Abstract
Cellular prion protein (PrPc) is a glycosylphosphatidylinositol (GPI)-anchored membrane glycoprotein that contains a putative membrane-spanning section. Patients with paroxysmal nocturnal hemoglobinuria (PNH) lack GPI proteins on the surface of somatically mutated hematopoietic stem cell and its progeny. Platelet expression of PrPc was studied in 8 PNH patients. Resting PNH (CD55−) platelets were devoid of surface PrPc, but activation of platelets resulted in the surface expression of PrPc. Expressed PrPc was detected by 2 monoclonal antibodies (mAbs) against the N-terminal part of the molecule but not by mAb 6H4, which binds at the C-terminus beyond the membrane-spanning section. However, 6H4 detected PrPc on Western blots of PNH platelets, demonstrating that the lack of 6H4 binding was not caused by PrPc truncation. Our results indicate that in the absence of GPI anchor, PrPc can be expressed intracellularly and up-regulated on the platelet membrane, likely in a transmembrane form with the C-terminal part of the molecule inserted into the cytoplasm.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a clonal disorder of hematopoietic cells caused by the cells' inability to synthesize glycosylphosphatidylinositol (GPI) anchor, and it is manifested by a lack of the GPI-anchored membrane proteins on a portion of peripheral blood cells.1,2 Various GPI-anchored proteins are missing from the surfaces of PNH platelets,3,4 and their deficiency may be implicated in the increased tendency of PNH patients to venous thrombosis.5,6 Cellular prion protein (PrPc) is a GPI-anchored membrane glycoprotein expressed on many cell types, including neurons, mononuclear leukocytes, and platelets.7,8 Recently, the decreased expression of PrPc was demonstrated on peripheral blood leukocytes of PNH patients.9 The physiological function of PrPc remains largely unknown10; however, its pathologic conformer plays a key role in neurodegenerative prion diseases.7
The primary structure of PrPc contains a putative membrane-spanning domain (residues, approximately110-135) that does not seem to be used in normal cells.10 However, the presence of transmembrane PrPc has been linked to neurodegeneration in Gerstmann-Straussler-Scheinker syndrome,11 an inherited form of prion disease, and transmembrane forms of PrPc have been reported in cultured cells expressing mutated PrPc.12,13Transmembrane PrPc can exist in 2 topologic variants: either with the C-terminus (CtmPrP) or with the N-terminus (NtmPrP) outside the cell membrane.11
We have previously demonstrated that normal human platelets contain an intracellular pool of PrPc, which is up-regulated on the plasma membrane after platelet activation.14 This up-regulated PrPc can be detected by monoclonal antibodies directed against the C-terminal and the N-terminal parts of the PrPc molecule. The aim of the present study was to investigate the expression of PrPc in platelets of patients with PNH.
Study design
Materials
The expression of PrPc on platelets of 8 PNH patients was studied and compared with the expression of PrPc on healthy donor platelets. Informed consent was obtained according to protocols approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute. PNH was diagnosed by a clinical history of hemolysis or marrow failure and by the presence of a GPI-anchored, protein-deficient clonal population of granulocytes in peripheral blood. Simultaneous absence of 2 GPI-anchored proteins, CD66b and CD16, was used to determine the abnormal phenotype.15 Five patients fulfilled criteria for primary hemolytic PNH; the other 3 were classified as having aplastic anemia/PNH syndrome.16 Blood was collected 9:1 into 3.8% sodium citrate and processed within 2 hours. Platelet-rich plasma was prepared, and platelets were activated with TRAP as described previously.14
Flow cytometry and Western blot analysis
Two-color flow cytometry on a FACScan flow cytometer (Becton Dickinson, San Jose, CA) equipped with CELLQuest software (Becton Dickinson, San Jose, CA) was used. Phycoerythrin-conjugated monoclonal antibody (mAb) CD55 (Chemicon International, Temecula, CA) was used to distinguish between a CD55− PNH clone of platelets lacking GPI proteins and normal CD55+ positive patient platelets. Monoclonal antibodies FH11 (Institute for Animal Health, Compton, England; donated by Dr C. R. Birkett), 1562 (Chemicon International), and 6H4 (Prionics AG, Zurich, Switzerland) were used for the detection of PrPc. Monoclonal antibodies were fluorescein conjugated, and their effective fluorescein–protein ratio was estimated as described previously.17 Binding of anti-PrPc mAbs to CD55− and CD55+ platelets was evaluated in FL1 versus FL2 fluorescence logarithmic plot. To allow direct comparison of the mAb binding, the geometric mean of measured platelet fluorescence was converted into relative fluorescence intensity (RFI).18 Results are presented after the subtraction of the platelet autofluorescence and corrected for fluorescein–protein ratio of the individual antibodies. Western blot analysis of PNH platelets with mAb 6H4 was performed as described previously.17
Results and discussion
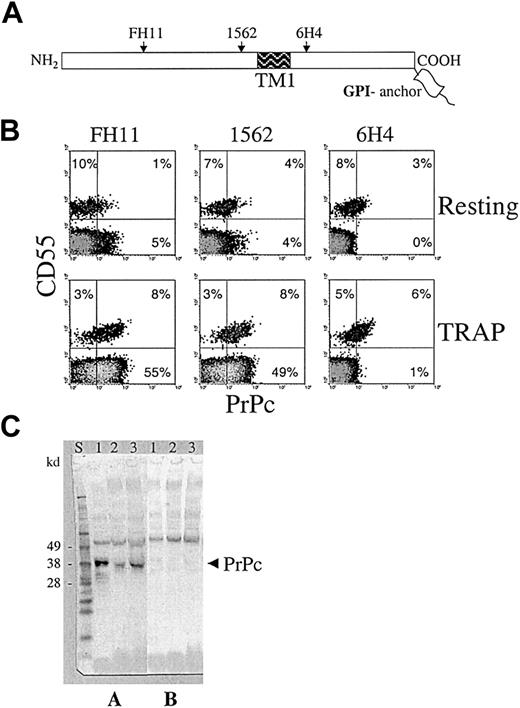
Monoclonal antibody CD55 distinguished a CD55− PNH clone of platelets lacking GPI proteins from normal CD55+patient platelets. All patients studied exhibited a bimodal distribution with type 1 (normal) and type 3 (complete deficiency) platelet populations.19 The size of the platelet PNH clone varied from 6% to 98% among different patients (median, 84%). Two-color flow cytometry with 3 mAbs against different parts of the PrPc molecule (Figure 1A) demonstrated the absence of PrPc on the surfaces of resting CD55− PNH platelets (Figure 1B). The surfaces of resting CD55+ PNH platelets was positive for PrPc, as expected, but the level of PrPc expression was approximately 4-fold lower than normal (Figure2), which was most likely a secondary phenomenon caused by recurrent disease.
Detection of PrPc on the surfaces of activated CD55− platelets of PNH patients.
(A) PrPc molecule schematic showing the location of mAb epitopes. Monoclonal antibodies FH11 and 1562 bind to sites on the N-terminal part of PrPc from a hydrophobic putative transmembrane segment (TM1), whereas 6H4 antibody binds to the C-terminal part of PrPc. (B) Two-color flow cytometry analysis of PrPc mAb binding to resting and TRAP-activated platelets of a PNH patient. CD55 antibody detected 2 platelet populations, CD55+ normal (11%) and CD55− PNH platelets (89%). Resting CD55+platelets displayed low levels of PrPc surface expression, but most resting CD55− platelets (depicted in gray) were negative for PrPc. Activation of platelets led to up-regulation of PrPc not only on CD55+ but surprisingly also on CD55−platelets. PrPc up-regulation on CD55− platelets, in contrast to CD55+ platelets, could not be detected by mAb 6H4, demonstrating that the C-terminal part of the molecule was not accessible for binding. Similar results were obtained in all PNH patients evaluated (n = 8). (C) Western blot analysis of PrPc expression in CD55− platelets of PNH patients. Immunoblots were developed using mAb 6H4 without (A) or in the presence of (B) competing peptide. Lane 1, normal platelets; lanes 2 and 3, platelets of 2 PNH patients with major PNH platelet clone (more than 95% of platelets were CD55−). PrPc present in PNH platelets contained the 6H4 epitope and exhibited slightly lower molecular weight than PrPc on normal platelets.
Detection of PrPc on the surfaces of activated CD55− platelets of PNH patients.
(A) PrPc molecule schematic showing the location of mAb epitopes. Monoclonal antibodies FH11 and 1562 bind to sites on the N-terminal part of PrPc from a hydrophobic putative transmembrane segment (TM1), whereas 6H4 antibody binds to the C-terminal part of PrPc. (B) Two-color flow cytometry analysis of PrPc mAb binding to resting and TRAP-activated platelets of a PNH patient. CD55 antibody detected 2 platelet populations, CD55+ normal (11%) and CD55− PNH platelets (89%). Resting CD55+platelets displayed low levels of PrPc surface expression, but most resting CD55− platelets (depicted in gray) were negative for PrPc. Activation of platelets led to up-regulation of PrPc not only on CD55+ but surprisingly also on CD55−platelets. PrPc up-regulation on CD55− platelets, in contrast to CD55+ platelets, could not be detected by mAb 6H4, demonstrating that the C-terminal part of the molecule was not accessible for binding. Similar results were obtained in all PNH patients evaluated (n = 8). (C) Western blot analysis of PrPc expression in CD55− platelets of PNH patients. Immunoblots were developed using mAb 6H4 without (A) or in the presence of (B) competing peptide. Lane 1, normal platelets; lanes 2 and 3, platelets of 2 PNH patients with major PNH platelet clone (more than 95% of platelets were CD55−). PrPc present in PNH platelets contained the 6H4 epitope and exhibited slightly lower molecular weight than PrPc on normal platelets.
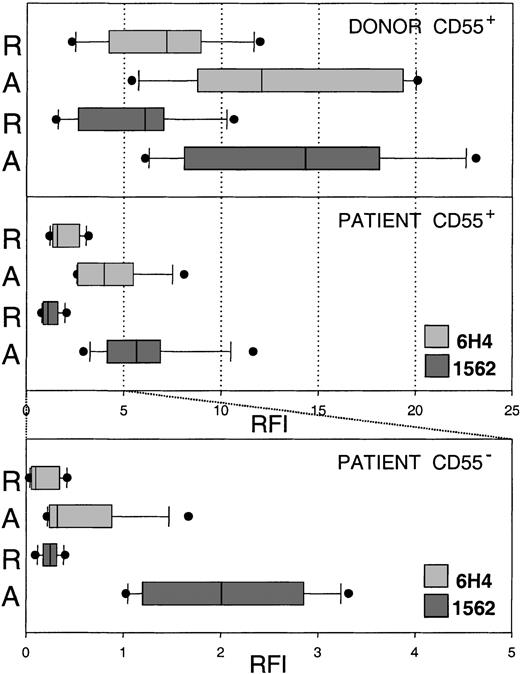
Comparison of mAb 1562 and 6H4 binding to resting and activated normal and PNH platelets.
Resting (R) and activated (A) platelets of healthy donors and PNH patients were double labeled with CD55 antibody and anti PrPc mAbs. Binding of mAbs 1562 and 6H4 to CD55+ donor (n = 6) and patient (n = 8) platelets and to CD55− patient platelets was evaluated by flow cytometry. Mean fluorescence values were converted to RFI. Results are presented as box and whiskers plots of RFI, indicating the median (full line) and the 25th to 75th and the 10th to 90th percentiles of the group distribution as boxes and error bars, respectively. Outlying values are plotted as full circles. Resting and activated normal platelets expressed more PrPc than did CD55+ patient platelets. Resting CD55−platelets were negative for PrPc (note different scale), whereas activated CD55− platelets expressed low levels of PrPc. This up-regulated PrPc on CD55− platelets was not detected by mAb 6H4 (with one exception), indicating that the C-terminal part of the molecule was not accessible to antibody.
Comparison of mAb 1562 and 6H4 binding to resting and activated normal and PNH platelets.
Resting (R) and activated (A) platelets of healthy donors and PNH patients were double labeled with CD55 antibody and anti PrPc mAbs. Binding of mAbs 1562 and 6H4 to CD55+ donor (n = 6) and patient (n = 8) platelets and to CD55− patient platelets was evaluated by flow cytometry. Mean fluorescence values were converted to RFI. Results are presented as box and whiskers plots of RFI, indicating the median (full line) and the 25th to 75th and the 10th to 90th percentiles of the group distribution as boxes and error bars, respectively. Outlying values are plotted as full circles. Resting and activated normal platelets expressed more PrPc than did CD55+ patient platelets. Resting CD55−platelets were negative for PrPc (note different scale), whereas activated CD55− platelets expressed low levels of PrPc. This up-regulated PrPc on CD55− platelets was not detected by mAb 6H4 (with one exception), indicating that the C-terminal part of the molecule was not accessible to antibody.
Normal platelets up-regulate PrPc on the plasma membrane during platelet activation.14,20 In the present study, the activation of platelets resulted in up-regulation of PrPc not only on the plasma membrane of CD55+ but surprisingly also on CD55− PNH platelets (Figure 1B). This result was obtained in all patients studied and revealed the existence of a pool of an intracellular form of PrPc in platelets lacking GPI-anchored proteins. Our data are in agreement with a recent report of an intracellular pool of PrPc in Burkitt lymphoma–derived Daudi cells that lack the surface PrPc and other GPI-anchored proteins because of deficient anchor formation.21 Surface expression of PrPc on activated CD55− PNH platelets was approximately 7 times lower than on activated normal platelets (Figure 2). PrPc up-regulated on the membrane of CD55− PNH platelets was detected by 2 mAbs, 1562 and FH11, directed against epitopes located N-terminally from the membrane-spanning region of PrPc (Figure 1A), but was not detected by mAb 6H4, which binds to the C-terminal part of the molecule (Figure1B). To clarify whether the lack of 6H4 binding was due to PrPc truncation, we performed Western blot analysis of platelets isolated from 2 patients with a large platelet PNH clone (larger than 95%) in whom contamination of the sample by normal platelets with GPI-linked PrPc was minimal. Monoclonal antibodies 6H4 (Figure 1C) and 1562 (not shown) detected a band of appropriate size for diglycosylated PrPc (approximately 35 kd), thus demonstrating the presence of the 6H4 epitope. Specificity of the mAbs binding was confirmed by its inhibition with competing peptides.17 The PrPc band in PNH platelets appeared to have a slightly lower molecular weight than normal platelet PrPc (Figure 1C), probably because of the missing GPI anchor.22 A comparable result was obtained in another PNH patient with a large platelet clone (90%) in whom CD55+platelets were depleted using immunomagnetic beads (not shown).
Our results indicate that in the absence of the GPI anchor, PrPc can be synthesized in a form that apparently is confined to intracellular compartments of resting cells, and, at least in platelets, can be expressed on the cell surface after activation. The absence of 6H4 binding to this membrane PrPc suggests that its epitope is hidden. An attractive explanation for inaccessibility is that the putative transmembrane domain of PrPc is used for insertion of the molecule in the membrane as a type 1 transmembrane protein, with the C-terminal part extended into the cytoplasm. A similar form of PrPc,NtmPrP, was demonstrated using in vitro translation systems and different cell cultures.11-13 An alternative explanation may be that the PrPc expressed on activated CD55− PNH platelets binds to the membrane by an interaction involving the 6H4 epitope, which blocks its accessibility. However, such interaction of PrPc with cells has not been described. The expression of intracellular form PrPc in other blood cells of PNH patients remains to be elucidated.
We thank Dr C. R. Birkett and the TSE Resource Center (Institute for Animal Health, Compton, England) for the donation of monoclonal antibody FH11.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jaroslav G. Vostal, Division of Hematology, Center for Biologics Evaluation and Research, FDA, Building 29, Room 323, HFM-335, 8800 Rockville Pike, Bethesda, MD 20892; e-mail:vostal@cber.fda.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal