Mice lacking the vascular endothelial growth factor (VEGF) receptor flt-1 die of vascular overgrowth, and we are interested in how flt-1 normally prevents this outcome. Our results support a model whereby aberrant endothelial cell division is the cellular mechanism resulting in vascular overgrowth, and they suggest that VEGF-dependent endothelial cell division is normally finely modulated by flt-1 to produce blood vessels. Flt-1−/− embryonic stem cell cultures had a 2-fold increase in endothelial cells by day 8, and the endothelial cell mitotic index was significantly elevated before day 8. Flt-1 mutant embryos also had an increased endothelial cell mitotic index, indicating that aberrant endothelial cell division occurs in vivo in the absence of flt-1. Theflt-1 mutant vasculature of the cultures was partially rescued by mitomycin C treatment, consistent with a cell division defect in the mutant background. Analysis of cultures at earlier time points showed no significant differences until day 5, whenflt-1 mutant cultures had increased β-galactosidase+ cells, indicating that the expansion of flt-1 responsive cells occurs after day 4. Mitomycin C treatment blocked this early expansion, suggesting that aberrant division of angioblasts and/or endothelial cells is a hallmark of theflt-1 mutant phenotype throughout vascular development. Consistent with this model is the finding that expansion of platelet and endothelial cell adhesion molecule+ and VE-cadherin+ vascular cells in theflt-1 mutant background first occurs between day 5 and day 6. Taken together, these data show that flt-1 normally modulates vascular growth by controlling the rate of endothelial cell division both in vitro and in vivo.
Introduction
Blood vessels form by coordinating several cellular processes, including cell division and morphogenesis (reviewed in Folkman & D'Amore,1 Weinstein,2 and Conway et al3). Some of the mitogenic signals that promote division of endothelial cells and their precursors are known, but how these signals are modulated to initiate cell divisions only when and where they are needed is not known in detail. After blood vessels initially form, maturation and remodeling steps involve the recruitment of ancillary cells, such as smooth muscle and pericytes. These cells and the extracellular matrix that is also produced can negatively modulate endothelial cell division.4-8 However, modulators of endothelial cell mitogenesis at the earliest stages of blood vessel formation have not been identified.
The vascular endothelial growth factor (VEGF) signaling pathway is clearly critical to both early endothelial cell division and morphogenesis, and its regulation is complex (reviewed in Ferrara & Davis-Smyth9 and Neufeld et al10). Mouse embryos lacking even one copy of the VEGF gene die in utero with severe vascular defects, and vascular development in differentiating embryonic stem (ES) cells is compromised in VEGF-A+/− andVEGF-A−/− ES cells in a dose-dependent manner.11-13 Moreover, even modestly elevated levels of VEGF lead to vascular abnormalities,14 and large doses of VEGF invariably severely compromise both vascular development and neovascularization in adult organisms.15-17 These findings suggest that VEGF signaling must be precisely controlled during vascularization to result in proper vessels. The location and duration of VEGF expression provide the first level of control,18-21 but other components of the pathway are likely to be involved in fine-tuning the signal.
Two high-affinity receptors, flk-1 and flt-1, participate in VEGF signal transduction and are candidates to be involved in fine-tuning mechanisms. Both receptors are membrane-spanning receptor tyrosine kinases that bind VEGF with high affinity,22-26 but their effects on VEGF signaling are very different. Mice or ES cells lacking flk-1 have little or no blood vessel formation, suggesting that many downstream effects of VEGF on endothelial cells are mediated through flk-1.27,28 Specifically, numerous studies show that VEGF signaling through flk-1 produces a strong mitogenic signal for endothelial cells.29-32
In contrast, VEGF binding to flt-1 does not produce a strong mitogenic signal, and flt-1−/− mice die at mid-gestation with vascular overgrowth and disorganization.23,29,33 This phenotype was reported to result from increased numbers of cells called hemangioblasts that can give rise to both hematopoietic and endothelial cells.34 However, invoking control of an early cell fate switch as the exclusive cellular mechanism of flt-1 action is inconsistent with evidence that flt-1 is expressed in mature endothelial cells, including tumor vasculature.35-37 It is also inconsistent with a molecular model of flt-1 action, suggesting that flt-1 can sequester VEGF ligand and, thus, modulate signaling through flk-1, because flk-1 signaling affects multiple endothelial processes, including cell division.38,39 Moreover, VEGF addition to flt-1–expressing trophoblast cells inhibits cell division, and 2 recent studies using chimeric receptors suggested that flt-1 signaling may counteract the positive mitogenic signal from flk.40-42
Thus, we asked if flt-1 could negatively modulate endothelial mitogenesis developmentally, and to address this question we analyzed the cellular mechanism responsible for theflt-1−/− phenotype in both ES cell cultures and embryos. The flt-1 mutant ES cell cultures and embryos had vascular overgrowth that was caused primarily by aberrant endothelial cell division, and this deregulated mitogenesis in the vascular lineage was seen throughout the stages of vascular development. Thus, flt-1 acts early in vascular development to modulate vessel formation by affecting the rate of cell division in embryonic endothelial cells and their precursors.
Materials and methods
Cell culture and in vitro differentiation
Wild type (WT, +/+), hemizygous mutant (flt-1+/−), and homozygous mutant for the targeted flt-1 mutation (flt-1−/−)33 ES cells were maintained and differentiated in vitro as attached cultures as described previously.43
For mitomycin C treatment, ES cell cultures were differentiated to day 6, then incubated with mitomycin C (Sigma) at 30 μg/mL diluted in differentiation media for 2 hours at 37°C. After incubation in fresh differentiation medium for 48 hours (to day 8), cultures were fixed and stained with the appropriate antibodies. For earlier times, cultures were incubated with mitomycin C as described earlier on day 4 or day 5, then incubated in fresh medium for 24 hours (to day 5 or day 6) before fixation and staining.
Antibody staining and image analysis
ES cell cultures were rinsed in phosphate-buffered saline (PBS) and fixed for 5 minutes in ice-cold methanol:acetone (50:50) or fresh 4% paraformaldehyde (for VE-cadherin staining). Fixed cultures were reacted with antibodies as described previously.13 43 In double-labeling experiments, cultures were first incubated with rabbit anti–β-galactosidase or rabbit antiphosphohistone H3 antibodies and the appropriate secondary, then blocked in staining media (3% fetal bovine serum [FBS], 0.1% NaN3 in PBS) with 5% donkey serum before the addition of rat antimouse platelet and endothelial cell adhesion molecule (PECAM). In triple-labeling experiments, rabbit polyclonal antiphosphohistone H3 incubation was followed by incubation with rat antimouse PECAM and, subsequently, staining with the DNA dye topro-3 (Molecular Probes) at 1:1000 for 5 minutes at room temperature. All cultures were rinsed in PBS and viewed with an Olympus IX-50 inverted microscope by using epifluorescence or a Zeiss LSM 410 confocal microscope.
Primary antibodies and dilutions used were rat antimouse PECAM at 1:1000 (MEC 13.3; Pharmingen); rat antimouse intercellular adhesion molecule 2 (ICAM-2) at 1:500 (3C4; Pharmingen), rabbit polyclonal anti–β-galactosidase at 1:300 (Cappel Labs), rabbit polyclonal antiphosphohistone H3 at 1:500 (Upstate Biotechnology), and rat antimouse VE-cadherin at 1:100 (11D4.1; Pharmingen). Secondary antibodies and dilutions used were donkey antirabbit immunoglobulin G (IgG; H + L) TRITC cross-absorbed at 1:100 (Jackson Immunoresearch) for antiphosphohistone H3 and β-galactosidase, donkey antirat IgG (H + L) B-phycoerythrin cross-absorbed at 1:300 (Jackson Immunoresearch) for PECAM and ICAM-2, donkey antirat IgG (H + L) fluorescein isothiocyanate (FITC) cross-absorbed at 1:100 (Jackson Immunoresearch) for PECAM, and goat antirat IgG (H + L) Alexa 488 cross-absorbed at 1:100 (Molecular Probes) for PECAM and VE-cadherin.
Quantitative image analysis of day 8 ES cell cultures reacted with the appropriate antibodies was performed as previously described.13 Sequential nonoverlapping areas completely covered with cells were photographed at ×10 magnification, so that the total area photographed per well was more than 60% of the well area. For earlier time points, β-galactosidase–stained wells were photographed, and only areas covered with cells were used for analysis. Digital images were generated and analyzed by using Adobe Photoshop (version 5.0, Adobe Systems). Quantitation of the stained area for each image was performed by using an Image Processing Tool Kit (Rev. 2.1; Reindeer Games, Asheville, NC). Stained area averages for each well were calculated, and the average of 3 to 4 wells for each condition was used to determine SD values.
β-Galactosidase detection
β-Galactosidase detection was performed by using a modified protocol.44 Cultures were rinsed twice in 0.1 M phosphate buffer (pH 7.3) and fixed with glutaraldehyde fix solution (0.2% glutaraldehyde, 5 mM EGTA [pH 7.3], 2 mM MgCl2 in 0.1 M phosphate buffer [pH 7.3]) for 5 minutes. After washing 3 times for 5 minutes with phosphate buffer, cultures were incubated for 3 hours (day 8 ES cultures) or 5 hours (early time course experiments) at 37°C in X-gal staining solution (0.625 mg/mL X-gal; Sigma), 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, in wash buffer (2 mM MgCl2, 0.02% Nonidet-P40 in 0.1 M sodium phosphate buffer [pH 7.3]), then rinsed and stored in wash buffer at 4°C.
RNA analysis
Total RNA was isolated from day 7 ES cell cultures by centrifugation through a CsCl gradient.45 RNase protection assays for PECAM were performed by using a modified protocol.13 46 In vitro transcription of PECAM-dCPa (nt 1425-1904) was used to generate a 32P-labeled antisense RNA probe. Overnight hybridization at 45°C with the PECAM probe and a β-actin internal control probe was followed by digestion with RNase A and RNase T1. Protected fragments were then electrophoresed through a 5% acrylamide urea (8 mM gel) and quantified by using a PhosphorImager (Molecular Dynamics).
Fluorescent-activated cell sorter analysis
Day 8 ES cell cultures were rinsed twice with PBS and dissociated with 0.2% collagenase (Sigma; 0.15% type II, 0.05% type XI in PBS) for approximately 2 hours with repeated passage through a 20-gauge needle. The cells were rinsed in FBS/PBS (1:1), resuspended in cold staining media (3% FBS + 0.01% sodium azide in PBS), and incubated on ice for 20 minutes. Cells were then incubated with 100 μg/mL biotin-coupled ICAM-2 antibody in staining medium for 45 minutes at 4°C. After 3 washes with cold staining medium, cells were resuspended in staining medium with 25 μg/mL streptavidin-phycoerythrin (Southern Biologicals) and incubated for 45 minutes at 4°C. After 3 washes with cold PBS, the cells were fixed and stored at 4°C in 1% paraformaldehyde. Flow cytometry data were collected with a Becton Dickinson FACSCAN.
Mitotic index calculations
WT and flt-1−/− ES cell cultures were differentiated in chamber slide wells (Nunc) to day 6 or 7, fixed, and triple-labeled with rabbit antiphosphohistone H3, rat antimouse PECAM, and the DNA binding dye topro-3. Slides were mounted in AquaPolymount (LifeSciences). Confocal images were analyzed by using Adobe Photoshop (version 5.0, Adobe Systems) software. Triple-labeled images were counted in the following 4 ways: (1) the total number of cells per field, (2) the total number of phosphohistone H3+ cells per field, (3) the number of PECAM+ cells with endothelial morphology per field, and (4) the number of PECAM+/phosphohistone H3+ cells with endothelial morphology per field. Endothelial mitotic indices were calculated on a per field basis by dividing the number of PECAM+, phosphohistone H3+ cells by the total number of PECAM+ cells. Nonendothelial mitotic indices were also calculated on a per field basis by dividing the number of PECAM−, phosphohistone H3+ cells by the total number of PECAM− cells. Data were collected from multiple fields of multiple wells and averaged for each day.
Embryo immunohistochemistry
Flt-1+/− mice maintained on the CD-1 background were intercrossed to obtain embryos. Embryos were dissected from the maternal decidua at day 8.5 (the morning of the plug is day 0.5), heads were removed and saved at −20°C for genotyping by using a modification of a published protocol,33 and the rest of the embryo was fixed in Serra fixative47 or cold 4% paraformaldehyde at 4°C overnight. The embryos were dehydrated through a methanol series and stored at −20°C in 100% methanol. Embryos were embedded in paraffin, sectioned at 10 μm on a Zeiss Microm, dewaxed in Histoclear, and rehydrated. Sections fixed in paraformaldehyde were incubated in 0.02% Protease XXIV (Sigma) in PBS for 4 minutes, then washed 3 times in PBS. After blocking in 0.25% H2O2 in PBS for 15 minutes, primary antibody (1:250 dilution in 5% goat serum/PBS) was added, and sections were incubated overnight at 4°C in humidified chambers. After 3 washes in PBS, secondary antibody (1:300 dilution of goat antirabbit or antirat IgG–horseradish peroxidase [Accurate] in 5% goat serum/PBS) was added, and incubation was overnight as before. After 3 washes in PBS, sections were incubated in 3′-diaminobenzidine tetrahydrochloride substrate to which 3 mg/mL NiSO4 was sometimes added (for blue color) for 15 minutes. Slides were rinsed in PBS, incubated in a 1:10 000 dilution of DAPI (1 mg/mL stock) in H2O for 10 minutes, mounted using Glycergel (Dako), and visualized with a Nikon Eclipse E800 microscope outfitted with DIC optics and epifluorescence. To count mitotic endothelial nuclei, alternate sections were stained with PECAM and phosphohistone H3. The DAPI-stained nuclei were used to overlay digital images.
Results
Flt-1−/−ES cell cultures have increased vascularization
ES cells undergo a differentiation program in vitro that mimics early murine yolk sac development, including primitive hematopoietic development and blood vessel formation.43,48-51Hematoendothelial development begins when a mesodermally derived hemangioblast population arises at days 2 to 3 of differentiation,52 and angioblasts can also differentiate directly from mesoderm. The endothelial cells of primitive blood vessels are differentiated from angioblasts by coexpression of PECAM and ICAM-2, both adhesion receptors of the immunoglobulin superfamily.13,53 54 We initially investigated the cellular mechanism of flt-1 in day 8 cultures, when the PECAM+/ICAM-2+ vasculature is well established.
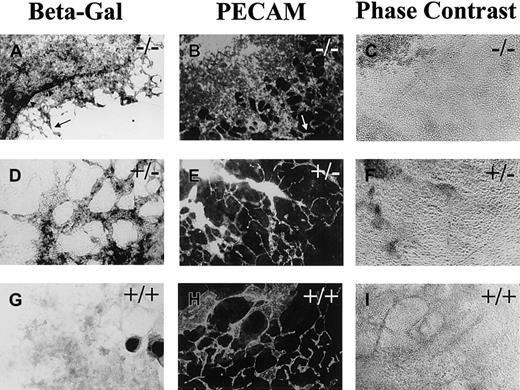
Flt-1+/− and flt-1−/−ES cells were engineered so that Escherichia coli lacZ is expressed under flt-1 regulatory control in the targeted gene.33 These ES cells and WT (+/+) controls were stained for β-galactosidase activity or for PECAM expression at day 8 (Figure 1). Theflt-1−/− cultures had a dramatically expanded β-galactosidase expression domain compared with theflt-1+/− cultures (Figure 1A). The β-galactosidase+ cells in theflt-1−/− cultures were found in large circular sheets, with areas of normal-looking vasculature at the edge of the sheets (Figure 1A,B, arrow). Immunofluorescent antibody staining for PECAM, ICAM-2, or VE-cadherin showed a similar pattern in theflt-1−/− cultures (Figure 1B and data not shown), suggesting that most of the β-galactosidase–expressing cells were endothelial cells. The β-galactosidase– and antibody-stained cells were elongated and interconnected, indicating that they were endothelial cells. This criterion is important, because subsets of hematopoietic cells can also react with the antibodies to PECAM or ICAM-2. Only a ring of intensely β-galactosidase+ cells (Figure 1A, arrowheads) did not appear to stain for PECAM or ICAM-2 by double-label immunofluorescent antibody staining (data not shown). These cells were found in both WT and mutant cultures, and they reacted with a flt-1 antisense RNA probe in the WT background (data not shown), indicating that they are nonvascular flt-1–expressing cells.
Flt-1−/−ES cell cultures have increased vascularization.
Day 8 differentiated flt-1−/− (A-C),flt-1+/− (D-F), and WT (G-I) cultures were processed for β-galactosidase detection (A,D,G) or reacted with an antibody to PECAM (B,E,H). A and B show one quadrant of the relatively large β-galactosidase+ (A) or PECAM+ (B) sheet of cells that characterizes the flt-1−/−phenotype. In contrast, an extensive vascular plexus is found in bothflt-1+/− (E) and WT (H) ES cell cultures. Arrowheads (A) outline an intensely stained β-galactosidase+ ring of cells that surrounds most of the β-galactosidase+ cells. Arrows (A,B) point toflt-1−/− vasculature that looks WT. (C,F,I) Phase contrast images of PECAM-labeled fields in B,E,H. Magnification is ×10.
Flt-1−/−ES cell cultures have increased vascularization.
Day 8 differentiated flt-1−/− (A-C),flt-1+/− (D-F), and WT (G-I) cultures were processed for β-galactosidase detection (A,D,G) or reacted with an antibody to PECAM (B,E,H). A and B show one quadrant of the relatively large β-galactosidase+ (A) or PECAM+ (B) sheet of cells that characterizes the flt-1−/−phenotype. In contrast, an extensive vascular plexus is found in bothflt-1+/− (E) and WT (H) ES cell cultures. Arrowheads (A) outline an intensely stained β-galactosidase+ ring of cells that surrounds most of the β-galactosidase+ cells. Arrows (A,B) point toflt-1−/− vasculature that looks WT. (C,F,I) Phase contrast images of PECAM-labeled fields in B,E,H. Magnification is ×10.
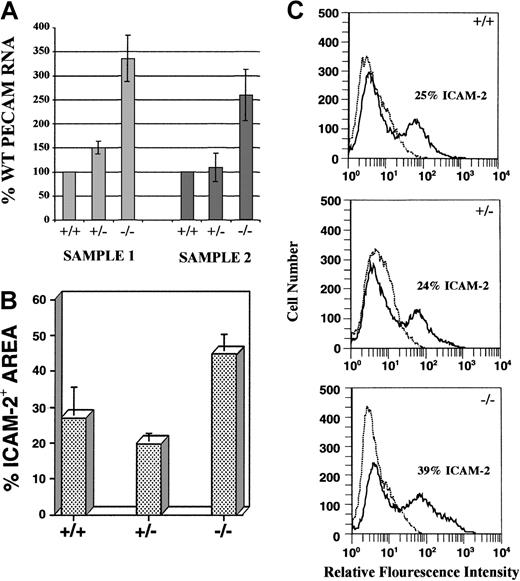
The increase in endothelial cells observed in mutant cultures was quantitated in several ways (Figure 2). RNase protection analysis of day 8 cultures with a PECAM antisense RNA probe revealed that PECAM RNA levels were 2.5- to 3.3-fold higher inflt-1−/− cultures compared with WT cultures (Figure 2A). Quantitative image analysis on day 8 ICAM-2–labeled cultures used digital images of vascular immunofluorescence to determine the percentage area stained, which approximates the amount of vasculature (see “Materials and methods” section for detailed protocols). Flt-1−/− cultures exhibited nearly a 2-fold increase in ICAM-2 staining area over WT, whereasflt-1+/− cultures had essentially WT levels (Figure 2B). ICAM-2 antibody-stained cultures were also processed for fluorescent-activated cell sorting (FACS; Figure 2C).Flt-1−/−–attached cultures contained a population of ICAM-2+ cells that was significantly increased over WT levels (compare 39% with 25%, respectively), whereas flt-1+/− cultures had WT numbers of ICAM-2+ cells. Similar FACS results were obtained with antibodies to PECAM (data not shown). Taken together, these data show that the lack of flt-1 results in increased numbers of vascular endothelial cells.
Flt-1−/− ES cell cultures have increased numbers of endothelial cells.
(A) RNase protection assay using an antisense PECAM RNA probe on day 8 WT, flt-1+/−, andflt-1−/− attached cultures. Protected fragments were separated on a polyacrylamide-urea gel and quantified by using a PhosphorImager. Protected PECAM signal was normalized to a β-actin signal, and the normalized PECAM band densities forflt-1+/− and flt-1−/−samples were compared with WT (+/+) samples. Sample 1 and sample 2 are RNAs from separate differentiations. Each bar is the average of 3 experiments performed on a particular sample. (B) Quantitative image analysis of the ICAM-2+ area on day 8 WT (+/+), flt-1+/−, andflt-1−/− attached cultures. Each bar represents the average area stained with ICAM-2 antibody from 3 wells. This experiment was repeated (data not shown), and similar quantitative trends were obtained. (C) Fluorescent cell sorting of ICAM-2–labeled day 8 WT (+/+), flt-1+/−, andflt-1−/− ES cell cultures. The plots in dotted lines are controls without primary antibody.
Flt-1−/− ES cell cultures have increased numbers of endothelial cells.
(A) RNase protection assay using an antisense PECAM RNA probe on day 8 WT, flt-1+/−, andflt-1−/− attached cultures. Protected fragments were separated on a polyacrylamide-urea gel and quantified by using a PhosphorImager. Protected PECAM signal was normalized to a β-actin signal, and the normalized PECAM band densities forflt-1+/− and flt-1−/−samples were compared with WT (+/+) samples. Sample 1 and sample 2 are RNAs from separate differentiations. Each bar is the average of 3 experiments performed on a particular sample. (B) Quantitative image analysis of the ICAM-2+ area on day 8 WT (+/+), flt-1+/−, andflt-1−/− attached cultures. Each bar represents the average area stained with ICAM-2 antibody from 3 wells. This experiment was repeated (data not shown), and similar quantitative trends were obtained. (C) Fluorescent cell sorting of ICAM-2–labeled day 8 WT (+/+), flt-1+/−, andflt-1−/− ES cell cultures. The plots in dotted lines are controls without primary antibody.
Lack of flt-1 leads to increased endothelial cell division
To investigate the cellular mechanism(s) responsible for the increased vascularization seen in the absence of flt-1, the hypothesis that flt-1−/− endothelial cells have a higher rate of cell division than WT endothelial cells was tested. Day 6 and day 7 ES cell cultures were labeled with antibodies to the vascular marker PECAM and to the mitotic marker phosphohistone H3,55 then stained with a DNA-binding dye (topro-3; Figure3). Visual observation suggested that day 6 and day 7 flt-1−/− ES cell cultures had more PECAM+ cells that colabeled with the antiphosphohistone H3 antibody than WT controls (compare Figure 3A,C with B,D and E with F).
Flt-1−/− ES cell cultures have mitotic endothelial cells.
Day 7 (A-D) or day 6 (E,F) WT (A,C,E), andflt-1−/− (B,D,F) attached cultures were labeled with antibodies to PECAM (green) and phosphohistone H3 (red), then stained with the nuclear marker topro-3 (blue). The arrowhead (B) shows a phosphohistone H3+ nonendothelial cell (PECAM−), whereas the arrow (B) points to a phosphohistone H3+ endothelial cell (PECAM+). Notice the increase in phosphohistone H3+/PECAM+ cells inflt-1−/− cultures relative to WT cultures. All panels are confocal images at ×40 magnification.
Flt-1−/− ES cell cultures have mitotic endothelial cells.
Day 7 (A-D) or day 6 (E,F) WT (A,C,E), andflt-1−/− (B,D,F) attached cultures were labeled with antibodies to PECAM (green) and phosphohistone H3 (red), then stained with the nuclear marker topro-3 (blue). The arrowhead (B) shows a phosphohistone H3+ nonendothelial cell (PECAM−), whereas the arrow (B) points to a phosphohistone H3+ endothelial cell (PECAM+). Notice the increase in phosphohistone H3+/PECAM+ cells inflt-1−/− cultures relative to WT cultures. All panels are confocal images at ×40 magnification.
To quantitate the apparent increase in mitotic PECAM+ cells in flt-1−/− cultures, confocal images from day 6 or day 7 fixed cultures processed as in Figure 3 were used to calculate cell counts and mitotic indices for both endothelial and nonendothelial cell populations (Table 1and Figure 4; see “Materials and methods” section for details). In all cases endothelial cells of theflt-1−/− cultures had a higher mitotic index than WT endothelial cells. To control for differential growth rates, a nonendothelial cell mitotic index was obtained for each experiment (Table 1). There was little difference between WT andflt-1−/− nonendothelial cell mitotic indices within a given experiment, in contrast to increases in theflt-1−/− endothelial cell mitotic index. Each endothelial cell mitotic index was normalized to its companion nonendothelial cell mitotic index (Figure 4; Table 1, far right column). Day 6 flt-1−/− cultures had normalized endothelial cell mitotic indices that were 3- to 4-fold higher than normal, and similar but less dramatic trends were observed in day 7 cultures (Figure 4, compare black bars with gray bars). These results indicate that the increased vascularization seen in day 8flt-1−/− ES cell cultures is caused, at least in part, by an increased endothelial cell division rate in the absence of flt-1.
Flt-1−/− ES cell cultures have an elevated endothelial cell mitotic index.
Days 6 and 7 WT (+/+) and flt-1−/−triple-labeled images were used to calculate nonendothelial cell and endothelial cell mitotic indices for 2 separate differentiation experiments (Table 1). Endothelial cell mitotic indices were expressed as a percentage of the nonendothelial cell mitotic index calculated for each experimental condition. The dotted black line represents the nonendothelial mitotic index for each experiment converted to 100%, and it was used as a baseline for comparison of endothelial mitotic indices.
Flt-1−/− ES cell cultures have an elevated endothelial cell mitotic index.
Days 6 and 7 WT (+/+) and flt-1−/−triple-labeled images were used to calculate nonendothelial cell and endothelial cell mitotic indices for 2 separate differentiation experiments (Table 1). Endothelial cell mitotic indices were expressed as a percentage of the nonendothelial cell mitotic index calculated for each experimental condition. The dotted black line represents the nonendothelial mitotic index for each experiment converted to 100%, and it was used as a baseline for comparison of endothelial mitotic indices.
If aberrant endothelial cell division contributes to theflt-1 mutant phenotype, then blocking cell division during ES cell differentiation may affect the phenotype. Thus, day 6 ES cell cultures were treated with the replication inhibitor mitomycin C before incubation for an additional 2 days (Figure5). Untreatedflt-1−/− cultures fixed on day 6 had slightly increased numbers of PECAM+ cells compared with day 6 WT cultures (compare Figure 5A with B). Treated day 8flt-1−/− had half as much vasculature as untreated genotype-matched controls, accompanied by a dramatic decrease in the labeling of nuclei with antiphosphohistone H3 (compare Figure 5D with F,H). In some cases, mitomycin C-treatedflt-1−/− vasculature at day 8 was branched and appeared WT in morphology (Figure 5G), suggesting that blocking cell division during days 6 to 8 of differentiation can partially compensate for the lack of flt-1 in vascular development. Mitomycin C treatment also affected vascular growth in WT cultures, which is predicted because blood vessel formation requires endothelial cell division. The treated WT cultures had 2- to 3-fold less vasculature and less branching than untreated controls (compare Figure 5C with E,H). Thus, treatment with mitomycin C, an inhibitor of replication, partially rescues the flt-1 mutant vascular phenotype.
Mitomycin C treatment partially rescues theflt-1−/− vascular phenotype.
Day 6 ES cell cultures were fixed (A,B), left untreated (C,D), or treated with mitomycin C (E-G). Some cultures (C-G) were differentiated for an additional 48 hours. Cultures were labeled with an antibody to PECAM (green), and some cultures (C-F) were also labeled with the mitotic marker antiphosphohistone H3 (red). Notice the abundance of phosphohistone H3–labeled figures in untreated (C-D) cultures compared with treated (E-F) cultures. (G) Example of a treatedflt-1−/− culture that morphologically resembled WT vasculature. (H) Quantitative image analysis of the PECAM+ area of day 8 WT (+/+) andflt-1−/− (−/−) cultures treated with mitomycin C (red) or left untreated (green). Each bar represents the average stained area from at least 3 wells stained with PECAM antibody. Magnification was ×10 except C (×20) and G (×4).
Mitomycin C treatment partially rescues theflt-1−/− vascular phenotype.
Day 6 ES cell cultures were fixed (A,B), left untreated (C,D), or treated with mitomycin C (E-G). Some cultures (C-G) were differentiated for an additional 48 hours. Cultures were labeled with an antibody to PECAM (green), and some cultures (C-F) were also labeled with the mitotic marker antiphosphohistone H3 (red). Notice the abundance of phosphohistone H3–labeled figures in untreated (C-D) cultures compared with treated (E-F) cultures. (G) Example of a treatedflt-1−/− culture that morphologically resembled WT vasculature. (H) Quantitative image analysis of the PECAM+ area of day 8 WT (+/+) andflt-1−/− (−/−) cultures treated with mitomycin C (red) or left untreated (green). Each bar represents the average stained area from at least 3 wells stained with PECAM antibody. Magnification was ×10 except C (×20) and G (×4).
Flt-1 mutation affects division of vascular precursor cells
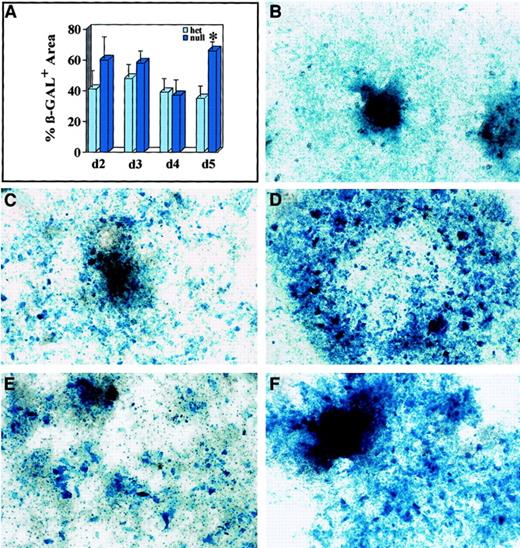
To determine when the flt-1 mutation first affects vascular development, we investigated earlier time points of ES cell differentiation. To establish when cells expressing lacZ under control of the flt-1 promoter were first affected by the lack of flt-1 protein, we analyzed an early time course of ES cell differentiation. We plated cells directly after dispase treatment, then processed wells of each genotype for lacZ expression on days 2 to 6 of differentiation (Figure 6). The percentage of lacZ-expressing cells was equivalent betweenflt-1+/− and flt-1−/−cultures on days 2 to 4, and only on day 5 was there a significant increase in the percentage of lacZ-expressing cells in the flt-1 mutant background (Figure 6A). To determine if this expansion was the result of aberrant cell division, wells were treated with mitomycin C on day 4 or day 5, then compared with control untreated wells 24 hours later. Day 5 flt-1−/− mutant cultures treated with mitomycin C 24 hours earlier had fewer lacZ-expressing cells than paired untreated controls (compare Figure 6C with D). The day 5 mitomycin C-treated wells were, in fact, similar to untreated wells fixed at day 4 (compare Figure 6B with C). Day 6flt-1−/− mutant cultures treated with mitomycin C 24 hours earlier also had fewer lacZ-expressing cells than paired untreated controls (compare Figure 6E with F). These results show that the earliest expansion of lacZ-expressing cells in theflt-1−/− mutant cultures can be inhibited by mitomycin C, suggesting that the expansion results from aberrant cell division.
Mitomycin C–sensitive expansion of β-galactosidase–expressing cells in flt-1−/− ES cell cultures at earlier times.
(A) Quantitative image analysis of the β-galactosidase+areas of flt-1+/− (light blue bars) andflt-1−/− (dark blue bars) ES cell cultures on days 2 to 5 of in vitro differentiation. For days 2 and 3, the bars represent the average β-galactosidase+ area for 9 individual attached ES cell clumps. For days 4 and 5, the bars represent the average β-galactosidase+ area for 2 culture wells. The asterisk (*) indicates significance atP < .001. (B-F) Days 4 to 6flt-1−/− ES cell cultures untreated (B,D,F) or treated with mitomycin C (C,E) and stained for β-galactosidase activity. (B) Day 4 flt-1+/− culture. (C) Day 5flt-1−/− culture treated on day 4 with mitomycin C. Note decrease in stained area relative to (D) untreated day 5 flt-1−/− culture. (E) Day 6flt-1−/− culture treated on day 5 with mitomycin C. Note decrease in stained area relative to (F) untreated day 6 flt-1−/− culture. Original magnification, ×20.
Mitomycin C–sensitive expansion of β-galactosidase–expressing cells in flt-1−/− ES cell cultures at earlier times.
(A) Quantitative image analysis of the β-galactosidase+areas of flt-1+/− (light blue bars) andflt-1−/− (dark blue bars) ES cell cultures on days 2 to 5 of in vitro differentiation. For days 2 and 3, the bars represent the average β-galactosidase+ area for 9 individual attached ES cell clumps. For days 4 and 5, the bars represent the average β-galactosidase+ area for 2 culture wells. The asterisk (*) indicates significance atP < .001. (B-F) Days 4 to 6flt-1−/− ES cell cultures untreated (B,D,F) or treated with mitomycin C (C,E) and stained for β-galactosidase activity. (B) Day 4 flt-1+/− culture. (C) Day 5flt-1−/− culture treated on day 4 with mitomycin C. Note decrease in stained area relative to (D) untreated day 5 flt-1−/− culture. (E) Day 6flt-1−/− culture treated on day 5 with mitomycin C. Note decrease in stained area relative to (F) untreated day 6 flt-1−/− culture. Original magnification, ×20.
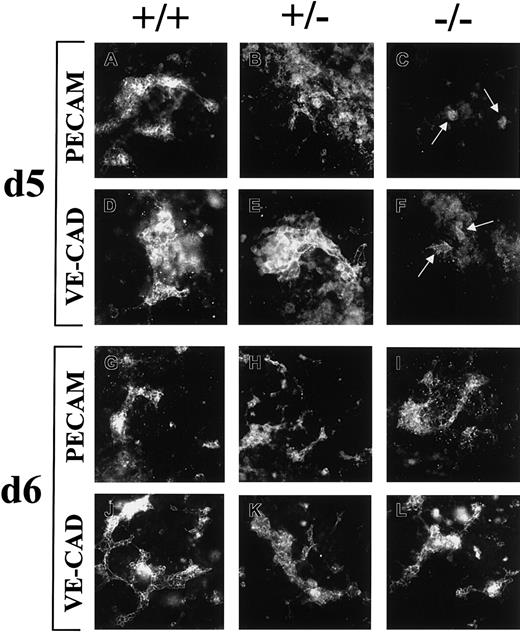
Because both endothelial cells and a nonendothelial cell population express flt-1 promoter-driven β-galactosidase, we investigated the expression of several vascular markers in the ES cell cultures. Cultures were stained with PECAM or VE-cadherin from days 2 to 6 of differentiation (Figure 7), because both markers are expressed early in vascular development. PECAM was expressed throughout the time course, but before day 5 only clumps of PECAM+ cells were seen, and no significant differences were seen among the different genotypes (data not shown). By day 5 both WT and flt-1+/− cultures had some areas of PECAM+ vasculature, but surprisingly theflt-1−/− mutant day 5 cultures had few PECAM+ cells and most were still in clumps (Figure 7A-C). By day 6 all cultures had PECAM+ vasculature, and theflt-1−/− mutant cultures had as much or more PECAM+ vessels compared with WT orflt-1+/− cultures (Figure 7G-I). Treatment offlt-1−/− cultures from days 5 to 6 with mitomycin C reduced the number of PECAM+ cells (data not shown). VE-cadherin+ cells were not seen in any cultures until day 5 (data not shown). Similar to the PECAM pattern, on day 5 WT and flt-1+/− cultures had VE-cadherin+ vasculature, whereas theflt-1−/− mutant cultures had only a few VE-cadherin+ cells that were not organized into vessels (Figure 7D-F). By day 6 cultures of all genotypes had VE-cadherin+ vessels (Figure 7J-L). These results show thatflt-1−/− mutant cultures did not have expansion of either PECAM+ or VE-cadherin+vascular cells until between days 5 and 6 of differentiation, when expansion of both β-galactosidase–expressing cells and PECAM-expressing cells was sensitive to mitomycin C.
Expression of vascular markers in differentiating ES cell cultures.
Wt (+/+) (A,D,G,J), flt-1+/−(B,E,H,K), and flt-1−/− (C,F,I,L) ES cell cultures were fixed on day 5 (A-F) or day 6 (G-L) and labeled with antibodies to PECAM (A-C,G-I) or VE-cadherin (D-F,J-L), and the appropriate fluorescent-labeled secondary antibody. Arrows (C,F) point to sparse PECAM+ and VE-cadherin+ cells in day 5 flt-1−/− cultures. Original magnifications ×20, except D-F at ×40.
Expression of vascular markers in differentiating ES cell cultures.
Wt (+/+) (A,D,G,J), flt-1+/−(B,E,H,K), and flt-1−/− (C,F,I,L) ES cell cultures were fixed on day 5 (A-F) or day 6 (G-L) and labeled with antibodies to PECAM (A-C,G-I) or VE-cadherin (D-F,J-L), and the appropriate fluorescent-labeled secondary antibody. Arrows (C,F) point to sparse PECAM+ and VE-cadherin+ cells in day 5 flt-1−/− cultures. Original magnifications ×20, except D-F at ×40.
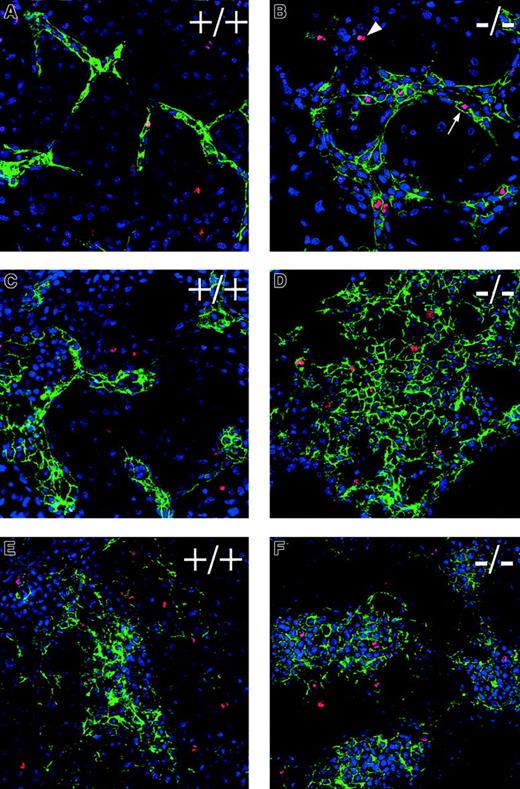
Flt-1−/−embryos have increased mitoses
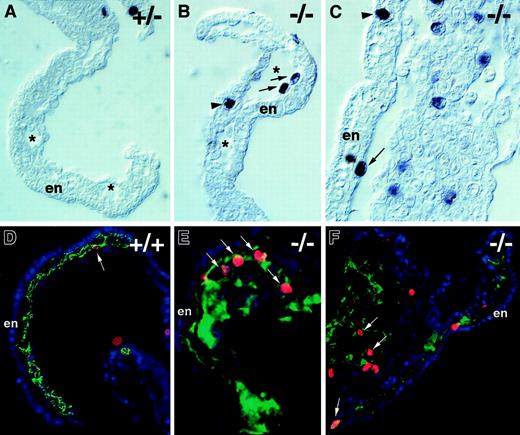
To determine if the aberrant endothelial cell division seen in the absence of flt-1 during ES cell differentiation also occurred in vivo, day 8.5 embryos were stained with the antiphosphohistone H3 antibody (Figure 8). Theflt-1−/− mutant embryos had numerous mitotic nuclei in several vascular areas, including the lining of yolk sac blood islands (Figure 8B,C,E,F) and the allantois (Figure 8F). In contrast, nonmutant embryos had far fewer mitotic nuclei in those areas (Figure 8A,D). The increase in mitotic nuclei was specific to vascular areas in vivo, because embryonic structures such as the neural tube and somites had roughly equivalent numbers of mitotic nuclei regardless of the genetic background (data not shown). Digital overlays of alternate sections stained with PECAM and phosphohistone H3 (Figure 8D-F) were used to calculate the endothelial mitotic indices in vivo. The endothelial mitotic index of flt-1−/− embryos was 2.8% (n = 1270), double that of WT+/+ embryos whose endothelial mitotic index was 1.4% (n = 425). Thus, the aberrant endothelial cell division documented during ES cell differentiation in the absence of flt-1 is also a hallmark of the mutant phenotype in vivo.
Flt-1 −/− embryos have increased mitoses in endothelial cells.
Transverse sections of day 8.5 embryos were processed for immunohistochemistry by using antiphosphohistone H3 to detect mitotic nuclei (A-C), and overlays of adjacent sections were processed individually (see “Materials and methods” section) for immunohistochemistry with antiphospohistone H3 (red), anti-PECAM (green), and DAPI (blue) (D-F). Visualization of yolk sacs offlt-1+/− (A) or WT (+/+) (D) embryos that were phenotypically normal showed few mitotic nuclei in vascular areas (arrow in D). In contrast,flt-1−/− embryos (B,C,E,F) exhibited vascular overgrowth and numerous mitotic nuclei (red; E,F) in PECAM+regions (green; E,F) of the yolk sac and allantois (F, left part of panel). (A-C) Asterisks denote the lumina of blood islands in the yolk sac, and arrows point to mitotic nuclei abutting the endoderm with the long axis perpendicular to the long axis of the endoderm cells, a characteristic of dividing endothelial cells. In contrast, the arrowhead in C points to a mitotic nucleus in the endoderm with the long axis parallel to the long axis of the endoderm cells, a characteristic of dividing endoderm. The arrowhead in B points to a mitotic nucleus of unknown cell type. (D-F) Arrows point to mitotic nuclei of PECAM+ cells. En, visceral endoderm of the yolk sac.
Flt-1 −/− embryos have increased mitoses in endothelial cells.
Transverse sections of day 8.5 embryos were processed for immunohistochemistry by using antiphosphohistone H3 to detect mitotic nuclei (A-C), and overlays of adjacent sections were processed individually (see “Materials and methods” section) for immunohistochemistry with antiphospohistone H3 (red), anti-PECAM (green), and DAPI (blue) (D-F). Visualization of yolk sacs offlt-1+/− (A) or WT (+/+) (D) embryos that were phenotypically normal showed few mitotic nuclei in vascular areas (arrow in D). In contrast,flt-1−/− embryos (B,C,E,F) exhibited vascular overgrowth and numerous mitotic nuclei (red; E,F) in PECAM+regions (green; E,F) of the yolk sac and allantois (F, left part of panel). (A-C) Asterisks denote the lumina of blood islands in the yolk sac, and arrows point to mitotic nuclei abutting the endoderm with the long axis perpendicular to the long axis of the endoderm cells, a characteristic of dividing endothelial cells. In contrast, the arrowhead in C points to a mitotic nucleus in the endoderm with the long axis parallel to the long axis of the endoderm cells, a characteristic of dividing endoderm. The arrowhead in B points to a mitotic nucleus of unknown cell type. (D-F) Arrows point to mitotic nuclei of PECAM+ cells. En, visceral endoderm of the yolk sac.
Discussion
Our data support a model whereby flt-1 normally affects early vascular development by negatively modulating cell division in the vascular lineage. The identification of this cellular mechanism of flt-1 action suggests that flt-1 is critical for the fine tuning of VEGF-mediated vessel growth that is required to form proper blood vessels. It also strongly suggests that flt-1 may affect blood vessel formation in similar ways in both the embryo and the adult. Embryos and differentiated ES cells lacking flt-1 have increased vascularization and numbers of endothelial cells accompanied by an increased endothelial cell mitotic index. In contrast, the nonendothelial cell mitotic index is similar in both genetic backgrounds, indicating that the increased mitotic rate in the flt-1−/−background is endothelial cell specific.
The ability of mitomycin C to partially rescue theflt-1−/− vascular phenotype further supports the conclusion that deregulated endothelial cell division is responsible for the flt-1 mutant phenotype. The WT cultures were also affected, which was expected because endothelial cell division is a critical component of normal blood vessel formation.56 A caveat is that mitomycin C inhibits division in all cells, so lack of division in nonendothelial cells could indirectly affect the endothelial cell phenotype. This scenario cannot be ruled out, but the increased endothelial mitotic index in theflt-1 mutant background and its diminution with mitomycin C suggest that a substantial part of the rescue is likely to result from direct effects on endothelial cell division. This model can be more precisely tested by expressing genes that modulate cell division under the control of endothelial-specific regulatory sequences in the mutant ES cells.
Flt-1 modulates cell division in the vascular lineage at the earliest stages of vascular development. The first documented difference in ES cell cultures was at day 5, when flt-1−/−mutant cultures had more cells expressing β-galactosidase under control of the flt-1 promoter than flt-1+/−cultures. The exact identity of these cells is unclear because we have identified a nonvascular, flt-1–expressing cell population in ES cell cultures, and several cell types such as trophoblasts and monocyte/macrophages express flt-1 in vivo.40 57-59However, because endothelial cells also express flt-1, it is likely that at least a subpopulation of these cells are vascular precursor cells. In any case, the expansion of β-galactosidase–expressing cells in the flt-1−/− mutant background could be blocked by mitomycin C from days 4 to 5 onward, indicating that the expansion of this cell population resulted from aberrant cell division. Interestingly, vascular cells expressing PECAM and/or VE-cadherin were much less prevalent in the flt-1−/− mutant cultures on day 5, suggesting that different subpopulations of vascular precursor cells may be affected by the flt-1 mutation at different times. The expansion of PECAM+ and/or VE-cadherin+ vascular cells was not evident until day 6 in the flt-1−/− mutant background, and this expansion was also blocked by mitomycin C. Thus, flt-1 has a major role in modulating cell division in the vascular lineage starting at days 4 to 5 of ES cell differentiation, just before formation of the first primitive blood vessels.
Other processes can also affect the number of endothelial cells, including cell fate decisions and programmed cell death. Appreciable endothelial cell death is not observed during days 5 to 8 of normal ES cell differentiation (V.L.B., unpublished observation), so inhibition of apoptosis is unlikely to make a major contribution to the flt-1 mutant phenotype. Our results do not formally exclude that, in addition to an effect on vascular cell division, flt-1 may alter cell fate by affecting hemangioblast formation,34 but our results are not consistent with this model. We see no significant differences between normal and mutant cultures until day 5, well beyond the peak of hemangioblast formation at days 2.5 to 3.0.52In the hemangioblast study, increased PECAM and β-galactosidase staining during differentiation of flt-1−/−EBs was interpreted as increased hemangioblast numbers, but the lack of a definitive hemangioblast marker makes it impossible to distinguish between hemangioblasts, angioblasts, and differentiated endothelial cells using these criteria. Moreover, in our hands the expansion of the vascular lineage was blocked by mitomycin C at its earliest detection on days 4 to 5, suggesting that the major effect of the flt-1 mutation on vascular growth results from aberrant cell division.
The identification of flt-1 as an early modulator of cell division in vascular development is consistent with several elegant studies showing that flt-1 affects endothelial cell mitogenesis in cultured endothelial cells.31,41,42 Extending this model of flt-1 action to the earliest stages of development has several implications. First, it suggests that deregulation of proliferation can be sufficient to disrupt developmental processes. Other recent investigations of the role of the cell cycle in development support this hypothesis.60 Second, the data suggest that flt-1 can modulate the endothelial cell cycle developmentally by affecting one or more molecular signaling pathways, although which pathways are affected is not entirely clear. Deletion of the flt-1 tyrosine kinase domain does not disrupt vascular development,58 suggesting that signaling through this domain is not necessary for flt-1 to affect the endothelial cell cycle developmentally. Signaling through flk-1 does produce a strong endothelial mitogenic signal, and flk-1 selective inhibitors partially rescue the flt-1−/−phenotype in ES cell cultures (D. Roberts and V.L.B., unpublished results). This finding suggests that flt-1 affects vascular development at least in part by modulating VEGF-mediated flk-1 signaling, and this modulation could occur in several ways.
A soluble form of flt-1, sflt-1, is expressed during development61 and ES cell differentiation (J.B.K. and V.L.B., unpublished results), and it can inhibit VEGF-dependent endothelial cell division.38,39 Thus, sflt-1 can bind VEGF and prevent ligand-induced dimerization of the flk-1 receptor. The full-length receptor can also theoretically form an inactive heterodimer with flk-1, as suggested by a recent study using chimeric receptors.41 In addition, ligand engagement of flt-1 may modulate flk-1 signaling at points downstream in the signal transduction pathway. This model is supported by the inhibitor sensitivity of chimeric receptors and a study implicating nitric oxide as a mediator of flt-1 effects on the flk-1 mitogenic pathway.42 62 Importantly, these models of flt-1 action are not mutually exclusive, and it is likely that flt-1 uses some combination of these actions to modulate endothelial cell division developmentally. The identification of the cellular mechanism of flt-1 action suggests ways to test these molecular models.
Flt-1−/− mutant embryos had increased mitoses in areas rich in endothelial cells and an increased mitotic index as well, indicating that aberrant endothelial cell division contributes to the mutant phenotype in vivo. To our knowledge this is the first demonstration that flt-1 affects endothelial cell division in vivo. The ability of flt-1 to negatively modulate endothelial cell division in vivo indicates that it is an endogenous negative regulator of blood vessel formation. Our data show that flt-1 regulates vascular growth from the earliest stages of vascular development, and it is likely to modulate angiogenesis in the adult organism by a similar cellular mechanism. Several molecules, such as angiostatin and endostatin, negatively regulate pathologic blood vessel formation when administered exogenously, and some of these regulators are likely to control pathologic vascularization by endogenous production.63-65However, these angiogenesis inhibitors have surprisingly little effect on normal blood vessel formation. Clearly, our knowledge of how blood vessel formation is negatively regulated is sparse compared with what is known of positive regulation.
VEGF expression is up-regulated in many pathologies with vascular components, such as cancer and chronic inflammation.66-70Thus, flt-1 could potentially negatively modulate pathologic vascularization, as described here for vascular development, and therapeutics that specifically block flt-1 action may help rather than hinder pathologic vascularization. Conversely, VEGF treatment can in some cases promote vascularization of ischemic limbs,71 72but our lack of understanding about how VEGF signaling is normally exquisitely fine-tuned has hampered our ability to produce functional vessels therapeutically. Flt-1 clearly participates in the modulation of VEGF-mediated vascular growth, and understanding the role of flt-1 in controlling this process should help in designing better therapies. In any case, defining the cellular mechanism of flt-1 action in endothelial cells developmentally suggests alternative ways to modulate blood vessel formation in vivo.
We thank Guo-Hua Fong for supplying the flt-1 mutant ES cell lines and mice and the pflt probe. We thank Bob Duronio, Anthony LaMantia, and Cam Patterson for critical reading of the manuscript; Susan Whitfield for artwork; and fellow lab members for fruitful discussion.
Supported by grants from the National Institutes of Health (HL43174) and Glaxo-Wellcome to V.L.B. V.L.B. was supported by a National Institutes of Health Career Development Award (HL02908), and J.B.K. was supported by a predoctoral fellowship from the Department of Defense (DAMD 17-00-1-0379).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Victoria L. Bautch, CB# 3280, The University of North Carolina at Chapel Hill, Chapel Hill, NC 27599; e-mail:bautch@med.unc.edu.