (A) or microscopic DAPI stain analysis. (A) Contour plots of the SSC image (x-axis) of PI-negative cells plotted against the forward scatter image (y-axis) as a parameter for cellular granularity versus cell size. (B) Quantitative fluorescence microscopy analysis of DAPI-stained endothelial cells. Results are given in percentage apoptotic HMECs (% apoptotic cells) ± SD (out of n = 10 microscopic fields with an average of 70 cells per field). Representatives of at least 5 independent experiments are shown. *P < .001 of untreated control versus F-Ara (10 μg/mL)–treated cells.
Defibrotide protects HMECs from the F-Ara–induced apoptosis
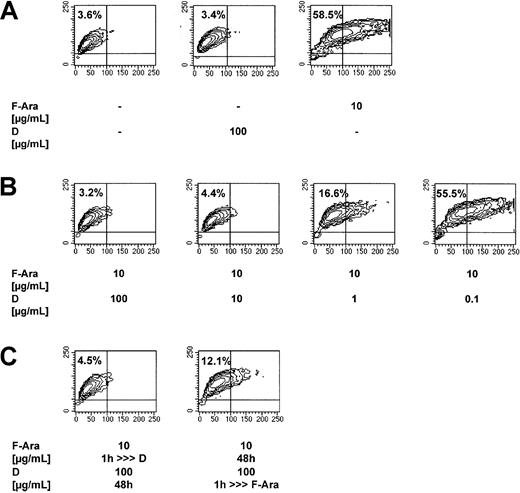
HMECs had either been left untreated or were treated with F-Ara in the presence or absence of varying concentrations of defibrotide (100 μg/mL-0.1 μg/mL) and were assessed for programmed cell death after 48 hours using flow cytometric analyses of the SSC image as described for Figure 1A. Figure 2A (mid-contour plot) shows that defibrotide alone, as a second control, did not influence endothelial cell viability. The apoptotic effect of F-Ara is reproduced in Figure 2A (right contour plot), whereas Figure 2B shows a dose-dependent protection of F-Ara–induced cell death by defibrotide. To exclude unspecific artifical extracellular interaction of F-Ara and defibrotide in vitro, HMECs were pretreated with defibrotide for 1 hour and subsequently, after 3 washing steps, were incubated with F-Ara for another 48 hours and vice versa. Figure 2C (right contour plot) revealed that pretreatment of HMECs for 1 hour was sufficient to protect cells from F-Ara–induced apoptosis. Similarly, pretreatment of HMEC with F-Ara for 1 hour (Figure 2C, left contour plot) and subsequent incubation with defibrotide did not lead to endothelial programmed cell death.
Defibrotide (D) inhibits F-Ara–induced apoptosis in HMECs.
Evidence for intracellular antagonism. F-Ara, 10 μg/mL. D, 100 μg/mL. Flow cytometric analysis of the SSC image of PI-negative cells. (A) Reproducible induction of apoptosis by F-Ara. (B) Dose-dependent inhibition of F-Ara–induced apoptosis by D. (C) (left plot) Incubation of HMECs with F-Ara for 1 hour; subsequent incubation with D for 48 hours after washing. (right plot) Incubation of HMECs with D for 1 hour; subsequent incubation with F-Ara for 48 hours after washing. For experimental details, see legend to Figure 1 and “Materials and methods.” Shown is 1 of 3 representative independent experiments.
Defibrotide (D) inhibits F-Ara–induced apoptosis in HMECs.
Evidence for intracellular antagonism. F-Ara, 10 μg/mL. D, 100 μg/mL. Flow cytometric analysis of the SSC image of PI-negative cells. (A) Reproducible induction of apoptosis by F-Ara. (B) Dose-dependent inhibition of F-Ara–induced apoptosis by D. (C) (left plot) Incubation of HMECs with F-Ara for 1 hour; subsequent incubation with D for 48 hours after washing. (right plot) Incubation of HMECs with D for 1 hour; subsequent incubation with F-Ara for 48 hours after washing. For experimental details, see legend to Figure 1 and “Materials and methods.” Shown is 1 of 3 representative independent experiments.
Effect of F-Ara on different epithelial cell lines: protective effect of defibrotide
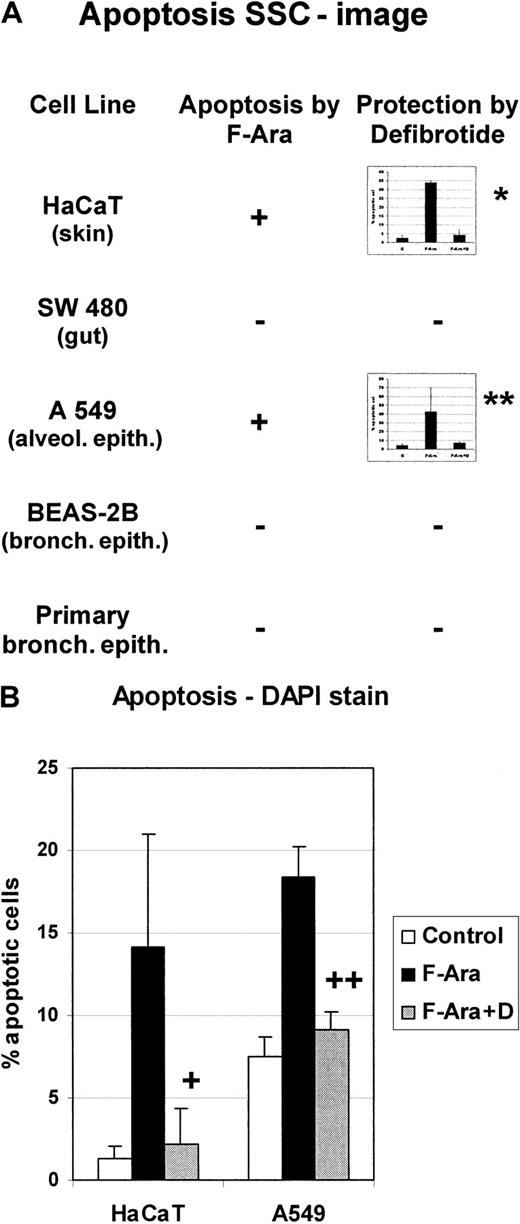
Skin, the gastrointestinal tract (GIT), and most likely the lung are among the primary targets of GVHD. Therefore, it was reasonable to test the influence of F-Ara on cell lines derived from these organs. Cells from keratinocyte (HaCaT), GIT (SW 480), alveolar (A549) cells lines, bronchial epithelial (BEAS-2B) cell lines, and primary bronchial epithelial cells were incubated with F-Ara (10 μg/mL) as for Figures1 and 2 and assayed in flow cytometric apoptosis analyses 48 hours after treatment. Figure 3A summarizes that gut and bronchial epithelial cells appeared to be resistant to the apoptotic stimuli of F-Ara, whereas keratinocytes (HaCaT) and alveolar epithelial cells (A549) showed signs of apoptosis, as determined by flow cytometry of the SSC image (34.0% ± 1.0% apoptotic cells for HaCaT and 42.9% ± 26.7% for A549, respectively). Again, the protective potential of defibrotide (100 μg/mL) was assessed. HaCaT (4.3% ± 3.0%) and A549 (5.4% ± 2.9%) cells were completely protected from programmed cell death after cotreatment with F-Ara and defibrotide (Figure 3A, inset bar graphs). To confirm these results, DAPI-stain apoptosis assays were performed for HaCAT (Figure 3B, left columns) and A549 cells (Figure 3B, right columns). As shown for endothelial cells, defibrotide alone did not influence the number of apoptotic cells in either cell line (data not shown).
F-Ara induces apoptosis in keratinocytes and alveolar epithelial cells, but not in gut or bronchial epithelial cells: protective effect of defibrotide.
F-Ara, 10 μg/mL. D, 100 μg/mL. Flow cytometric analysis of the SSC image of PI-negative cells (A) and DAPI stain analysis of apoptotic cells (B). Results are given in mean percentage apoptotic cells ± SD of 3 different experiments. HaCaT, human keratinocyte cell line; SW 480, gut epithelial cells line; A549, lung carcinoma cell line from the alveolar epithelium; BEAS-2B, bronchial epithelial cell line. Primary bronchial epithelial cells have been derived from a bronchoscopic brush procedure. (A) *P = .005 of F-Ara versus F-Ara+D-treated HaCaT cells. **P = .116 of F-Ara versus F-Ara+D-treated A 549 cells. −: No apoptosis induction. (B) +: P = .026 of F-Ara versus F-Ara+D-treated HaCaT cells. ++: P = .001 of F-Ara versus F-Ara+D-treated A549 cells. For experimental details, see the legend to Figure 1 and “Materials and methods.” Three representative experiments are summarized for each cell line.
F-Ara induces apoptosis in keratinocytes and alveolar epithelial cells, but not in gut or bronchial epithelial cells: protective effect of defibrotide.
F-Ara, 10 μg/mL. D, 100 μg/mL. Flow cytometric analysis of the SSC image of PI-negative cells (A) and DAPI stain analysis of apoptotic cells (B). Results are given in mean percentage apoptotic cells ± SD of 3 different experiments. HaCaT, human keratinocyte cell line; SW 480, gut epithelial cells line; A549, lung carcinoma cell line from the alveolar epithelium; BEAS-2B, bronchial epithelial cell line. Primary bronchial epithelial cells have been derived from a bronchoscopic brush procedure. (A) *P = .005 of F-Ara versus F-Ara+D-treated HaCaT cells. **P = .116 of F-Ara versus F-Ara+D-treated A 549 cells. −: No apoptosis induction. (B) +: P = .026 of F-Ara versus F-Ara+D-treated HaCaT cells. ++: P = .001 of F-Ara versus F-Ara+D-treated A549 cells. For experimental details, see the legend to Figure 1 and “Materials and methods.” Three representative experiments are summarized for each cell line.
Defibrotide does not interfere with the antileukemic and anti-PBMC effects of F-Ara
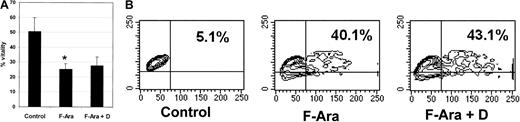
Next to its desirable protective capacity for endothelial and epithelial cells against F-Ara–induced apoptosis, it was important to investigate whether defibrotide would also interfere with the antileukemic properties of F-Ara. To address this question, primary peripheral blood–derived acute myeloid leukemic (AML) cells with a blast amount of 70% were thawed, kept in culture for 24 hours, and subsequently treated with F-Ara in the presence or absence of defibrotide for another 48 hours. Figure4A demonstrates that already almost 50% of the cells died spontaneously of necrotic cell death. However, F-Ara induced cell death in up to 80% of the cells. In contrast to its effect on endothelial and epithelial cells, defibrotide was unable to protect AML cells from F-Ara–mediated toxicity. It is of note that Figure 4A describes percentage vitality of the cells, not percentage apoptotic cells, because F-Ara directly caused necrosis rather than apoptosis in AML cells. This could be observed as early as 24 hours of incubation. Still, Figure 4A clearly shows that defibrotide does not interfere with the desirable toxicity of F-Ara against leukemic cells. We next asked whether defibrotide might modulate the effect of F-Ara against normal hematopoietic cells and performed apoptosis assays (SSC image) with PBMCs from healthy human blood donors. As could be learned from a representative experiment depicted in Figure 4B, F-Ara induced apoptosis in 40.1% of the cells compared with 5.1% apoptotic cells in the untreated control. Again, defibrotide did not interfere with the apoptotic activity of F-Ara against PBMCs (43.1% apoptotic cells), suggesting that the immunosuppressive properties of F-Ara are not harmed by cotreatment with defibrotide.
Defibrotide does not interfere with the antileukemic and the anti-PBMC effects of F-Ara.
F-Ara, 10 μg/mL. D, 100 μg/mL. (A) PI staining of primary AML cells derived from a patient in blast crisis (70% blasts of total PBMC count). Results are given in mean percentage vitality of 3 independent experiments. *P = .008 of control versus F-Ara–treated AML cells. (B) Flow cytometric analysis of the SSC image of PI-negative PBMCs. Shown is 1 of 5 independent experiments with different blood donors.
Defibrotide does not interfere with the antileukemic and the anti-PBMC effects of F-Ara.
F-Ara, 10 μg/mL. D, 100 μg/mL. (A) PI staining of primary AML cells derived from a patient in blast crisis (70% blasts of total PBMC count). Results are given in mean percentage vitality of 3 independent experiments. *P = .008 of control versus F-Ara–treated AML cells. (B) Flow cytometric analysis of the SSC image of PI-negative PBMCs. Shown is 1 of 5 independent experiments with different blood donors.
F-Ara up-regulates ICAM-1 on HMECs with antagonistic effects of defibrotide
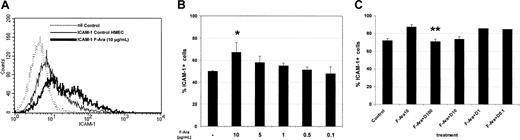
Based on previous observations that pretransplantation conditioning not only damages but also leads to proinflammatory activation of endothelial cells in terms of adhesion molecule induction,15 we next investigated the expression of ICAM-1 under the influence of F-Ara. As depicted in Figure5A-B, flow cytometric analyses demonstrated that F-Ara, after 24 hours of incubation, significantly enhances the expression on HMECs in a dose-dependent manner, similar to that observed for apoptosis induction. Concentrations as small as 1 μg/mL F-Ara were effective in inducing ICAM-1. We next asked whether defibrotide would also be functional as an antagonist of F-Ara in this experimental setting. HMECs were treated with F-Ara, as given and incubated in the presence or absence of descending concentrations of defibrotide. Figure 5C summarizes 3 independent experiments showing that defibrotide in fact antagonized F-Ara–induced ICAM-1 expression in concentrations of 100 μg/mL and 10 μg/mL. Defibrotide alone did not activate endothelial cells, and ICAM-1 expression remained unchanged with every concentration tested (data not shown).
F-Ara induces ICAM-1 expression on HMECs: protective effect of defibrotide.
Flow cytometric analysis of ICAM-1–positive cells. HMECs were either left untreated or were incubated with F-Ara (10 μg/mL or descending concentrations in B) in the presence or absence of descending concentrations of D. (A) Histogram plot of ICAM-1 expression from a representative experiment. (dotted line) Background staining (nil Control). (thin line) ICAM-1 expression of untreated control cells. (thick line) ICAM-1 expression of F-Ara–treated cells. (B) Dose-dependent induction of ICAM-1 expression by F-Ara. Summary of 3 independent experiments. Results are given as mean percentage ICAM-1–positive cells ± SD. *P = .075 of F-Ara versus untreated control cells. (C) Dose-dependent inhibition of F-Ara–induced ICAM-1 expression by defibrotide (D). Results are given as mean percentage ICAM-1–positive cells ± SD. **P = .004 of F-Ara versus F-Ara+D-treated HMECs.
F-Ara induces ICAM-1 expression on HMECs: protective effect of defibrotide.
Flow cytometric analysis of ICAM-1–positive cells. HMECs were either left untreated or were incubated with F-Ara (10 μg/mL or descending concentrations in B) in the presence or absence of descending concentrations of D. (A) Histogram plot of ICAM-1 expression from a representative experiment. (dotted line) Background staining (nil Control). (thin line) ICAM-1 expression of untreated control cells. (thick line) ICAM-1 expression of F-Ara–treated cells. (B) Dose-dependent induction of ICAM-1 expression by F-Ara. Summary of 3 independent experiments. Results are given as mean percentage ICAM-1–positive cells ± SD. *P = .075 of F-Ara versus untreated control cells. (C) Dose-dependent inhibition of F-Ara–induced ICAM-1 expression by defibrotide (D). Results are given as mean percentage ICAM-1–positive cells ± SD. **P = .004 of F-Ara versus F-Ara+D-treated HMECs.
Because a proinflammatory activation of target cells is often associated with increased expression of MHC class I and class II, we conducted further flow cytometric analyses for these antigens after incubation with F-Ara in various concentrations for 24 hours. Despite its well-described immunosuppressive properties, F-Ara surprisingly induced MHC class I molecules on HMECs dose dependently (1.5-fold induction of mean fluorescence intensity at 10 μg/mL, 1.3-fold induction at 5 μg/mL), whereas MHC class II remained unchanged (data not shown).
F-Ara increases the antigenicity of endothelial cells toward allogeneic peripheral blood cells: protection by defibrotide
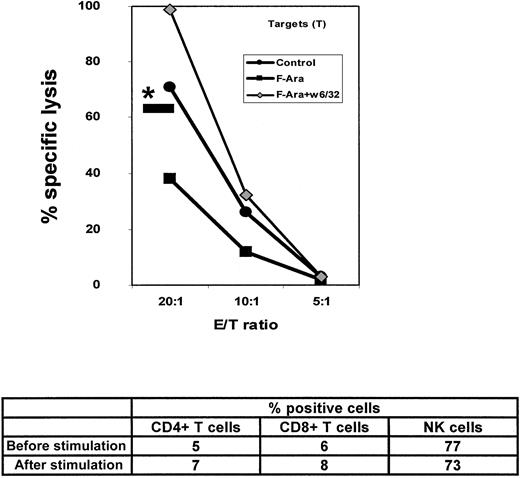
The induction of MHC class I molecules on HMECs by F-Ara prompted us to examine whether F-Ara would also enhance the capacity of HMECs to stimulate allocytotoxic responses. PBMCs as effectors were either derived from heparinized blood of healthy human volunteers of from buffy coat preparations, stimulated with irradiated (20 Gy) HMECs in the presence of 50 U/mL IL-2 for 7 days, and subsequently subjected to a standard 51Cr release assay (for details, see “Materials and methods”). At day −1, fresh HMECs as targets were either left unstimulated or were incubated with F-Ara (10 μg/mL) in the presence or absence of an anti-MHC class I neutralizing antibody (w6/32). Autologous effector Epstein-Barr virus–transformed B-LCL and K562 cells as classical NK cell targets served as controls. Figure6A demonstrates that F-Ara indeed increased the antigenicity of HMECs toward allogeneic PBMCs at all effector: target (E/T) ratios tested. The lack of specific lysis of K562 and autologous effector B-LCLs verified the involvement of MHC-restricted cytotoxic T lymphocytes (CTLs). In addition, lysis of either untreated or F-Ara–treated HMECs could almost fully be blocked after coincubation of these cells with the anti–MHC class I antibody w6/32 (Figure 6A, *—). To further confirm that CD8+ CTLs were responsible for the antiendothelial cytotoxic activity, PBMCs were selected for CD8+ and CD4+ T cells (non-CD8– and non-CD4–depleted PBMCs, respectively) using magnetic bead separation with MACS bead kits. Purity of the preparations was 93% or more in all cases with a complete absence of the other cell populations (not shown). Separated T cells were stimulated with HMECs and IL-2 exactly as described for unselected PBMCs (see above). As shown in Figure 6B, lysis of F-Ara–treated HMECs by CD8+ CTLs was, again, significantly higher than that of control HMECs. Furthermore, pretreatment of target HMECs with F-Ara and defibrotide (F-Ara+D) down-regulated specific lysis even below control levels, suggesting that defibrotide also protects endothelial cells against the lysis of allogeneic effector lymphocytes. HMEC-stimulated CD4+ T cells did not show any signs of cytotoxic activity in this experimental setting (data not shown). Flow cytometric analyses of F-Ara versus F-Ara+D–treated HMECs resulted in a significant down-regulation of MHC class I molecules by defibrotide, suggesting that MHC class I expression is the critical element in regulating the cytotoxic response induced by F-Ara (data not shown).
F-Ara increases the allogenicity of HMECs for CD8+ CTLs: protective effect of defibrotide.
(A) PBMCs were stimulated with irradiated HMECs in the presence of IL-2 (50 U/mL) for 7 days and were subjected to a 51Cr release assay with untreated (Control) and F-Ara (10 μg/mL)–treated HMECs (24-hour incubation) as target cells. Autolog B-LCL, autologous (effector) Epstein-Barr virus (EBV)–transformed B-lymphoblastoid cells; K 562, target cells for NK cells. Results are given as percentage specific lysis as described in “Materials and methods.” *— : percentage specific lysis of F-Ara–treated HMECs in the presence of anti-MHC class I antibody w6/32. E/T ratio: effector–target ratio. (B) Down-regulation of F-Ara–induced allogenicity of HMECs toward CD8+ CTLs by defibrotide (D). CD8+ PBMCs have been negatively selected (non-CD8+-cell–depleted) by magnetic bead separation. For experimental details, see legend to Figure 6A.
F-Ara increases the allogenicity of HMECs for CD8+ CTLs: protective effect of defibrotide.
(A) PBMCs were stimulated with irradiated HMECs in the presence of IL-2 (50 U/mL) for 7 days and were subjected to a 51Cr release assay with untreated (Control) and F-Ara (10 μg/mL)–treated HMECs (24-hour incubation) as target cells. Autolog B-LCL, autologous (effector) Epstein-Barr virus (EBV)–transformed B-lymphoblastoid cells; K 562, target cells for NK cells. Results are given as percentage specific lysis as described in “Materials and methods.” *— : percentage specific lysis of F-Ara–treated HMECs in the presence of anti-MHC class I antibody w6/32. E/T ratio: effector–target ratio. (B) Down-regulation of F-Ara–induced allogenicity of HMECs toward CD8+ CTLs by defibrotide (D). CD8+ PBMCs have been negatively selected (non-CD8+-cell–depleted) by magnetic bead separation. For experimental details, see legend to Figure 6A.
Antiendothelial CTLs display a Tc1-like phenotype
To gain information about the nature of antiendothelial CTLs, PBMCs and CD8+ T cells were stimulated as given above, and supernatants were collected for the assessment of IFN-γ and IL-4 using ELISA analyses. As depicted in Table1, stimulation with HMECs and IL-2 obviously led to the outgrowth of Tc1-like T cells, as could be told from the unique expression of IFN-γ, whereas no IL-4 was produced.
Antiendothelial CTLs elicit a Tc1-like phenotype
| Effector . | IFN-γ . | IL-4 . |
|---|---|---|
| PBMC | 319 (± 176) | 0 |
| CD8+ | 524 (± 174) | 0 |
| Effector . | IFN-γ . | IL-4 . |
|---|---|---|
| PBMC | 319 (± 176) | 0 |
| CD8+ | 524 (± 174) | 0 |
ELISA for the production of IFN-γ and IL-4 in the supernatants of stimulated effector cells (7 days, irradiated HMECs, 50 U/mL IL-2). PBMCs were left unseparated or were negatively selected for CD8+ T cells as given for the experiments in Figure 6. Results are given as mean pg/mL cytokine ± SD of 3 independent experiments.
F-Ara down-regulates lysis of HMECs by allogeneic NK cells
Another interesting question was how F-Ara–induced modulations of MHC class I expression affects the cytolytic response of NK cells against endothelial cells. PBMCs from healthy persons were negatively selected for NK cells (non-NK–cell depleted) and were stimulated for 4 days with irradiated HMECs in the presence of IL-2, as it was described for the experiment in Figure 6B. At day 4, HMECs as target cells were left untreated or were incubated with F-Ara (10 μg/mL) for 24 hours and subjected to a standard 51Cr release assay with the stimulated NK cells as effectors. Figure7 demonstrates that F-Ara significantly down-regulated the allogenicity of HMEC toward NK cells. As a positive control for NK cell activity, lysis of MHC class I–negative K562 cells could be observed (Figure 7, *—). Pretreatment of F-Ara–stimulated HMECs with the anti-MHC class I antibody w6/32 completely abrogated the effect of F-Ara and led to almost 100% specific lysis of HMECs (Figure7), suggesting that MHC class I on the surface of HMECs is, again, the critical switch for the regulation of the cytotoxic response of NK cells. The role of killer cell inhibitory receptors, found to be negatively regulated by high expression levels of MHC class I molecules,24 might be responsible for the decreased cytolytic response of NK cells.
F-Ara decreases the allogenicity of HMECs for NK cells: enhancement of lysis by blockade of MHC class I.
NK cells have been negatively selected (non-NK-cell–depleted) by magnetic bead separation and stimulated with irradiated HMECs in the presence of IL-2 (50 U/mL) for 4 days and subsequently subjected to a51Cr release assay as described for Figure 6. The data below the graph shows flow cytometric analysis of the effector cell population before and after stimulation with HMECs. NK cells were characterized as CD3−/CD16+CD56+. *—: percentage specific lysis of K 562 cells at an E/T ratio of 20:1.
F-Ara decreases the allogenicity of HMECs for NK cells: enhancement of lysis by blockade of MHC class I.
NK cells have been negatively selected (non-NK-cell–depleted) by magnetic bead separation and stimulated with irradiated HMECs in the presence of IL-2 (50 U/mL) for 4 days and subsequently subjected to a51Cr release assay as described for Figure 6. The data below the graph shows flow cytometric analysis of the effector cell population before and after stimulation with HMECs. NK cells were characterized as CD3−/CD16+CD56+. *—: percentage specific lysis of K 562 cells at an E/T ratio of 20:1.
Discussion
Clinical results with fludarabine-containing reduced-intensity conditioning regimens obtained so far show a clear down-regulation of conditioning-related toxicity without affecting immune reconstitution.25 The incidence of acute GVHD in patients receiving reduced-intensity conditioning is comparable or even less than that in patients receiving the classical conditioning regimen.26 However, equally severe or even increased late effects such as osteonecrosis (E. H., personal communication, June 2001), pulmonal complications,27 and more cases of chronic GVHD arise.28 Despite its well-documented immunosuppressive properties, fludarabine, in our study, activated and damaged endothelial and epithelial cells. This observation might, at least in part, explain the undesired clinical side effects described above because osteonecrosis is an expression of endothelial dysfunction, and fludarabine appears to be toxic for alveolar epithelial cells. It is interesting to note that the harmful effects of fludarabine on lung cells seem to be compartment specific—bronchial epithelial cells did not undergo apoptosis in response to this immunosuppressant. The fact that a keratinocyte cell line (HaCaT) was also sensitive to fludarabine suggests that it might also be involved in cutaneous disorders after SCT. Given that the pathogenesis of late complications is multifactorial and might also be influenced by increasing age of the patients undergoing SCT and the use of peripheral stem cells, further evaluation in clinical analyses of pulmonal and dermatologic complications is needed.
In many pretransplantation protocols fludarabine is used in combination with ionizing radiation, so it was important to test whether these 2 compounds would cooperate in affecting endothelial cells. Interestingly, we could not find any enhancement of radiation-induced cell death by fludarabine or vice versa (data not shown). This suggests differential mechanisms of how the apoptotic death signal is transferred to endothelial cells. In addition, it remains to be investigated how far other conditioning agents such as cyclophosphamide, BCNU, or melphalan affect the viability and allogenicity of endothelial cells alone or in combination with fludarabine. This is subject to ongoing studies in our laboratory.
The precise mechanism regarding how fludarabine induces apoptosis in endothelial and epithelial cells remains to be elucidated. It is likely that fludarabine, as a purin analogue, integrates into the DNA and thus causes mutations that lead to gene deletion like those reported previously.29 It has also been suggested that fludarabine can cooperate with cytochrome c and apoptosis protein-activating factor-1 in triggering the apoptotic caspase pathway.30
Fludarabine increases the allogenicity of endothelial cell targets for CD8+ T cells. In contrast, fludarabine significantly down-modulates endothelial lysis by allogeneic NK cells. MHC class I expression seems to be critical for the regulation of any of these immune responses because a blockade of class 1 fully abrogated CTL lysis and tremendously up-regulated lysis by NK cells. These opposing effects of fludarabine, taken together with the clinical observation that fludarabine shows less acute and equal or even more chronic toxicity than the classical conditioning regimen, raises the speculation that NK cells and CTLs might be active in different phases of GVHD pathophysiology—that is, NK cells would primarily act in the earlier phase (suppressed by fludarabine) and CTLs would act in the later phase (enhanced by fludarabine) after transplantation. It is uncertain whether this holds true in clinical practice.
With regard to the nature of antiendothelial CTLs, it is an interesting question whether these CTLs are endothelial-specific or simply allo-specific. The existence of endothelial-specific effector lymphocytes has been described.31 In contrast to the CTLs we characterized as displaying a Tc1-like phenotype, many of the CTL clones reported show little, if any, IFN-γ and unusually express CD40 ligand at rest that might enhance cytolytic activity.32 These data do not rule out the existence of additional allogeneic CTLs with a specificity for nonhematopoietic targets.
Defibrotide is a well-tolerated drug that is successfully used for the treatment of veno-occlusive disease as one major hepatic complication after SCT.33 In addition, an increasing number of preclinical and clinical reports show its efficacy in treating ischemia–reperfusion injury, atherosclerosis, and recurrent thrombotic thrombocytopenic purpura.34-36 Defibrotide is known to act directly on endothelial cells without the requirement for further metabolism37 and could, therefore, be used in our in vitro studies. Defibrotide fully protected endothelial and epithelial cells from fludarabine-mediated apoptosis. Additional experimentation is needed to assess the precise mechanism of protection by which defibrotide antagonizes fludarabine, but one can imagine a role for defibrotide in the inhibition of DNA integration of fludarabine or the aforementioned caspase activation. Besides its antiapoptotic effects, defibrotide was able to down-regulate antiendothelial CTL responses by regulating MHC class I expression. In contrast, defibrotide did not affect the desirable antileukemic effect of fludarabine, as shown by the lack of protection of AML cells. This promising result still has to be confirmed by testing other hematologic malignancies to exclude that defibrotide does not interfere with the antitumor response. However, another important observation was that defibrotide could not block fludarabine-mediated apoptosis of PBMC. This suggests that the immunosuppressant effect of fludarabine mandatory for conditioning would not be influenced by cotreatment with defibrotide.
Defibrotide did not protect against radiation-induced endothelial cell damage, suggesting that its effect was specific for fludarabine-mediated cellular changes (data not shown).
Based on these results and with respect to its few, if any, side effects,38 we conclude that defibrotide might be a good candidate used in combination with fludarabine during conditioning before SCT, especially in patients at risk for veno-occlusive disease. Studies analyzing endothelial protection against further conditioning agents should help clarify whether defibrotide can be used as a broad protective agent.
We thank Drs Christian Schulz and Petra Smyczek for providing primary bronchial epithelial cells and the cell lines A549 and BEAS-2B, Dr Klaus Schlottmann for providing SW 480 cells, and Karin Fischer for providing primary AML cells.
Supported by grants Ei68/1-1 and 2-1-2 from the Deutsche Forschungsgemeinschaft.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Günther Eissner, Department of Hematology and Oncology, University of Regensburg, Franz-Josef-Strauss-Allee 11, D-93053 Regensburg, Germany; e-mail:guenther.eissner@klinik.uni-regensburg.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal