Our inability to purify hematopoietic stem cells (HSCs) precludes direct study of many aspects of their behavior in the clinical hematopoietic stem cell transplantation (HSCT) setting. We indirectly assessed stem/progenitor cell behavior in the first year after HSCT by examining changes in neutrophil telomere length, X-inactivation ratios, and cycling of marrow progenitors in 25 fully engrafted allogeneic HSCT recipients. Donors were sampled once and recipients at engraftment and 2 to 6 months and 12 months after HSCT. Telomere length was measured by an in-gel hybridization technique, X-inactivation ratios were measured by the human androgen receptor assay, and cell cycle status was determined by flow cytometric analysis of pyronin Y- and Hoechst 33342–stained CD34+CD90+ and CD34+CD90− marrow cells. Compared with their donors, recipients' telomeres were shortened at engraftment (−424 base pairs [bp]; P < .0001), 6 months (−495 bp; P = .0001) after HSCT, and 12 months after HSCT (−565 bp; P < .0001). There was no consistent pattern of change in telomere length from 1 to 12 months after HSCT; marked, seemingly random, fluctuations were common. In 11 of 11 informative recipients, donor X-inactivation ratios were faithfully reproduced and maintained. The proportion of CD34+CD90+ progenitors in S/G2/M was 4.3% in donors, 15.7% at 2 to 6 months (P < .0001) after HSCT, and 11.5% at 12 months after HSCT (P < .0001, versus donors; P = .04, versus 2-6 months). Cycling of CD34+ CD90−progenitors was largely unchanged. We infer that (1) HSCT-induced accelerated telomere loss is temporary and unlikely to promote graft failure or clonal hematopoietic disorders and (2) the striking fluctuations in telomere length and variation in pattern of telomere loss reflect stochastic determination of HSC fate after HSCT.
Introduction
Hematopoietic stem cell transplantation (HSCT) is increasingly used to treat patients with a variety of malignant and nonmalignant diseases. Its clinical application has evolved in the absence of rigorous understanding of many aspects of its biology. In particular, the phenomena of engraftment and hematopoietic reconstitution remain poorly understood.
The marrow repopulating hematopoietic stem cells (HSCs) in an allograft represent a small fraction of the donor's HSCs, yet they are required to reconstitute hematopoiesis and sustain it for the lifetime of the recipient. Such a burden imposes a “replicative stress” on hematopoietic stem/progenitor cells. Superficially, hematopoietic reconstitution after HSCT appears complete, with peripheral blood cell counts and bone marrow cellularity normalizing in most recipients within a few months of transplantation. However, studies of recipients' hematopoietic progenitors, defined functionally (according to clonogenic potential) or phenotypically (according to surface antigen expression), have revealed marked deficits persisting for more than 10 years after HSCT.1-5
Most human hematopoietic stem/progenitor cells express the CD34 antigen.6 Most primitive hematopoietic progenitors, and virtually all CD34+ HSCs, are contained within the CD90+ subset.7-10 Although markedly enriched for primitive progenitors, the CD34+CD90+population is heterogeneous and contains some lineage-committed progenitors. Most lineage-committed progenitors, however, reside in the larger CD34+CD90− subset.7,8 The CD34+ cell population is diminished in HSCT recipients.5 To meet hematopoietic demands, these cells must increase their rate of proliferation.1 We have previously shown that the mitotic activity of CD34+CD90+ progenitors is increased nearly 4-fold over steady-state levels in the first 6 months after transplantation.11
In recent years, we and others have noted excessive telomere shortening in leukocytes of HSCT recipients.12-19 Telomeres, the nonencoding regions of DNA at each end of eukaryotic chromosomes, consist of stereotyped oligonucleotide repeats and associated protein.20 They shorten with each round of mitosis in normal human somatic cells, in contrast to the encoding DNA sequences.20 Such shortening is due to the “end replication problem,” whereby a stretch of DNA is lost from the 5′ end of each new DNA strand.21 This DNA loss is estimated at 40 to 120 base pairs (bp) per division.20 Thus, telomeres have been likened to a “mitotic clock.”22 The ribonucleoprotein reverse transcriptase, telomerase, may restore telomere length.23 It is variably present in hematopoietic progenitors and in monocytes and lymphocytes,24 but in these cells its presence does not completely attenuate telomere shortening.25,26 Cross-sectional studies of healthy subjects older than 4 years have shown a yearly decrease in peripheral blood leukocyte telomere length of approximately 30 to 60 bp.13 26-28 It has been inferred that this decrease represents the yearly aggregate telomere loss in HSCs.
Excessive leukocyte telomere shortening has been observed in virtually all short- and long-term survivors of autologous and allogeneic HSCTs studied and has amounted to between 300 and 2000 bp.12-19It occurs in neutrophils, monocytes, and T cells.12,16,19Notaro et al12 found an inverse relationship between the extent of post-HSCT telomere loss and the number of nucleated cells in the graft. Another study found a correlation between donor age and the extent of telomere shortening in allogeneic recipients.14Other studies have failed to identify these, or any other, associations.13,15 16 Most investigators have invoked HSC replicative stress as the cause of HSCT-induced accelerated telomere shortening. Some have concluded that transplantation induces accelerated HSC “aging.” Direct study of telomere changes in HSCs has been precluded by our current technical inability to isolate a pure HSC population for analysis.
HSC behavior can be studied indirectly by examining X chromosome inactivation ratios in circulating leukocytes of female subjects.29-31 Polymorphisms existing at distinct loci on the X chromosome can be used to distinguish paternally and maternally derived X chromosomes. The active and inactive X chromosome can be distinguished by differential DNA methylation patterns at these loci.32 The human androgen receptor (HUMARA) locus has been useful, because it is amenable to polymerase chain reaction (PCR)–based analysis, and approximately 90% of women are heterozygous and, therefore, informative.31,32 Skewed X-inactivation ratios (arbitrarily defined as inactivation of one allele in more than 75% of cells) have been demonstrated in leukocytes of hematologically normal women. The incidence of such skewing rises with age.31 It is likely that most cases of acquired skewing in apparently healthy individuals are due to a growth advantage conferred by parent-specific X-linked alleles (hemizygous selection),33 although other causes, such as stem cell depletion and the expansion of premalignant clones, have been suggested.29,34 Autologous HSCT experiments in female Safari cats have shown that low stem cell doses can result in skewed X-inactivation ratios, suggestive of oligoclonal hematopoiesis, and an extended period of clonal instability after transplantation.29 Polyclonal hematopoiesis has been demonstrated in most human allogeneic HSCT recipients studied.18,35 36
The central aim of this study was to investigate the fate of HSCs in human allogeneic HSCT recipients. Because these cells are currently not amenable to direct study, surrogate markers of HSC behavior were examined. Specifically, we determined the kinetics of telomere shortening in neutrophils in the year after HSCT and sought correlations with changes in X-inactivation ratios and in the mitotic activity of CD34+CD90+ and CD34+CD90− hematopoietic progenitors. Our findings shed new light on hematopoietic reconstitution after HSCT and have important implications for the clinical application of ex vivo HSC expansion and gene therapy.
Patients, materials, and methods
Patients and transplantation characteristics
A total of 25 subjects (R1-R25) undergoing allogeneic HSCT at Princess Margaret Hospital or the Hospital for Sick Children in Toronto, together with their related donors (D1-D25), entered the study between September 1999 and January 2001. The study was approved by the Research Ethics Board at each institution, and informed consent was obtained from all participants. The recipients' clinical and transplantation characteristics are outlined in Table1.
Clinical and transplant characteristics of HSCT recipients
| ID . | Recipient age (y)/sex . | Diagnosis . | Donor age (y)/sex . | Conditioning regimen . | Cell dose × 108/kg* . | ANC/platelet† recovery (d) . | Acute/chronic GVHD . | Outcome‡ . |
|---|---|---|---|---|---|---|---|---|
| 1 | 51/F | AML | 29/F | BU/CY | 4.0 | 15/22 | I/0 | Died |
| 2 | 45/F | NHL | 50/F | BU/CY | 6.8 | 19/36 | III/E | EFS |
| 3 | 53/M | AML | 56/F | CY/TBI | 2.5 | 21/33 | I/E | EFS |
| 4 | 43/M | CLL | 55/F | BU/CY | 2.6 | 19/24 | II/0 | EFS |
| 5 | 58/M | CML | 62/F | CY/TBI/AraC | 1.7 | 23/18 | II/0 | EFS |
| 6 | 56/F | NHL | 61/F | BU/CY | 3.0 | 27/25 | 0/0 | EFS |
| 7 | 42/M | CML | 36/F | BU/CY | 3.0 | 26/22 | I/E | EFS |
| 8 | 55/F | NHL | 43/F | BU/CY | 2.6 | 22/37 | II/0 | EFS |
| 9 | 23/M | NHL | 30/F | CY/TBI | 2.5 | 34/23 | II/0 | EFS |
| 10 | 12/M | AML | 10/F | CY/TBI | 2.5 | 14/13 | 0/E | EFS |
| 11 | 52/F | CML | 46/F | BU/CY | 3.2 | 28/22 | II/E | EFS |
| 12 | 11/M | ALL | 13/M | CY/TBI | 2.7 | 14/24 | 0/0 | EFS |
| 13 | 18/M | AML | 45/M | BU/CY | 2.8 | 16/63 | III/E | EFS |
| 14 | 40/M | ALL | 56/F | CY/TBI | 2.8 | 17/17 | II/E | EFS |
| 15 | 38/M | MF | 37/F | CY/TBI | 3.4 | 19/23 | II/E | EFS |
| 16 | 55/F | CML | 52/M | CY/TBI/AraC | 1.0 | 21/19 | III/E | Died |
| 17 | 50/M | NHL | 42/M | BU/CY | 3.8 | 15/20 | II/E | EFS |
| 18 | 55/M | CML | 45/M | BU/CY | 2.9 | 25/20 | I/L | EFS |
| 19 | 49/M | CML | 46/F | BU/CY | 2.6 | 19/15 | I/L | EFS |
| 20 | 54/M | AML | 49/M | BU/CY | 2.2 | 21/23 | I/0 | EFS |
| 21 | 36/M | MDS | 30/M | CY/TBI | 3.5 | 24/— | 0/— | Died |
| 22 | 42/F | MDS | 40/M | BU/CY | 2.8 | 24/— | II/E | Died |
| 23 | 52/M | PLL | 65/M | BU/CY | 2.0 | 27/24 | 0/— | EFS |
| 24 | 52/F | AML | 50/M | VP-16/TBI | 3.8 | 19/28 | II/— | EFS |
| 25 | 46/M | CML | 49/M | CY/TBI | 2.9 | 18/19 | II/— | EFS |
| ID . | Recipient age (y)/sex . | Diagnosis . | Donor age (y)/sex . | Conditioning regimen . | Cell dose × 108/kg* . | ANC/platelet† recovery (d) . | Acute/chronic GVHD . | Outcome‡ . |
|---|---|---|---|---|---|---|---|---|
| 1 | 51/F | AML | 29/F | BU/CY | 4.0 | 15/22 | I/0 | Died |
| 2 | 45/F | NHL | 50/F | BU/CY | 6.8 | 19/36 | III/E | EFS |
| 3 | 53/M | AML | 56/F | CY/TBI | 2.5 | 21/33 | I/E | EFS |
| 4 | 43/M | CLL | 55/F | BU/CY | 2.6 | 19/24 | II/0 | EFS |
| 5 | 58/M | CML | 62/F | CY/TBI/AraC | 1.7 | 23/18 | II/0 | EFS |
| 6 | 56/F | NHL | 61/F | BU/CY | 3.0 | 27/25 | 0/0 | EFS |
| 7 | 42/M | CML | 36/F | BU/CY | 3.0 | 26/22 | I/E | EFS |
| 8 | 55/F | NHL | 43/F | BU/CY | 2.6 | 22/37 | II/0 | EFS |
| 9 | 23/M | NHL | 30/F | CY/TBI | 2.5 | 34/23 | II/0 | EFS |
| 10 | 12/M | AML | 10/F | CY/TBI | 2.5 | 14/13 | 0/E | EFS |
| 11 | 52/F | CML | 46/F | BU/CY | 3.2 | 28/22 | II/E | EFS |
| 12 | 11/M | ALL | 13/M | CY/TBI | 2.7 | 14/24 | 0/0 | EFS |
| 13 | 18/M | AML | 45/M | BU/CY | 2.8 | 16/63 | III/E | EFS |
| 14 | 40/M | ALL | 56/F | CY/TBI | 2.8 | 17/17 | II/E | EFS |
| 15 | 38/M | MF | 37/F | CY/TBI | 3.4 | 19/23 | II/E | EFS |
| 16 | 55/F | CML | 52/M | CY/TBI/AraC | 1.0 | 21/19 | III/E | Died |
| 17 | 50/M | NHL | 42/M | BU/CY | 3.8 | 15/20 | II/E | EFS |
| 18 | 55/M | CML | 45/M | BU/CY | 2.9 | 25/20 | I/L | EFS |
| 19 | 49/M | CML | 46/F | BU/CY | 2.6 | 19/15 | I/L | EFS |
| 20 | 54/M | AML | 49/M | BU/CY | 2.2 | 21/23 | I/0 | EFS |
| 21 | 36/M | MDS | 30/M | CY/TBI | 3.5 | 24/— | 0/— | Died |
| 22 | 42/F | MDS | 40/M | BU/CY | 2.8 | 24/— | II/E | Died |
| 23 | 52/M | PLL | 65/M | BU/CY | 2.0 | 27/24 | 0/— | EFS |
| 24 | 52/F | AML | 50/M | VP-16/TBI | 3.8 | 19/28 | II/— | EFS |
| 25 | 46/M | CML | 49/M | CY/TBI | 2.9 | 18/19 | II/— | EFS |
ANC, absolute neutrophil count; GVHD, graft-versus-host disease; acute GVHD graded I-IV39; chronic GVHD classified L (limited) or E (extensive)40; AML, acute myeloid leukemia; BU, busulphan; CY, cyclophosphamide; NHL, non-Hodgkin lymphoma; EFS, event-free survival; TBI, total body irradiation (fractionated when combined with CY or VP-16 alone, otherwise single dose); CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; AraC, cytarabine; ALL, acute lymphoblastic leukemia; MF, myelofibrosis; MDS, myelodysplasia; PLL, prolymphocytic leukemia; —, not performed; VP-16, etoposide.
Nucleated cell dose (all bone marrow grafts, except mobilized peripheral blood for R19).
Recovery of ANC to > 0.5 × 109/L and unsupported platelets of > 20 × 109/L.
Patients followed until death or for 1 year, except R23-R25 followed for 60 days; deaths were transplantation related in R16 and R21, and because of relapse in R1 and R22.
The median age of the recipients was 49 years (range, 11 to 58). The indications for HSCT were chronic myelogenous leukemia in 7 patients, acute myelogenous leukemia in 6, non-Hodgkin lymphoma in 5, acute lymphoblastic leukemia in 2, myelodysplasia in 2, acute prolymphocytic leukemia in 1, chronic lymphocytic leukemia in 1, and myelofibrosis in 1. The median age of the donors was 45 years (range, 10 to 64). All donors were hematologically normal as established by history, examination of peripheral blood cell counts, and bone marrow morphology.
Patients and donors underwent serologic typing for human leukocyte antigens (HLA) A and B and high-resolution molecular HLA-DRB1 typing. Twenty-two donor/recipient pairs were fully HLA-matched, and 3 (D/R11, D/R13, and D/R21) were mismatched for one antigen. All patients received myeloablative conditioning: 14 with busulphan and cyclophosphamide37 and 11 with radiation-based regimens.38 Hematopoietic cytokines were not administered to bone marrow donors before harvest. Twenty-four patients received bone marrow grafts; one received mobilized peripheral blood. The donor of mobilized peripheral blood (D19) was mobilized with recombinant human granulocyte colony-stimulating factor administered for 5 days (5 μg/kg/d), with apheresis performed on days 4 and 5. Grafts were not T-cell depleted and contained a median of 2.8 × 108nucleated cells per kilogram recipient weight (range, 1 to 6.8 × 108). Three recipients received granulocyte colony-stimulating factor after HSCT (5 μg/kg/d): R12 from day +1 until the second of 2 consecutive days with an absolute neutrophil count (ANC) more than 1.0 × 109/L, R9 from day +28 until day +38 because of delayed engraftment, and R13 from day +13 to day +20 in the setting of severe sepsis. Cyclosporine and short-course methotrexate were used in all patients for graft-versus-host disease (GVHD) prophylaxis.38 Clinical grading of acute GVHD was performed according to the criteria of Glucksberg et al,39and chronic GVHD was diagnosed and classified according to standard criteria.40 Twenty-two recipients were followed until time of death or 1 year after HSCT. The other 3 (R23-R25) were studied in greater detail immediately after engraftment and were followed for 60 days. Four patients died: R1 and R22 because of relapse at 176 and 233 days, respectively; R16 because of complications of chronic GVHD at 248 days; and R21 from regimen-related toxicity at 38 days.
Specimen collection
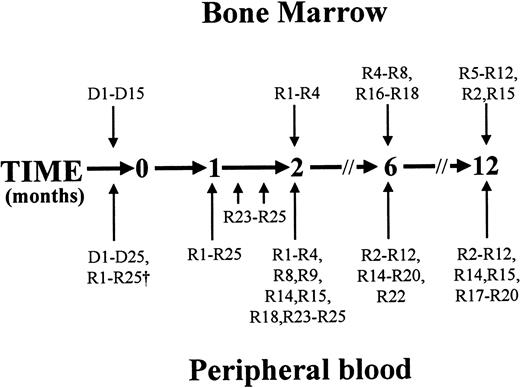
Peripheral blood and bone marrow were collected and tested as outlined in Figure 1. The core requirement was for blood samples to be drawn from donors and recipients before HSCT and from recipients at the time of engraftment and at 6 and 12 months after HSCT. The only surviving recipient who was not tested 6 and 12 months after HSCT (R13) suffered pulmonary insufficiency that precluded air travel to attend follow-up appointments at the transplantation center.
Timing of specimen collection.
Peripheral blood (20-40 mL) was obtained from all donors before bone marrow harvest or mobilized peripheral blood collection and from recipients before conditioning. Microsatellite analysis was performed on whole blood leukocytes from these samples to allow for subsequent assessment of hematopoietic chimerism in recipients. Donors' neutrophils were isolated, and their telomere length and X-inactivation pattern (when the donor was female) were assessed. HSCT was performed at time zero. All recipients were sampled within 2 weeks of attainment of an ANC more than 0.5 × 109/L after HSCT (represented by the arrow at 1 month). Three recipients (R23, R24, and R25) underwent further peripheral blood sampling on 3 occasions at weekly intervals from the time of engraftment. Nineteen of 20 surviving patients were sampled 6 months after HSCT, and 17 of 18 surviving patients were sampled 12 months after HSCT. In addition, 9 patients were tested 2 months after HSCT, as shown. Complete blood cell counts and differential white blood cell counts, hematopoietic chimerism, neutrophil telomere length, and X-inactivation patterns in neutrophils (when the donor was female) were determined at these times. Bone marrow specimens (5-10 mL) were obtained from 15 donors at the time of bone marrow harvest, and from 4 recipients 2 months after HSCT, 7 recipients 6 months after HSCT, and 10 recipients 12 months after HSCT, as indicated. The proportion of CD34+ cells expressing CD90 was determined, and the cell cycle status of CD90+/−subsets was examined.
Timing of specimen collection.
Peripheral blood (20-40 mL) was obtained from all donors before bone marrow harvest or mobilized peripheral blood collection and from recipients before conditioning. Microsatellite analysis was performed on whole blood leukocytes from these samples to allow for subsequent assessment of hematopoietic chimerism in recipients. Donors' neutrophils were isolated, and their telomere length and X-inactivation pattern (when the donor was female) were assessed. HSCT was performed at time zero. All recipients were sampled within 2 weeks of attainment of an ANC more than 0.5 × 109/L after HSCT (represented by the arrow at 1 month). Three recipients (R23, R24, and R25) underwent further peripheral blood sampling on 3 occasions at weekly intervals from the time of engraftment. Nineteen of 20 surviving patients were sampled 6 months after HSCT, and 17 of 18 surviving patients were sampled 12 months after HSCT. In addition, 9 patients were tested 2 months after HSCT, as shown. Complete blood cell counts and differential white blood cell counts, hematopoietic chimerism, neutrophil telomere length, and X-inactivation patterns in neutrophils (when the donor was female) were determined at these times. Bone marrow specimens (5-10 mL) were obtained from 15 donors at the time of bone marrow harvest, and from 4 recipients 2 months after HSCT, 7 recipients 6 months after HSCT, and 10 recipients 12 months after HSCT, as indicated. The proportion of CD34+ cells expressing CD90 was determined, and the cell cycle status of CD90+/−subsets was examined.
Cell processing and DNA extraction
Neutrophils were obtained by aspiration of the cell layer immediately above the red cell pellet after sequential centrifugation of peripheral blood samples on 58% Percoll (Amersham Pharmacia Biotech [APB], Piscataway, NJ) and Ficoll-Paque (APB). Purity, confirmed by examination of Wright-stained smears, always exceeded 95%. Neutrophils were placed in lysis buffer (1% sodium dodecyl sulfate, 10 mM tris-HCl, 1 mM EDTA, and 100 mM NaCl) and incubated overnight at 37°C with proteinase K (100 μg/mL). DNA was extracted with phenol/chloroform/isoamyl alcohol, precipitated in 95% ethanol, and dissolved in TrisEDTA, as described.13 16 The integrity of each DNA sample was confirmed visually after electrophoresis of 1 μg in ethidium bromide–stained 0.8% agarose minigels with control lanes of phage λ DNA digested with HindIII (λHIII). Low-density mononuclear cells were isolated from bone marrow samples after centrifugation on 58% Percoll.
Chimerism studies
Leukocyte DNA was extracted and amplification of 8 microsatellite regions was performed by PCR, as described.41 After gel electrophoresis, hematopoietic chimerism was determined by comparing the size of amplified bands from the recipient at the time of blood sampling after HSCT with the size of bands from donor and recipient samples before HSCT. The test allows detection of admixtures of donor and recipient DNA down to the level of 5% to 10%.
Measurement of mean terminal restriction fragment length of neutrophils
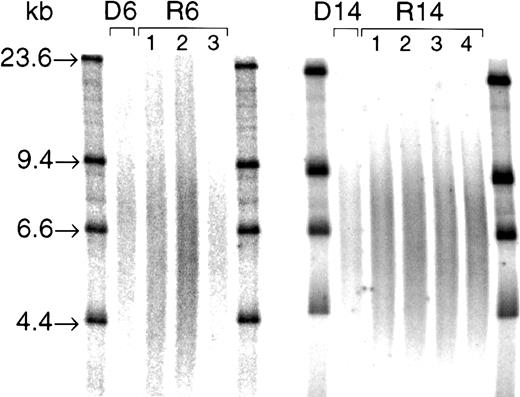
Genomic DNA was digested by using the restriction enzymesRsaI and HinfI. The completeness of digestion was confirmed by electrophoresis and visualization of aliquots (1 μg) on ethidium bromide–stained 0.8% agarose minigels. Digested DNA (3 μg) was loaded into 0.5% agarose gels and electrophoresed overnight alongside a 32P-labeled size marker (λHIII). Samples from donor/recipient pairs were run in adjacent lanes in the same gel. After drying, denaturing, and neutralization, gels were hybridized to a γ-32P end-labeled 5′-(CCCTAA)3 telomeric probe at 37°C overnight, as described.13,16 Gels were then washed in decreasing concentrations of sodium chloride/sodium citrate and exposed to a PhosphorImager plate (Molecular Dynamics, Sunnyvale, CA). Lanes from representative gels are shown in Figure2. Each terminal restriction fragment (TRF) smear was quantified by densitometry with the use of ImageQuant software (Molecular Dynamics) by an investigator blinded to the identity of samples. Mean TRF length was calculated as previously described,13,16 using the following equation: mean TRF length = Σ(ODi × MWi)/Σ(ODi) where ODi is the optical density at position “i,” and MWi is the molecular weight of DNA at point “i.” Mean TRF lengths were corrected for the running of inter-gel control samples, as previously described.26 Paired control samples were run in adjacent lanes of 15 gels to establish methodologic variance in determination of differences in mean TRF length; derived differences between these control samples varied by 93 ± 59 bp. Thus, the smallest true difference in mean TRF length reliably detected by this method was 270 bp (the mean + 3 SD of the variance observed among paired controls).12
TRF smears from 2 donor/recipient pairs (D/R6 and D/R14).
Three micrograms RsaI and HinfI digests were loaded into a 0.5% agarose gel and electrophoresed overnight alongside 32P-labeled size markers (λHIII; with bands at 23.6, 9.4, 6.6, and 4.4 kb, as indicated). After gel drying, denaturing, and neutralization, gels were hybridized to a γ-32P end-labeled 5′-(CCCTAA)3 telomeric probe at 37°C overnight. After washing, the gel was exposed to a PhosphorImager plate, and each TRF smear was quantified by densitometry using ImageQuant software. Mean TRF lengths for these samples were as follows: D6, 9.2 kb; R6, 8.4 kb, 8.7 kb, and 8.6 kb, at engraftment (1), 6 months (2), and 12 months (3), respectively; D14, 8.6 kb; R14, 8.5 kb, 8.4 kb, 8.4 kb, and 8.4 kb, at engraftment (1), 2 months (2), 6 months (3), and 12 months (4), respectively.
TRF smears from 2 donor/recipient pairs (D/R6 and D/R14).
Three micrograms RsaI and HinfI digests were loaded into a 0.5% agarose gel and electrophoresed overnight alongside 32P-labeled size markers (λHIII; with bands at 23.6, 9.4, 6.6, and 4.4 kb, as indicated). After gel drying, denaturing, and neutralization, gels were hybridized to a γ-32P end-labeled 5′-(CCCTAA)3 telomeric probe at 37°C overnight. After washing, the gel was exposed to a PhosphorImager plate, and each TRF smear was quantified by densitometry using ImageQuant software. Mean TRF lengths for these samples were as follows: D6, 9.2 kb; R6, 8.4 kb, 8.7 kb, and 8.6 kb, at engraftment (1), 6 months (2), and 12 months (3), respectively; D14, 8.6 kb; R14, 8.5 kb, 8.4 kb, 8.4 kb, and 8.4 kb, at engraftment (1), 2 months (2), 6 months (3), and 12 months (4), respectively.
X-inactivation analysis with the HUMARA assay
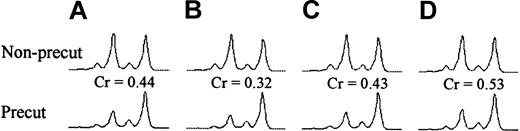
Neutrophil DNA was digested with RsaI orRsaI/HpaII (digest 2) at 37°C overnight. Restriction enzymes were inactivated at 65°C for 10 minutes. Digested DNA (50 ng) was added to a PCR mix (containing buffer, primer HUMARA I, primer HUMARA II, platinum Taq, and water) and amplified on a programmable thermal cycler. The PCR product was then mixed with deionized formamide and the GeneScan 500 TAMRA size standard (Applied Biosystems, Foster City, CA) and denatured at 95°C for 10 minutes. After rapid cooling, the denatured PCR product was run on an automated sequencer (PerkinElmer 310 CE; PerkinElmer, Shelton, CT) for sequence detection. Fragment sizes and peak heights were determined automatically by using GeneScan Analysis 3.1.2 and Genotyper 2.5 software (Applied Biosystems). Fragment peaks from a representative series are shown in Figure 3. Samples were deemed noninformative if only one allele was identified. In informative samples, the shorter of the 2 alleles was termed “allele 1,” according to convention. The ratio between the 2 alleles (allele 1:allele 2) was measured for each sample. The allelic ratio was defined as the ratio between expressions of the 2 alleles and was corrected for potential preferential amplification of one of the alleles by dividing the allelic ratio of the precut sample by the allelic ratio of the non-precut sample of the same specimen (Figure 3). Allelic ratios of 3.0 and 0.33 both describe a 75%/25% split in X-inactivation in a given cellular population, but allele 1 is dominant in the first instance and allele 2 is dominant in the second. Excessive skewing was defined as an allelic ratio more than 3.0 or less than 0.33 (corresponding to > 75% expression of one allele).31
X-inactivation ratios in bone marrow donor (D5) and fully engrafted recipient (R5) in the first year after HSCT.
Corrected HUMARA allelic ratios (Cr) in neutrophils were determined by dividing the allelic ratio of the precut sample by the allelic ratio of the non-precut sample of the same specimen. (A) Donor. (B) Recipient at the time of engraftment. (C) Recipient 6 months after HSCT. (D) Recipient 12 months after HSCT.
X-inactivation ratios in bone marrow donor (D5) and fully engrafted recipient (R5) in the first year after HSCT.
Corrected HUMARA allelic ratios (Cr) in neutrophils were determined by dividing the allelic ratio of the precut sample by the allelic ratio of the non-precut sample of the same specimen. (A) Donor. (B) Recipient at the time of engraftment. (C) Recipient 6 months after HSCT. (D) Recipient 12 months after HSCT.
Isolation of CD34+ cells and fluorescence-activated sorting of CD34+CD90+ and CD34+CD90− progenitors
CD34+ cells were obtained by positive immunomagnetic selection from bone marrow mononuclear cells, using Minimacs separation columns (Miltenyi Biotec, Auburn, CA) following the manufacturer's guidelines. CD34+-selected cells were stained with CD34 fluorescein isothiocyanate (FITC; clone 581; Beckman-Coulter, Miami, FL), CD45 phycoerythrin (PE)/CY5 (clone J33, Beckman-Coulter), and CD90PE (clone 5e10, Pharmingen, Mississauga, ON). CD34+cells were identified by using CD34 and CD45 staining and sequential gating,42 and gated CD34+ cells were analyzed for CD90 expression by using standardized assays for CD34+cell subsets.43 44 CD34+CD90+ and CD34+CD90− cells were sorted on a FACSVantage (Becton Dickinson Immunocytometry Systems, San Jose, CA) cell sorter equipped with Coherent Enterprise (Innova Technologies, Palo Alto, CA) and Helium-Neon (Spectra-Physics, Mountain View, CA) lasers. Small aliquots of sorted cells were reanalyzed to establish sort purity, which always exceeded 94%.
Assessment of cell cycle status of CD34+CD90+ and CD34+CD90− subsets
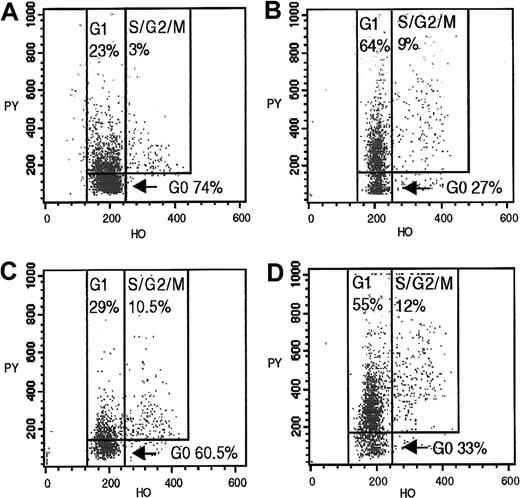
CD34+CD90+ and CD34+CD90− cells were incubated with 1 μg/mL Hoechst 33342 (Sigma, St Louis, MO) (binds DNA) in Hoechst buffer (Hanks balanced salt solution, 20 mM HEPES pH 7.2, glucose 1 g/L, and 10% fetal bovine serum) at 37°C for 45 minutes. Samples were then incubated with 1 μg/mL pyronin Y (Sigma) (binds RNA) at 37°C for 45 minutes. After one wash in RPMI containing 10% fetal bovine serum at 4°C, cells were resuspended in Hoechst buffer, and the cell cycle status of these subsets was ascertained by flow cytometry, as described.44,45 A mean of 1035 events (range, 300 to 2960) were gated for CD34+CD90+ cells. Results of cell cycle analysis of donors' (D1-D14) cells and of recipients' (R1-R8 and R16-R18) cells tested at 2 and 6 months after HSCT have been previously published.11 Control experiments performed to validate G0/G1 discrimination have been previously reported.43 Representative dot plots from a donor/recipient pair are shown in Figure4.
Cycling profiles of CD34+CD90+and CD34+CD90− bone marrow progenitors from a donor (D11) at the time of bone marrow harvest and a fully engrafted matched recipient (R11) 12 months after HSCT.
CD34+CD90+/− subsets, obtained by fluorescence-activated sorting of preselected CD34+ marrow cells, were analyzed by flow cytometry after incubation with Hoechst 33342 and pyronin Y. (A) Donor CD34+CD90+cells. (B) Donor CD34+CD90− cells. (C) Recipient CD34+CD90+ cells 12 months after HSCT. (D) Recipient CD34+CD90− cells 12 months after HSCT.
Cycling profiles of CD34+CD90+and CD34+CD90− bone marrow progenitors from a donor (D11) at the time of bone marrow harvest and a fully engrafted matched recipient (R11) 12 months after HSCT.
CD34+CD90+/− subsets, obtained by fluorescence-activated sorting of preselected CD34+ marrow cells, were analyzed by flow cytometry after incubation with Hoechst 33342 and pyronin Y. (A) Donor CD34+CD90+cells. (B) Donor CD34+CD90− cells. (C) Recipient CD34+CD90+ cells 12 months after HSCT. (D) Recipient CD34+CD90− cells 12 months after HSCT.
Statistical analysis
Changes in neutrophil telomere length and cycling of marrow progenitors are expressed as mean ± SD. Two-tailed paired Studentt tests were used to compare donor and recipient telomere lengths and progenitor cycling status. Linear regression analysis was used to explore the relationship between changes in telomere length over time within recipients. To discern whether a nonlinear or seemingly random relationship was evident, we plotted residuals (the difference between the actual and predicted value for each observation) against time and checked for a systematic pattern. Univariable analyses were then performed to search for predictors of the extent of telomere loss in recipients at 12 months after HSCT and for predictors of the pattern of telomere loss over the 12 months. Donor age, nucleated cell dose, the extent of GVHD, and the proportion of CD34+CD90+ progenitors in S/G2/M in recipients were examined in multivariable models. All analyses were performed by using the SAS statistical program (SAS-PC, Version 8.0; SAS Institute, Cary, NC).
Results
Chimerism and recovery of peripheral blood cell counts
All HSCT recipients were complete hematopoietic chimeras (> 90% donor hematopoiesis) at the time each blood and bone marrow sample was obtained after HSCT, as determined by analysis of donor- and recipient-specific microsatellite DNA markers.
In all but one patient, the ANC recovered to more than 0.5 × 109/L before day +28. In R9, neutrophil engraftment did not occur until day +34. One recipient (R23) was mildly neutropenic at 2 months, and another (R18) at 6 months after HSCT; neutrophil counts were otherwise in the normal range from 2 to 12 months after HSCT in this cohort. Sustained platelet engraftment (defined as transfusion-independent platelet counts of more than 20 × 109/L) occurred in 23 patients after a median of 23 days (range, 13 to 63); it had not occurred in R21 and R22 at the time of their deaths 38 and 232 days after HSCT, respectively. All surviving recipients were platelet transfusion-independent at 6 and 12 months of follow-up.
Changes in neutrophil telomere length
Overall, the mean TRF length in recipients' neutrophils was significantly shorter than in their respective donors at engraftment (−424 ± 350 bp; P < .0001), 6 months (−495 ± 436 bp; P < .0001), and 12 months (−565 ± 420 bp;P < .0001) after HSCT. The degree of telomere shortening at 2 months after HSCT (−325 ± 520 bp) did not achieve statistical significance (P = .054); however, only 12 of 24 surviving recipients were tested at this time.
Considering recipient telomere length in isolation from donor values, there was no significant difference between telomere length at engraftment, 2, 6, and 12 months by paired t tests, and the slope of the regression line derived from these telomere lengths was not significantly different from zero (P = .16). To determine if a nonlinear model would fit the data better, residuals from the regression line expressing change in telomere length were plotted against time; their random distribution suggested random determination of the pattern of change in telomere length. The pattern of change in the mean TRF length of each recipient is shown in Figure5. The variation among this cohort was striking. Many recipients showed marked fluctuations in telomere length over the 12-month period of observation; in some, pronounced fluctuations were observed in the first 60 days after HSCT (Figure 5F). In 5 instances, telomere lengths exceeding donor values were transiently recorded in recipients (R2, R3, R4, R8, and R9). Six recipients (R1, R10, R13, R14, R17, and R21) showed no significant change from donor telomere length over the period of follow-up.
Changes in mean TRF length in neutrophils of fully engrafted HSCT recipients.
Telomere length in donors is indicated at time zero. (A) R1 to R4. (B) R5 to R7 and R10. (C) R8, R9, R14, R15, and R18. (D) R11 to R13, R16, and R17. (E) R19 to R22. (F) R23 to R25. To facilitate comparison, (A,C) recipients sampled 2 months after HSCT (in addition to other times) are presented together; (F) those sampled on 4 occasions in the first 60 days are shown together. On the basis of analysis of paired control samples, changes in mean TRF length exceeding 270 bp may be regarded as significant. Note that, although telomeres in recipients tended to be shorter than in their respective donors, they were not always so. Many recipients showed marked fluctuations in telomere length over the 12-month period of observation. The timing and extent of these fluctuations appear highly variable and random.
Changes in mean TRF length in neutrophils of fully engrafted HSCT recipients.
Telomere length in donors is indicated at time zero. (A) R1 to R4. (B) R5 to R7 and R10. (C) R8, R9, R14, R15, and R18. (D) R11 to R13, R16, and R17. (E) R19 to R22. (F) R23 to R25. To facilitate comparison, (A,C) recipients sampled 2 months after HSCT (in addition to other times) are presented together; (F) those sampled on 4 occasions in the first 60 days are shown together. On the basis of analysis of paired control samples, changes in mean TRF length exceeding 270 bp may be regarded as significant. Note that, although telomeres in recipients tended to be shorter than in their respective donors, they were not always so. Many recipients showed marked fluctuations in telomere length over the 12-month period of observation. The timing and extent of these fluctuations appear highly variable and random.
By using univariable and multivariable linear regression, we sought associations between the degree of telomere shortening at 12 months and donor age, nucleated cell dose, the extent of GVHD, and the proportion of CD34+CD90+ progenitors in S/G2/M after HSCT. No associations were identified. In addition, univariable and multivariable analyses were performed to assess for associations between these factors and the pattern of change in telomere length. For these analyses, recipients were divided into 2 groups (“positive” and “negative”) for each of 2 categorical features of the pattern of change: significant telomere loss (> 270 bp) at any time over the 12 months of follow-up, and significant fluctuation (> 270 bp) in telomere length over the 12 months. Again, no associations were identified.
Stability of X-inactivation ratios
Fourteen recipients had a female donor; 11 of these were informative at the HUMARA locus. X-inactivation ratios were nonskewed in 8, and skewed in 3 informative donors. As can be seen from Table2, ratios in each recipient tended to show little variation over the period of observation. They approximated those in respective donors, with the same allelic dominance. Two recipients (R1 and R5) with nonskewed donors with X-inactivation ratios close to 3:1 had ratios that exceeded 3:1 at 2 months and transiently at engraftment, respectively. There was no clear evidence for acquired oligoclonal hematopoiesis in any of this cohort of HSCT recipients.
X-inactivation ratios in informative donor/recipient pairs
| ID . | Donor . | Recipient . | |||
|---|---|---|---|---|---|
| Engraftment* . | 2 mo . | 6 mo . | 12 mo . | ||
| 1 | 2.17 | 2.69 | 3.36 | — | — |
| 2 | 0.75 | 0.67 | 0.62 | 0.75 | 0.85 |
| 4 | 0.75 | 0.79 | 1.12 | 0.91 | 0.83 |
| 5 | 0.44 | 0.32 | — | 0.43 | 0.53 |
| 6 | 0.27 | 0.19 | — | 0.21 | 0.18 |
| 8 | 0.73 | 0.72 | 0.69 | 0.62 | 0.62 |
| 9 | 0.59 | 0.47 | 0.52 | 0.55 | 0.53 |
| 11 | 2.46 | 2.72 | — | — | 2.42 |
| 14 | 4.62 | 11.93 | 5.12 | 6.38 | 10.42 |
| 15 | 1.77 | 2.13 | 1.71 | 1.70 | 1.91 |
| 19 | 0.29 | 0.30 | — | 0.32 | 0.28 |
| ID . | Donor . | Recipient . | |||
|---|---|---|---|---|---|
| Engraftment* . | 2 mo . | 6 mo . | 12 mo . | ||
| 1 | 2.17 | 2.69 | 3.36 | — | — |
| 2 | 0.75 | 0.67 | 0.62 | 0.75 | 0.85 |
| 4 | 0.75 | 0.79 | 1.12 | 0.91 | 0.83 |
| 5 | 0.44 | 0.32 | — | 0.43 | 0.53 |
| 6 | 0.27 | 0.19 | — | 0.21 | 0.18 |
| 8 | 0.73 | 0.72 | 0.69 | 0.62 | 0.62 |
| 9 | 0.59 | 0.47 | 0.52 | 0.55 | 0.53 |
| 11 | 2.46 | 2.72 | — | — | 2.42 |
| 14 | 4.62 | 11.93 | 5.12 | 6.38 | 10.42 |
| 15 | 1.77 | 2.13 | 1.71 | 1.70 | 1.91 |
| 19 | 0.29 | 0.30 | — | 0.32 | 0.28 |
Excessive skewing, corresponding to more than 75% expression of one allele, is defined by ratios less than 0.33 or more than 3. Ratios in D14 (4.62) and in R14 (11.93) at the time of engraftment correspond to expression of the dominant allele in approximately 83% and 92% of cells, respectively. Note that D6, D14, and D19 have skewed ratios and that R1 and R5 acquire skewed ratios at 2 months after HSCT and transiently at the time of engraftment, respectively. Overall, consistency in X-inactivation ratios within donor/recipient pairs is demonstrated.
Sample taken within 2 weeks of attainment of absolute neutrophil count of 0.5 × 109/L; most recipients were red cell and platelet transfusion–dependent at this time.
Composition and cycling of CD34+ marrow progenitors
CD90 expression among CD34+ marrow cells and progenitor cell cycling in 4 recipients at 2 months after HSCT were very similar to that observed in 8 recipients at 6 months. A single set of data was derived for the one recipient (R4) tested at both 2 and 6 months (by calculating the mean values), and results from all recipients from these 2 times were combined for the sake of analysis, as previously published.11
In steady-state donor marrow, 20.3% ± 5.7% of CD34+cells expressed the CD90 antigen. At 2 to 6 months after HSCT, this proportion was significantly lower (10.3% ± 4%;P = .01). By 12 months after HSCT, the proportion had fallen further to 5.6% ± 2.7% (P < .0001, compared with donors; P = .02, compared with 2-6 months).
In donor marrow, CD34+CD90+ cells were more quiescent than CD34+CD90− cells, with 72.5% ± 12.8% in G0 and 4.3% ± 1.6% in S/G2/M, compared with 44% ± 19.8% and 9.2% ± 2% (P < .0001 for both). At 2 to 6 months after HSCT, there was a marked increase in proliferation of CD34+CD90+ progenitors, with 42.4% ± 12.3% in G0 and 15.7% ± 3.1% in S/G2/M (P = .0008 and < .0001, respectively). In contrast, there was only a slight increase in proliferation among CD34+CD90− progenitors, with 38.5% ± 13.9% in G0 and 11% ± 1% in S/G2/M (P = .4 and .04, respectively). At 12 months after HSCT, the mitotic activity of CD34+CD90+ cells was significantly decreased from that at 2 to 6 months but remained higher than observed in steady-state marrow; 61% ± 12.9% were in G0 and 11.5% ± 3.4% were in S/G2/M (P = .04 and < .0001, respectively, compared with donors; P = .07 and .04 compared with 2-6 months). Again, there was little change in cycling of CD34+CD90− progenitors; 36.7% ± 14.4% were in G0 and 10.9% ± 2.5% were in S/G2/M (P = NS, compared with donors or 2-6 months). At 2 to 6 months and 12 months after HSCT there was deviation from the normal relationship of cycling profiles in CD90+/− subsets, with the proliferative rate of CD34+CD90+ cells exceeding that of the CD34+CD90− population at 2 to 6 months (P = .0005), and approximating it at 12 months (P = .59). The proportions of progenitors in G0 and S/G2/M in donor and recipient marrow samples are shown in Table 3.
Percentage of cells in CD90+/− subsets of CD34+ bone marrow progenitors in G0 and S/G2/M phases of the cell cycle in donors and recipients
| ID . | CD34+subset . | Donor . | Recipient . | ||
|---|---|---|---|---|---|
| G0/S/G2/M . | 2 mo3-150G0/S/G2/M . | 6 mo3-150G0/S/G2/M . | 12 mo G0/S/G2/M . | ||
| 1 | CD90+ | 92/3 | 49/15 | — | — |
| CD90− | 69/7 | 71/9 | — | — | |
| 2 | CD90+ | 85/5 | 37/13 | — | 74/6 |
| CD90− | 65/10 | 33/12 | — | 46/8 | |
| 3 | CD90+ | 58/6 | 30/19 | — | — |
| CD90− | 33/7 | 49/11 | — | — | |
| 4 | CD90+ | 69/4 | 55/13 | 65/15 | — |
| CD90− | 38/12 | 34/10 | 22/12 | — | |
| 5 | CD90+ | 91/2 | — | 41/22 | 64/12 |
| CD90− | 81/7 | — | 28/12 | 37/12 | |
| 6 | CD90+ | 65/5 | — | 21/19 | 80/9 |
| CD90− | 29/8 | — | 53/10 | 45/13 | |
| 7 | CD90+ | 58/6 | — | 49/14 | 48/11 |
| CD90− | 33/7 | — | 29/12 | 40/8 | |
| 8 | CD90+ | 80/5 | — | 27/15 | 38/15 |
| CD90− | 60/13 | — | 27/11.5 | 15.5/10.5 | |
| 9 | CD90+ | 77/5 | — | — | 49/15 |
| CD90− | 56/10 | — | — | 60/9 | |
| 10 | CD90+ | 62/6 | — | — | 61/13 |
| CD90− | 18/12 | — | — | 49/10 | |
| 11 | CD90+ | 74/3 | — | — | 60.5/10.5 |
| CD90− | 27/9 | — | — | 33/12 | |
| 12 | CD90+ | 58/7 | — | — | 63/16 |
| CD90− | 38/9 | — | — | 18/16 | |
| 13 | CD90+ | 58/4 | — | — | — |
| CD90− | 17/10 | — | — | ||
| 14 | CD90+ | 71/2 | — | — | — |
| CD90− | 35/8 | — | — | — | |
| 15 | CD90+ | 90/2 | — | — | 72/7 |
| CD90− | 62/9 | — | — | 23/10 | |
| 16 | CD90+ | — | — | 48/11 | — |
| CD90− | — | — | 40/10 | — | |
| 17 | CD90+ | — | — | 49/15 | — |
| CD90− | — | — | 30/11 | — | |
| 18 | CD90+ | — | — | 55/16 | — |
| CD90− | — | — | 36/11 | — | |
| Mean (SD) | CD90+ | 72.5 (12.8)/4.3 (1.6)3-151,3-152 | 42.4 (12.3)/15.7 (3.1)3-151,3-153 | 61 (13)/11.5 (3.4)3-152,3-153 | |
| Mean (SD) | CD90− | 44 (19.8)/9.2 (2)3-155 | 38.5 (13.9)/11 (1)3-155 | 36.7 (14.4)/10.9 (2.5) | |
| ID . | CD34+subset . | Donor . | Recipient . | ||
|---|---|---|---|---|---|
| G0/S/G2/M . | 2 mo3-150G0/S/G2/M . | 6 mo3-150G0/S/G2/M . | 12 mo G0/S/G2/M . | ||
| 1 | CD90+ | 92/3 | 49/15 | — | — |
| CD90− | 69/7 | 71/9 | — | — | |
| 2 | CD90+ | 85/5 | 37/13 | — | 74/6 |
| CD90− | 65/10 | 33/12 | — | 46/8 | |
| 3 | CD90+ | 58/6 | 30/19 | — | — |
| CD90− | 33/7 | 49/11 | — | — | |
| 4 | CD90+ | 69/4 | 55/13 | 65/15 | — |
| CD90− | 38/12 | 34/10 | 22/12 | — | |
| 5 | CD90+ | 91/2 | — | 41/22 | 64/12 |
| CD90− | 81/7 | — | 28/12 | 37/12 | |
| 6 | CD90+ | 65/5 | — | 21/19 | 80/9 |
| CD90− | 29/8 | — | 53/10 | 45/13 | |
| 7 | CD90+ | 58/6 | — | 49/14 | 48/11 |
| CD90− | 33/7 | — | 29/12 | 40/8 | |
| 8 | CD90+ | 80/5 | — | 27/15 | 38/15 |
| CD90− | 60/13 | — | 27/11.5 | 15.5/10.5 | |
| 9 | CD90+ | 77/5 | — | — | 49/15 |
| CD90− | 56/10 | — | — | 60/9 | |
| 10 | CD90+ | 62/6 | — | — | 61/13 |
| CD90− | 18/12 | — | — | 49/10 | |
| 11 | CD90+ | 74/3 | — | — | 60.5/10.5 |
| CD90− | 27/9 | — | — | 33/12 | |
| 12 | CD90+ | 58/7 | — | — | 63/16 |
| CD90− | 38/9 | — | — | 18/16 | |
| 13 | CD90+ | 58/4 | — | — | — |
| CD90− | 17/10 | — | — | ||
| 14 | CD90+ | 71/2 | — | — | — |
| CD90− | 35/8 | — | — | — | |
| 15 | CD90+ | 90/2 | — | — | 72/7 |
| CD90− | 62/9 | — | — | 23/10 | |
| 16 | CD90+ | — | — | 48/11 | — |
| CD90− | — | — | 40/10 | — | |
| 17 | CD90+ | — | — | 49/15 | — |
| CD90− | — | — | 30/11 | — | |
| 18 | CD90+ | — | — | 55/16 | — |
| CD90− | — | — | 36/11 | — | |
| Mean (SD) | CD90+ | 72.5 (12.8)/4.3 (1.6)3-151,3-152 | 42.4 (12.3)/15.7 (3.1)3-151,3-153 | 61 (13)/11.5 (3.4)3-152,3-153 | |
| Mean (SD) | CD90− | 44 (19.8)/9.2 (2)3-155 | 38.5 (13.9)/11 (1)3-155 | 36.7 (14.4)/10.9 (2.5) | |
Percentage of cells in G1 = 100 − (%G0 + %S/G2/M).
Results from 2 and 6 months combined for comparative analyses (for R4, a mean of 2 and 6 month results derived).
P = .0008 (G0) and < .0001 (S/G2/M) (by two-tailed paired t test).
P = .04 (S/G2/M) (by two-tailed paired t test).
P = .04 (G0) and < .0001 (S/G2/M) (by two-tailed paired t test).
P = .04 (S/G2/M) (by two-tailed paired t test).
Discussion
By examining surrogate markers of stem cell behavior in recipients of allogeneic HSCT, we sought to gain new insights into the processes of engraftment and early hematopoietic reconstitution in the clinical transplantation setting. We have demonstrated excessive neutrophil telomere shortening in fully engrafted recipients in the first year after HSCT. However, when recipient telomere length was considered in isolation from telomere length in donors, we found no consistent change in telomere length between 1 and 12 months after HSCT; many recipients had wide, and apparently random, fluctuations in telomere length during this time. The stochastic nature of these fluctuations contrasted strikingly with the consistency of trends in circulating blood cell counts, cycling kinetics of bone marrow progenitors, and X-inactivation ratios in neutrophils.
Neutrophils were chosen for study because they are homogeneous, short-lived, and easily obtained. Unlike lymphocytes, they have no potential for significant postmaturational proliferation. On each occasion, we assessed the mean TRF length in DNA derived from at least 3 × 107 circulating neutrophils. It is most likely that this population contained progeny of the full complement of HSCs contributing to granulopoiesis at the time of sampling.
The length of a neutrophil's telomeres is determined by several factors: (1) the telomere length in the HSC from which it is derived, (2) the number of cell divisions required to complete its development, (3) the extent of telomere loss with each round of mitosis, and (4) the degree of attenuation of telomere loss by telomerase. If an increase in the number of cell divisions from HSC to mature neutrophil were the sole factor underlying the excessive telomere shortening in HSCT recipients, we would have expected changes in telomere length to parallel changes in the mitotic activity of hematopoietic progenitors. No such relationship was demonstrated. We did not measure telomerase activity in marrow progenitors in this study and, therefore, cannot exclude impaired telomerase expression or function as a cause of the telomere shortening. However, there is indirect evidence to suggest that telomerase activity may be normal or up-regulated in HSCT recipients. Telomerase has been found to be up-regulated in response to ex vivo expansion of hematopoietic progenitors25,46 and in the setting of accelerated telomere shortening in Fanconi anemia47 and HIV infection.48 Although there are no strong grounds to suspect telomerase dysfunction after HSCT, the demonstration of excessive telomere shortening in more than one hematopoietic lineage of HSCT recipients in previous studies16 19 strongly suggests excessive telomere loss occurring at the level of the pluripotent stem cell. It is likely that accelerated HSC proliferation is the dominant cause of the telomere shortening we observed in these HSCT recipients.
Few studies have prospectively assessed changes in leukocyte telomere length within healthy subjects. Aside from age-associated decline, telomere length has fluctuated over time in most leukocyte subsets studied.19,49,50 In the setting of steady-state hematopoiesis and constant rates of postmaturational telomere loss, true fluctuations (ie, those exceeding the method's limit of detection of approximately 300 bp) can be attributed to differences in the clonal origin of the cells analyzed. We did not track changes in telomere length in the marrow donors in this study, and, therefore, lack a rigorous control for variation in telomere length over the 12-month period. However, the fluctuations in telomere length in many recipients were particularly striking when compared with the limited data for leukocytes of normal individuals19,49 50—the fluctuation in R23 in the month after engraftment is a good example (Figure 5F). The propensity for such wide fluctuations was probably conferred by a significant reduction in the number of HSC clones contributing to hematopoiesis after HSCT. The variation in the pattern of telomere loss among recipients was also striking.
Some cross-sectional studies of allogeneic HSCT recipients have suggested that nucleated cell dose12 and donor age14 can affect the extent of telomere loss, and it is conceivable that GVHD-associated immune assault on hematopoietic cells could affect telomere loss. In this cohort, we sought associations between these transplant-related factors and both the extent and pattern of telomere shortening and found none in univariable or multivariable analyses. It is possible that other factors, such as infection, drugs, and cytokine administration, may influence the extent and pattern of telomere loss, but in this small and heterogeneous cohort it was not feasible to test for all potential interactions. Given the weight of evidence for stochastic determination of HSC behavior,30 we believe it is likely that the pattern of telomere loss was determined randomly.
The lack of correlation between the extent of leukocyte telomere shortening and time from HSCT has been a consistent finding in previous studies.12-19 This finding implies that HSCT-induced acceleration in leukocyte telomere loss is neither progressive nor sustained. One recent longitudinal study that specifically addressed the kinetics of telomere shortening after HSCT was that of Rufer et al.19 They examined telomere length in monocytes and lymphocytes obtained from 4 allogeneic HSCT recipients up to 12 years after HSCT and found that telomeres in the recipients shortened at a comparable rate to that of their donors from the second year after HSCT. This finding led them to suggest that acceleration in telomere shortening was limited to the first year after HSCT. We found that many recipients entered their second year after HSCT with no evidence of sustained acceleration in telomere loss, in accordance with the findings of Rufer et al.19 Two other groups have recently presented preliminary results of prospective analyses of changes in leukocyte telomere length in HSCT recipients.51 52 Like us, they found wide variation in the pattern of telomere changes.
That clonal instability could reflect small HSC numbers was elegantly demonstrated by Abkowitz et al29 through studies of X-inactivation ratios in transplanted Safari cats and computer modeling of HSC behavior.30 In their longitudinal studies of hematologically normal women, Prchal et al53 established that X-inactivation ratios remained remarkably constant in multiple blood lineages over a period of 2 to 3 years. Furthermore, they estimated the number of HSCs required to be actively engaged in hematopoiesis to ensure ratio stability. Of note, their model did not take into account the effects of hemizygous selection. The relative constancy in X-inactivation ratios observed in this cohort of HSCT recipients suggests the active involvement of a sufficient number of HSCs in granulopoiesis to ensure ratio stability. The X-inactivation ratio that has been widely used to define the limit of polyclonal hematopoiesis, 3:1, was only marginally exceeded in 2 recipients with donors exhibiting nonskewed ratios. We are reluctant to infer the development of oligoclonal hematopoiesis from these 2 isolated results, because all other X-inactivation ratios obtained in these 2 donor/recipient pairs were very close to this arbitrary cutoff. Recipients of grafts from donors with skewed ratios tended to exhibit skewing to the same extent, and with the same dominant allele as their donors. If hemizygous selection is the cause of most cases of acquired ratio skewing in apparently healthy older women, as suggested,33 then it appears to be manifested similarly in human HSC-replete marrow donors and their comparatively HSC-deficient engrafted recipients.
We used indirect methods to assess HSC behavior. The advantage of these methods is that a global picture of HSC activity may be captured. A more direct method involves marking HSCs with retroviral vectors and following the fate of specific clones distinguished by their possession of unique proviral insertion sites. Recently, Kim et al54showed in Rhesus macaques that hematopoiesis was polyclonal in the first year after autologous HSCT. Analysis of individual clones in colony-forming units on multiple occasions in the first year showed that several clones contributed for prolonged periods. By tracking the function of individual clones of genetically marked human severe combined immunodeficient (SCID)–repopulating cells (SRCs) in nonobese diabetic (NOD)–SCID mice, Guenechea et al55 observed marked variation in the timing of appearance, life span, proliferative capacity, and self-renewal potential of individual SRC clones. They concluded that their data supported the existence of discrete human HSCs with short- or long-term repopulating capabilities. There is strong data to support the existence of these cells among murine HSCs.56 Our study, with its absence of a gene-marking component, clearly has little to add to the ongoing debate about the existence of short-term and long-term repopulating cells in humans. However, their existence would have profound implications for the interpretation of our results. If Guenechea et al55 are correct in their assumption that human long-term repopulating cells read out after 3 months in NOD-SCID mice, then we may have captured the activity of both short-term and long-term repopulating cells in this study. Most importantly, we believe that the highly variable nature of HSC behavior after HSCT revealed by the studies of Kim et al54 and Guenechea et al55 and by our own observations in these human HSCT recipients constitutes increasingly strong evidence for stochastic determination of HSC contribution to mature blood cell formation and hematopoietic reconstitution after HSCT.
Drawing on all our data, we propose the following paradigm for early hematopoietic reconstitution after human allogeneic HSCT. Immediately after grafting, maximal proliferation of donor HSCs is induced in the myeloablated recipient and results in accelerated telomere loss. The contribution of individual HSCs to active hematopoiesis (through differentiation) or hematopoietic repopulation (through extensive self-renewal) is determined stochastically. When HSCs with shortened telomeres contribute to granulopoiesis, the accelerated telomere loss may be detected in circulating neutrophils. Over the next 2 to 6 months, the number of HSC clones contributing to mature blood cell formation is significantly reduced compared with normal numbers, as evidenced by a propensity for marked fluctuations in neutrophil telomere length and a nearly 4-fold increase in the mitotic rate of CD34+CD90+ marrow progenitors. However, the threshold HSC number that would be expected to result in significant variation in X-inactivation ratios is seldom crossed. By 12 months, a significant degree of hematopoietic repopulation has been accomplished. The mitotic rate of CD34+CD90+ bone marrow progenitors, although still significantly higher than in steady-state marrow, is lower than at 2 to 6 months after HSCT because more HSC clones are now contributing to mature blood cell formation at any given time. HSC proliferative rates have slowed, and leukocyte telomere loss is no longer accelerated. However, a marked reduction in the proportion of CD34+ cells expressing the CD90 antigen betrays a persistent proximal hematopoietic deficit—a deficit exposed by studies of the clonogenic potential of marrow from HSCT recipients.3-5
To conclude, there is now strong evidence that HSCT-induced accelerated telomere loss, occurring at the HSC level, is finite in terms of duration and magnitude. Here, and in 2 previous studies,13,16 we have shown HSCT-induced telomere loss of 400 to 600 bp. This magnitude of telomere loss is within the normal interindividual range at any given age13,26-28 and may be less than the normal telomere loss experienced in the first year of life.26,51,57,58 Thus, it is unlikely to pose a threat to graft survival or appreciably increase the risk of donor-derived clonal hematopoietic disorders in most allogeneic recipients. Our results provide further indirect evidence for stochastic stem cell behavior in HSCT recipients, underscoring the importance of maximizing transfection and sustained transgene expression in gene therapy protocols. With regards to the clinical-grade ex vivo expansion of HSCs, a note of caution is warranted. The demonstration that telomere length in a population of CD34+ cells is unaffected by 2 weeks of ex vivo culture46 should not be taken as evidence for maintenance of telomere length in HSCs. HSCs constitute such a small minority of the cells assayed that any change in their telomere length will not affect the mean TRF length of the population as a whole. The change will only be detected when large numbers of their progeny are tested. Stochastic HSC behavior in vivo may ensure the relative protection of a proportion of stem cells from the burden of HSCT-induced replicative stress. With ex vivo culture techniques capable of inducing proliferation of all repopulating cells in a very short space of time,59 we must be careful not to overly stress an entire population of HSCs before it has begun its work in a myeloablated recipient. Markers of HSC replicative stress should be monitored very closely in this setting.
We thank Roxanne Macaskill and Martha Rolland for support; Elizabeth Sexsmith, Susan Chilton-MacNeill, Jodi Lees, Nazir Jamal, and Wilma Vanek for invaluable technical assistance; Peter Ray for chimerism studies; and David Malkin for providing equipment and space.
Supported by an award from the Aplastic Anemia Association of Canada and a Seed Grant from the Hospital for Sick Children Research Institute. I.T. was supported by a Young Investigator Award from the American Society of Clinical Oncology and a Duncan L. Gordon Fellowship from the Hospital for Sick Children Foundation. L.S. is supported by a Canadian Institutes of Health Research postdoctoral Fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hans A. Messner, Department of Medical Oncology and Hematology, Rm 5107, 610 University Ave, Toronto, Ontario M5G 2M9, Canada; e-mail: hans.messner@uhn.on.ca.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal