It is known from nutritional studies that vitamin A is an important factor for normal hematopoiesis, though it has been difficult to define its precise role. The vitamin A–deficient (VAD) quail embryo provides an effective ligand “knockout” model for investigating the function of retinoids during development. The VAD embryo develops with a significant reduction in erythroid cells, which has not been noted previously. Activation of the primitive erythroid program and early expression of the erythroid marker GATA-1 occurs, though GATA-1 levels eventually decline, consistent with the erythropoietic and hemoglobin deficits. However, from its early stages, the GATA-2 gene fails to be expressed normally in VAD embryos. The bone morphogenetic protein (BMP)–signaling pathway regulates GATA-2, and BMP4 expression becomes reduced in the caudal embryonic region of VAD embryos. Adding BMP4 to cultured VAD-derived explants rescues the production of erythroid cells, whereas normal embryos cultured in the presence of the BMP antagonist noggin are defective in primitive hematopoiesis. We find that cell clusters of primitive blood islands undergo an inappropriate program of apoptosis in the VAD embryo, which can explain the deficit in differentiated primitive blood cells. We propose that vitamin A–derived retinoids are required for normal yolk sac hematopoiesis and that an embryonic retinoid–BMP–GATA-2 signaling pathway controls progenitor cell survival relevant to primitive hematopoiesis.
Introduction
The first hematopoietic cells develop on the extra-embryonic yolk sac in close association with the initial endothelial cells. Mesoderm-derived cell clusters or “blood islands” contain the embryonic or “primitive” erythrocytes and the surrounding endothelial structures that form the extra-embryonic vascular plexus. The association of embryonic blood and endothelial cells led to a hypothesis that both derive from a common progenitor, the hemangioblast.1 This concept has been supported recently by overlapping gene expression programs and by in vitro lineage analysis using embryonic stem cells.2 The developmental origin and the relationship between the hemangioblast, the hematopoietic progenitors, and the hematopoietic stem cells that ultimately seed the bone marrow for adult or “definitive” hematopoiesis are not completely defined. Meanwhile, the signaling molecules that control blood island development and primitive hematopoiesis are unknown and may be distinct from definitive hematopoietic cytokines. Primitive erythrocytes are a transient population of relatively large, hyperchromatic cells expressing embryonic globins and are not dependent on the same set of regulatory genes that are required for definitive hematopoiesis. For example, definitive but not primitive erythropoiesis is critically dependent on erythropoietin.3
Vitamin A-derived retinoids, including retinoic acid (RA), are required for normal embryogenesis and tissue maintenance.4Retinoids function as signaling ligands through the activation of RAR and RXR nuclear receptors that regulate target genes important for numerous developmental programs, most notably the nervous, cardiovascular, and reproductive systems. In the mouse, disruption of the gene encoding RXRα or compound mutations in the genes encoding RAR isoforms have confirmed many of the developmental functions ascribed originally to retinoids based on the phenotype caused by a deficiency of vitamin A.5,6 A role for retinoids in primitive hematopoiesis has not been described in the mouse receptor knockouts, though RA signaling is apparently not essential. RXRα knockout mice do not die until approximately embryonic day 15.5 from severe cardiac defect. It was determined that there is a transient defect in definitive hematopoiesis in the mutant mouse caused by a delay in fetal liver hematopoietic development.7
In contrast, considerable evidence from nutritional studies implicate a function for vitamin A during hematopoiesis.8 Clinical studies using human volunteers and nutritional surveys of populations from underdeveloped countries find a statistically significant correlation between vitamin A deficiency and anemia, independent of total iron-binding capacity. Studies using a vitamin A–deficient (VAD) rat model led to contrasting results that support either increased9 or decreased8 hemoglobin and hematocrit (it has been suggested that such studies can be complicated by dehydration).10 Mouse bone marrow hematopoiesis is affected by a lack of retinoids, resulting in myeloid cell expansion.11 Many in vitro studies have analyzed the effect of RA on hematopoietic progenitors and differentiation and, in some cases, indicate a positive role for clonal proliferation of progenitors.12 However, it is unclear whether the in vitro approaches reflect the normal physiological function of retinoids.
An effective model system for studying the normal function of retinoid signaling is the VAD avian embryo.4,13 This system overcomes several of the inherent problems in studying RA function because the embryo represents a complete knockout of the nonredundant ligand. In this model, the only source of retinoids provided to the adult is RA that is not transferred to the egg.14,15Therefore, the embryo develops in the absence of retinoids, and the phenotype of the VAD embryo is completely rescued by administering RA to the embryo at or before the 5-somite stage. The VAD quail embryo displays developmental defects in the heart and nervous system, but initially the most dramatic disturbance is the failure of the early vascular system, particularly development of the omphalomesenteric veins required to establish extra-embryonic circulation and connection with the posterior heart tube. Although analyzed less extensively, it was noted that the development of intra-embryonic vasculature and yolk sac blood islands (extra-embryonic area vasculosa) initiates normally in the VAD quail embryo.16 Complete failure in embryonic circulation complicated any further analysis of hematopoiesis.
We have revisited the relationship between retinoids and yolk sac hematopoiesis, and we show that the VAD quail embryos are significantly anemic. Studies in amphibians28 29 indicate that bone morphogenetic proteins (BMPs) are required for the specification of mesoderm from which primitive blood is derived, and it is thought that BMPs provide or activate signals that regulate primitive erythropoiesis. We found using the VAD quail embryo that retinoids are required for normal primitive erythropoiesis, and we provide evidence that the mechanism of RA action is through a BMP-dependent pathway controlling early expression of the hematopoietic regulatory gene,GATA-2. Blood island development initiates normally, but in the absence of RA many of the primitive clusters initiate a program of apoptosis before terminal differentiation, resulting in significantly reduced numbers of primitive erythrocytes.
Materials and methods
Embryo culture and manipulation
VAD quail eggs were obtained from birds fed with a special diet, as described14; chicken eggs were purchased from Spafas (Norwich, CT). Fertilized quail and chicken eggs were incubated at 38 ± 1°C for the appropriate period and were staged as described.17 For in ovo rescue experiments, 10 ng all-trans retinoic acid (Sigma, St Louis, MO) was injected in VAD embryo blastoderms at stage 6, as described.18Chicken embryos were cultured in vitro by the method of New.19 In vitro culture of VAD embryos is described elsewhere.20
Embryos were isolated in freshly prepared phosphate-buffered saline (PBS). For benzidine staining, embryos were incubated in a solution of 0.2% acetic acid containing 0.2% o-dianisidine (3, 3′ dimetylbenzidine), and 1% hydrogen peroxide for 10 minutes at room temperature. Stained embryos were fixed in methanol. For counting cell numbers, embryos or explants were dissociated in 200 μL collagenase B (2 mg/mL in 0.7X PBS). Twenty microliterso-dianisidine solution (described above) was added to the cell suspension, and the reaction was incubated for 1 minute at room temperature. Cells were pelleted by centrifugation at 4000 rpm and were resuspended in 250 μL 0.7% PBS. Cell suspensions were concentrated onto slides using Cytospin 3 (Shandon Scientific, Cheshire, England) at 1000 rpm for 3 minutes before fixation in methanol.
To quantify hemoglobin levels, embryos were dissociated in a Dounce homogenizer using a loose-fitting piston to avoid nuclear breakage, as described.21 The cell suspension was overlaid on a cushion of 80% Ficoll-Hypaque Plus (Amersham Pharmacia Biotech, Uppsala, Sweden) and was centrifuged 5000 rpm at room temperature for 10 minutes. The pellet containing erythroid cells was resuspended in 1.0 mL Drabkin reagent (Sigma) and was allowed to stand for 15 minutes at room temperature. Absorbance was recorded at 540 nm, and hemoglobin was measured using standards (Sigma) described by the manufacturer.
For explant assays, posterior nodal pieces were isolated through the bisection of stage 4 embryos at the level of the mid-primitive streak. Posterior nodal pieces were then transferred onto vitelline membranes in New19 culture. Explants prepared from VAD embryos were cultured in 50 μL of a 2.5 μg/mL solution of human recombinant BMP4. Explants were incubated at 37°C for 72 to 96 hours. Fresh BMP4 solution was added every 24 hours. Explants were stained with benzidine to detect the presence of hemoglobin-expressing cells. For some experiments, embryos were cultured in the presence of conditioned media, prepared from control Chinese hamster ovary (CHO) cells or from CHO cells expressing noggin as described.22 For each embryo, 0.1 mL media from control or noggin-expressing cells was used during culture for 24 hours (most embryos reaching stage 11) before benzidine staining.
Gene expression analysis
Whole-mount in situ hybridization was performed as described.23 Digoxigenin-labeled sense and antisense riboprobes were prepared from the linearized plasmids containing the cDNAs encoding cGATA-1,24 cGATA-2,23BMP2,25 or BMP425 using T3 or T7 RNA polymerase. Hybridization was carried out overnight at 70°C. After stringent washing, embryos were incubated overnight in anti-digoxigenin (AP-conjugate) antibody, and probe was detected by a chromogenic reaction using NBT and BCIP. Embryos were postfixed in 4% formaldehyde and stored in this solution.
Terminal dUTP nick-end labeling assays
Embryos isolated from eggs or after in vitro culture were fixed in freshly prepared 4% paraformaldehyde, dehydrated, cleared of xylene, embedded in paraffin (Paraplast), and sectioned in 10-μm pieces. Terminal dUTP nick-end labeling (TUNEL) assays were performed using an in situ cell death detection kit (fluorescein-based) according to the manufacturer's protocol (Boehringer Mannheim, Indianapolis, IN). Briefly, paraffin sections were deparaffinized using Hemo-De (Fisher Scientific, Pittsburgh, PA), sequentially hydrated with ethanol and PBS (pH 7.2), washed twice in PBS, and digested with a solution of 10 mg/mL proteinase K for 5 minutes at room temperature. Sections were washed 3 times with PBS and overlaid with the in situ cell death reaction mix. Samples were incubated for 1 hour at 37°C, washed once, cover-slipped, and observed for fluorescence using a Nikon (Tokyo, Japan) epifluorescence microscope.
Results
VAD quail embryos have impaired primitive erythropoiesis
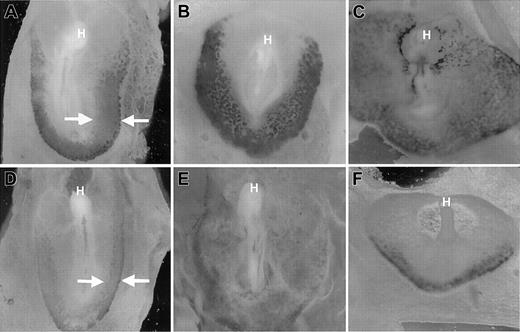
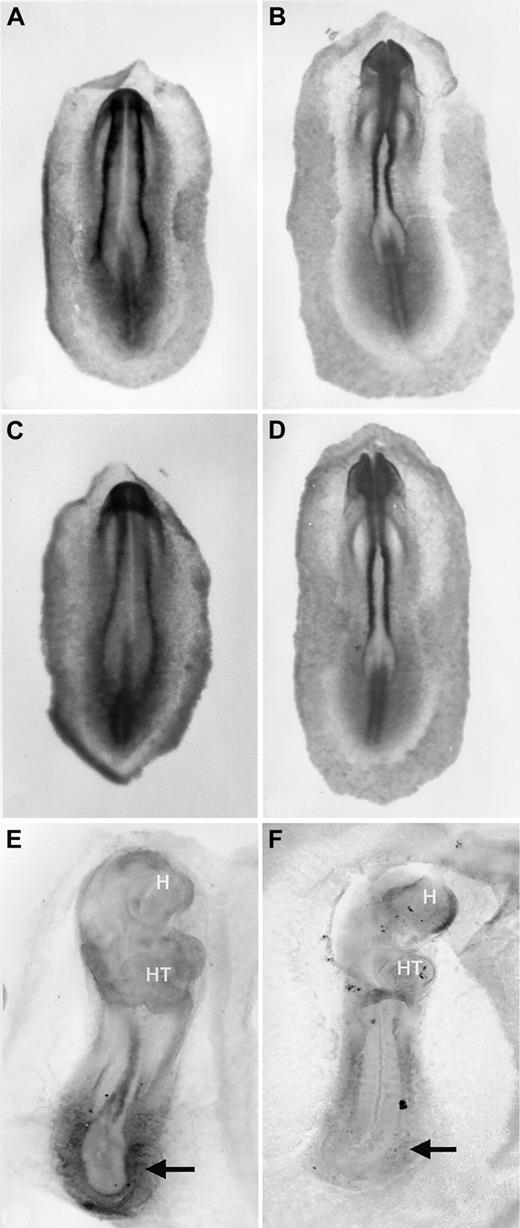
Early blood island development in VAD quail embryos was described originally as essentially normal, at least in terms of vascular morphology.16 The failure to establish blood circulation is caused primarily by the lack of omphalomesenteric vein formation. Therefore, the blood island vasculature does not connect with the primitive heart tube, and the endocardium ends in a blind pouch in the mid-region of the embryo. However, initial studies did not formally address blood cell development beyond yolk sac vascular morphology. We observed a consistent paleness of the VAD embryos compared to normal control embryos. To more carefully analyze primitive erythropoiesis during blood island development, we compared VAD and normal embryos after staining with benzidine, which identifies hemoglobin-synthesizing cells. We analyzed embryos between 1 and 2 days of development, when differentiated primitive erythroid cells emerge.17 At stages 9 to 11, benzidine-positive blood islands were detected readily in normal embryos throughout the extraembryonic area opaca (Figure1A,B). As expected, the erythroid cells were most abundant at the caudal periphery of the area pellucida, but the pattern extended laterally alongside the embryo in a classic horseshoe shape. By stage 12, circulation was established in normal embryos, and blood cells were detected in the embryo proper (Figure1C). In contrast, stages 9 to 11 VAD embryos stained weakly with benzidine, and the signal was restricted primarily to the caudal periphery (Figure 1D,E). Erythroid cells were detected in stage 12 VAD embryos (Figure 1F), but they were significantly reduced in number and were restricted to the most peripheral regions, and they failed to enter the embryo because of the vascular defect. As expected, the defect was caused by retinoid deficiency because benzidine staining patterns are entirely normal if VAD embryos are provided with RA at stage 6 (not shown).
Reduced erythropoiesis in blood islands of VAD embryos.
Normal (A-C) or VAD (D-F) quail embryos were stained witho-dianisidine to identify (dark stained) globin-expressing cells at stages 9 (A, D), 11 (B, E), or 12 (C, F). Views are ventral, and the anterior portion is at the top; H indicates the head of each embryo. By stage 12, blood can be seen intra-embryonically in the normal embryo. Arrows (A, D) indicate the differences in the globin-expressing caudo-lateral domains. VAD embryos show significantly reduced staining, particularly limited to the extreme caudo-lateral regions by stage 12. Original magnification × 32.
Reduced erythropoiesis in blood islands of VAD embryos.
Normal (A-C) or VAD (D-F) quail embryos were stained witho-dianisidine to identify (dark stained) globin-expressing cells at stages 9 (A, D), 11 (B, E), or 12 (C, F). Views are ventral, and the anterior portion is at the top; H indicates the head of each embryo. By stage 12, blood can be seen intra-embryonically in the normal embryo. Arrows (A, D) indicate the differences in the globin-expressing caudo-lateral domains. VAD embryos show significantly reduced staining, particularly limited to the extreme caudo-lateral regions by stage 12. Original magnification × 32.
To analyze the phenotype quantitatively, we pooled dissociated cells from several whole embryos (VAD or normal), enriched for erythrocytes by Ficoll-Hypaque gradient centrifugation, and quantified the total hemoglobin content. Results from several independent measurements showed that in 55-hour VAD embryos, there was an average decrease in globin expression of approximately 3-fold compared to normal control embryos (Figure 2). Embryos were dissociated, and erythroid cells were counted directly after staining with benzidine, demonstrating that at 55 hours the number of globin-positive cells was reduced by half. However, there was also a difference in staining intensity consistent with a differentiation defect in those erythroid cells that developed. In addition, a significant fraction of erythroid cells from VAD embryos had abnormal membrane morphology (Figure 2, inset). We concluded that there were defects in the numbers of primitive erythroid cells and in the differentiation program that together contributed to a severely anemic phenotype of the VAD embryo.
Quantification of hemoglobin content and morphology of erythroid cells in VAD and normal embryos.
Erythroid cells were isolated from normal or VAD embryos at approximately stage 15 (55-hour incubation), and the total number of benzidine-staining cells was counted using a hemacytometer (inset, n = 3 embryos each). Alternatively, total hemoglobin was extracted and measured as shown in the graph (n = 21 [normal] or 33 [VAD]). As indicated by the representative cell in the inset (arrow), there is a high incidence from VAD embryos of cells with abnormal membrane morphology (17.6% compared to 2.2% for normal embryos; n = 1000 cells). Original magnification × 1000.
Quantification of hemoglobin content and morphology of erythroid cells in VAD and normal embryos.
Erythroid cells were isolated from normal or VAD embryos at approximately stage 15 (55-hour incubation), and the total number of benzidine-staining cells was counted using a hemacytometer (inset, n = 3 embryos each). Alternatively, total hemoglobin was extracted and measured as shown in the graph (n = 21 [normal] or 33 [VAD]). As indicated by the representative cell in the inset (arrow), there is a high incidence from VAD embryos of cells with abnormal membrane morphology (17.6% compared to 2.2% for normal embryos; n = 1000 cells). Original magnification × 1000.
GATA-2 fails to be expressed normally in the VAD embryo
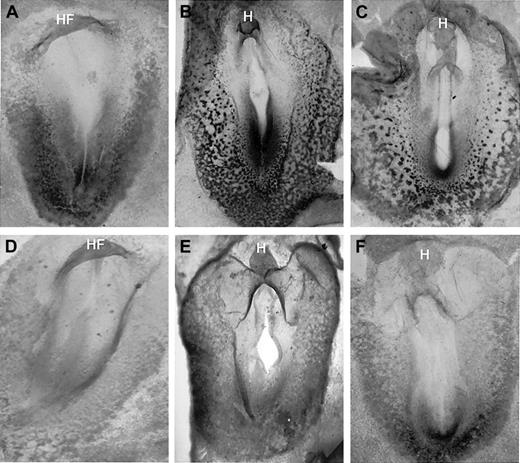
Previous studies using VAD rats indicated that erythropoiesis was defective in the absence of retinoids, but they could not rule out a late indirect effect from failure in iron metabolism or uptake. Therefore, we investigated whether the expression of early hematopoietic regulatory genes is normal in the VAD quail embryo. Our studies revealed that the GATA-2 gene failed to be expressed normally in the absence of retinoids (Figure3). The GATA-2 gene was expressed as early as stage 7 broadly in the caudal region, including the presumptive blood islands, and the expression was significantly reduced but not entirely eliminated in equivalently staged VAD embryos.
GATA-2 expression is significantly decreased in the VAD embryo.
Normal (A-C) and VAD embryos (D-F) were analyzed by whole-mount in situ hybridization for GATA-2 transcripts at stages 7 (A, D), 9 (B, E), and 11 (C, F). All embryos are arranged anterior to the top, with a ventral view. HF indicates head-fold; H, head. Note that GATA-2 transcripts are detected readily in the caudal region of the embryo and blood island region of normal embryos by stage 7, whereas these levels are significantly decreased in the VAD embryos. Original magnification × 32.
GATA-2 expression is significantly decreased in the VAD embryo.
Normal (A-C) and VAD embryos (D-F) were analyzed by whole-mount in situ hybridization for GATA-2 transcripts at stages 7 (A, D), 9 (B, E), and 11 (C, F). All embryos are arranged anterior to the top, with a ventral view. HF indicates head-fold; H, head. Note that GATA-2 transcripts are detected readily in the caudal region of the embryo and blood island region of normal embryos by stage 7, whereas these levels are significantly decreased in the VAD embryos. Original magnification × 32.
The precise function of GATA-2 is unknown, but it is thought to be required for regulating growth factor responsiveness of early hematopoietic cells.26 27 The GATA-2 expression pattern is relatively complex because the gene is also transcribed in cells outside the hematopoietic system, including the primitive ectoderm, endothelial cells, and perhaps the hemangioblast. GATA-1 is an erythroid transcription factor required intrinsically for the development and differentiation of primitive erythroid cells. In contrast to GATA-2, the pattern of GATA-1 expression was relatively normal in the early (stage 8) VAD embryo (Figure4). The pattern was uneven compared with the horseshoe pattern in normal embryos, but distinct clusters of GATA-1–expressing cells were found in the expected domain. By stage 11, the GATA-1 transcript pattern correlated essentially with benzidine staining and was restricted now to the more caudal and lateral regions of the VAD embryo. Northern blotting experiments confirmed that GATA-2 transcript levels were significantly decreased in the early stage VAD embryo, whereas GATA-1 transcripts were less affected (Figure5). Because these findings were consistent with the blood island morphology, we concluded that primitive erythropoiesis was initiated normally in the absence of RA but that the cells failed to proliferate, fully differentiate, or survive.
GATA-1 is expressed in the early blood island regions of VAD embryos.
Normal (A, C) or VAD (B, D) embryos were analyzed at stage 8 (A, B) or stage 11 (C, D) as in Figure 3, except that a probe was used to detect GATA-1 transcripts. In contrast to GATA-2, the pattern at stage 8 is similar comparing normal and VAD embryos, though GATA-1 expression becomes restricted to lateral regions by stage 11. Arrows indicate GATA-1–expressing blood islands. Original magnification × 32.
GATA-1 is expressed in the early blood island regions of VAD embryos.
Normal (A, C) or VAD (B, D) embryos were analyzed at stage 8 (A, B) or stage 11 (C, D) as in Figure 3, except that a probe was used to detect GATA-1 transcripts. In contrast to GATA-2, the pattern at stage 8 is similar comparing normal and VAD embryos, though GATA-1 expression becomes restricted to lateral regions by stage 11. Arrows indicate GATA-1–expressing blood islands. Original magnification × 32.
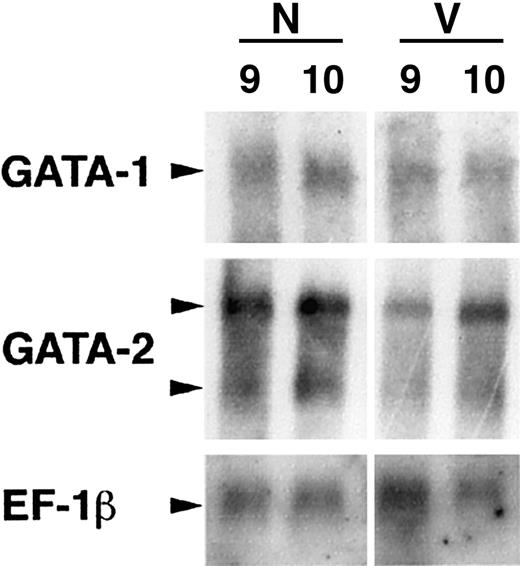
Northern blotting experiments confirm the quantitative decrease in GATA-2 transcript levels.
RNA was isolated from normal (N) or VAD (V) embryos at stage 9 or 10, and mRNA was analyzed by Northern blotting using probes to GATA-1, GATA-2, or control EF-1β cDNA as indicated. Note that there are 2 GATA-2 transcripts (arrowheads), and levels of both are decreased significantly at these stages relative to either GATA-1 or EF-1β.
Northern blotting experiments confirm the quantitative decrease in GATA-2 transcript levels.
RNA was isolated from normal (N) or VAD (V) embryos at stage 9 or 10, and mRNA was analyzed by Northern blotting using probes to GATA-1, GATA-2, or control EF-1β cDNA as indicated. Note that there are 2 GATA-2 transcripts (arrowheads), and levels of both are decreased significantly at these stages relative to either GATA-1 or EF-1β.
Expression of BMP4 in the VAD embryo
Experiments in Xenopus and mouse indicate that BMPs are required for the proper patterning of mesoderm that contributes to hematopoiesis. This includes a BMP-dependent step for the activation of GATA-2, because blocking BMP signaling inhibits GATA-2 expression and primitive erythropoiesis.28,29 Therefore, we considered whether the expression of BMP ligands was affected in the VAD embryos. The closely related genes BMP2 andBMP4 are both expressed during early avian embryogenesis, and the patterns have been described.30,31 Neither gene product has been associated specifically with blood island development. BMP2 transcripts are found in endoderm associated with the precardiac mesoderm,31 but they are also present in the posterior primitive streak from which mesoderm, including primitive hematopoietic cells, must migrate. However, the pattern of BMP2 transcripts in the blood island region was not altered in the VAD embryo (data not shown).
BMP4 was expressed in ectoderm associated with the precardiac mesoderm, on the right side of the Hensen node, in neural plate ectoderm, and also in the posterior domain, but it extended more broadly and laterally than did BMP2. During early blood island development, the BMP4 transcript pattern appeared similar in normal and VAD embryos (Figure 6A-D). Transcripts were abundant in the developing neural folds and were also present in the caudal presumptive posterior domain. The gene was not expressed at high levels in the blood island region, though the pattern overlapped precisely with the strong caudal embryonic domain of GATA-2 expression. However, this pattern failed to be maintained in the caudal region of the VAD embryo. Hence, by stage 14 transcripts were not detected in the posterior domain compared to normal embryos (Figure 6E,F), and this correlated with low levels of GATA-2. The failure to maintain expression of BMP4 may be related to the yolk sac hematopoietic defect, but we were cautious in this interpretation because the BMP4 defect was late compared with primitive erythropoiesis. It was, however, interesting that the phenotype determined by benzidine staining reflected a failure in blood development mostly near this posterior domain given that erythroid cells accumulated at the most lateral periphery of the extra-embryonic VAD tissues (Figure 1).
BMP4 transcript patterns in normal and VAD embryos.
Compared to normal embryos (A, B), the pattern in VAD embryos (C, D) is relatively unchanged in stage 7 (A, C) or stage 8+ (B, D) embryos. However, BMP4 expression is not maintained normally in the VAD embryo, which by stage 14 (E, normal; F, VAD) lacks BMP4 RNA in the caudal embryonic domain (arrows). All views are anterior to the top. In panels A to D, the views are dorsal, whereas in panels E to F, it is ventral. H indicates head; HT, heart. Original magnification × 32.
BMP4 transcript patterns in normal and VAD embryos.
Compared to normal embryos (A, B), the pattern in VAD embryos (C, D) is relatively unchanged in stage 7 (A, C) or stage 8+ (B, D) embryos. However, BMP4 expression is not maintained normally in the VAD embryo, which by stage 14 (E, normal; F, VAD) lacks BMP4 RNA in the caudal embryonic domain (arrows). All views are anterior to the top. In panels A to D, the views are dorsal, whereas in panels E to F, it is ventral. H indicates head; HT, heart. Original magnification × 32.
Regulation of primitive erythropoiesis by BMP4
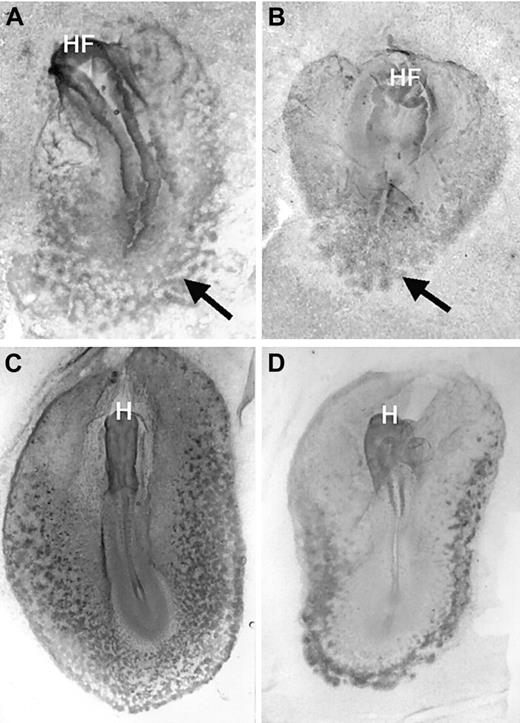
To test whether the erythroid phenotype of the VAD embryo was related to defective BMP4 expression, we cultured posterior explants derived from VAD embryos in the presence or absence of BMP4. The posterior nodal piece assay is an established method for analyzing the ability of a tissue to respond to inducing molecules,32 analogous to the more familiarXenopus animal cap assay. Explants were isolated from stage 4 embryos and consisted of the posterior nodal region and the surrounding presumptive blood islands. When isolated from normal stage 4 embryos, these explants developed large numbers of benzidine-positive cells, whereas similar explants isolated from VAD embryos had significantly fewer erythroid cells (Figure7A,B). Therefore, these explants could be used to mimic the blood phenotype of the VAD embryos. We added purified BMP4 to the VAD embryo cultures during the known period of sensitivity18 to retinoids. After culture, benzidine staining of dissociated explants provided a reliable and quantitative assay for primitive erythroid cell differentiation. As shown in Figure7C, the addition of BMP4 to the culture media of VAD embryo explants resulted in a significant rescue of erythropoiesis. The rescue occurred even if explants were taken as late as stage 8 (not shown). This supported the notion that the exogenous BMP4 rescued cell survival or differentiation rather than specification or migration of progenitors.
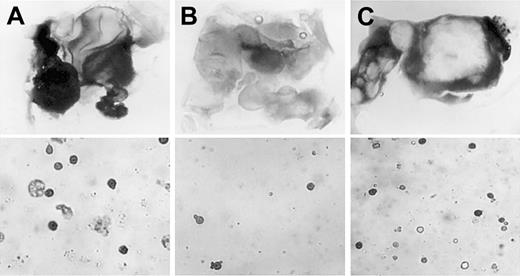
Rescue of primitive erythropoiesis in VAD explants by exogenous BMP4.
Posterior nodal piece explants were taken at stage 4 from normal (A) or VAD (B, C) embryos and were cultured either alone (A, B) or in the presence of 2.5 μg/mL recombinant purified BMP4 (C). Shown are representative photographs of explants that were stained in whole-mount preparations using benzidine (top panels, dark stain indicates erythroid cells; original magnification × 32.) or in cytospin preparations from explants that were first dissociated and then stained with benzidine (lower panels, original magnification × 200.). With whole-mount staining, large benzidine-positive regions were identified in 93% of the normal explants (n = 15), 22% of the VAD explants (n = 9), or 76% of the VAD explants cultured with BMP4 (n = 21). The recovery of erythroid cell number is similarly reflected quantitatively by the cytospin preparations.
Rescue of primitive erythropoiesis in VAD explants by exogenous BMP4.
Posterior nodal piece explants were taken at stage 4 from normal (A) or VAD (B, C) embryos and were cultured either alone (A, B) or in the presence of 2.5 μg/mL recombinant purified BMP4 (C). Shown are representative photographs of explants that were stained in whole-mount preparations using benzidine (top panels, dark stain indicates erythroid cells; original magnification × 32.) or in cytospin preparations from explants that were first dissociated and then stained with benzidine (lower panels, original magnification × 200.). With whole-mount staining, large benzidine-positive regions were identified in 93% of the normal explants (n = 15), 22% of the VAD explants (n = 9), or 76% of the VAD explants cultured with BMP4 (n = 21). The recovery of erythroid cell number is similarly reflected quantitatively by the cytospin preparations.
If the rescue of the VAD phenotype by BMP4 reflected the normal process of primitive cell differentiation, we expected that blocking BMP signaling at similar stages of normal embryos would be sufficient to generate a corresponding erythropoietic defect, even in the presence of retinoids. The BMP antagonist noggin has been shown to block BMP signaling by directly binding to BMP ligands,22 and it has been used extensively to define functions for BMPs in neural and cardiac development. We treated normal chick embryos with conditioned medium derived from control CHO cells or from CHO cells expressing noggin. We used the New19 culture technique, which allows normal development of whole embryos. Embryos treated with control media developed, by stage 12, normal blood islands with abundant clusters of benzidine-positive erythroid cells (Figure8A). In contrast, embryos cultured between stages 5 and 12 in the presence of noggin-containing media were significantly deficient for benzidine-staining cells (Figure 8B). Because the noggin was added after gastrulation, results were consistent with a retinoid-dependent requirement of BMP signaling for cell proliferation, differentiation, or survival of primitive erythrocytes.
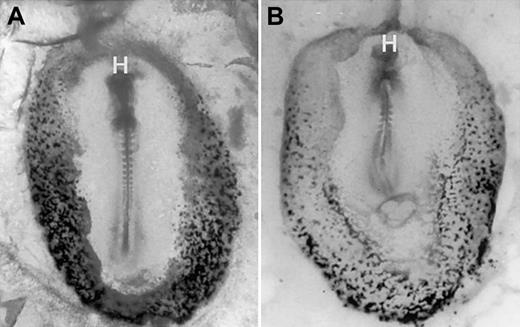
Blood island erythropoiesis is inhibited by noggin, an antagonist of BMP signaling.
Normal chicken embryos were isolated at stage 4 and were cultured according to New19 technique for 24 hours, followed by benzidine staining. Shown are representative embryos cultured in control medium (A) or noggin-conditioned medium (B). Embryos are oriented anterior to the top, with a ventral view. Original magnification × 32.
Blood island erythropoiesis is inhibited by noggin, an antagonist of BMP signaling.
Normal chicken embryos were isolated at stage 4 and were cultured according to New19 technique for 24 hours, followed by benzidine staining. Shown are representative embryos cultured in control medium (A) or noggin-conditioned medium (B). Embryos are oriented anterior to the top, with a ventral view. Original magnification × 32.
Lack of vitamin A results in failure of cell survival in blood islands
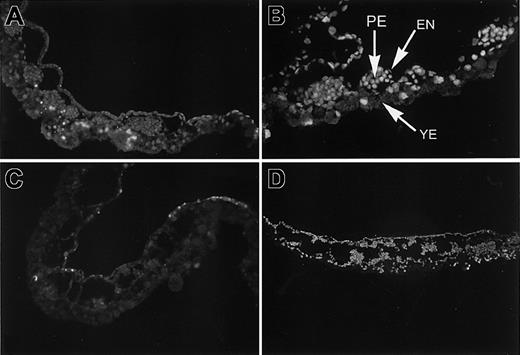
Because VAD embryos failed to produce normal numbers of globin-expressing primitive blood cells, we considered whether the erythroblasts failed to survive. We compared patterns of apoptosis in blood island regions of normal and VAD embryos. In sections of normal stage 8 embryos were clusters of blood cells surrounded by an endothelium and an underlying extra-embryonic endoderm. A few TUNEL-positive endoderm cells were detected, but most cells in the clusters were not apoptotic (Figure 9A). In striking contrast, entire blood island clusters were TUNEL-positive at the same stage in the VAD embryos (Figure 9B). Again, a few endoderm cells appeared to be undergoing apoptosis, but this pattern was similar to that of normal embryos. TUNEL-positive cells comprised the presumptive blood cells in the inner cluster and might also have included the surrounding endothelial cells. A similar comparison at stage 11 demonstrated that normal embryos were largely negative for apoptotic cells (Figure 9C), whereas widespread apoptosis was detected by the TUNEL assay throughout the blood island region of VAD embryos (Figure 9D).
Inappropriate apoptosis is present in blood island regions of VAD embryos.
Sections derived from normal (A, C) or VAD (B, D) embryos isolated at stage 8 (A, B) or stage 11 (C, D) were analyzed by TUNEL staining to identify cells undergoing apoptosis. Large clusters of dying cells are evident in the VAD blood islands, indicated by bright yellow. A few positive cells are found in the yolky endoderm (YE) of normal and VAD embryos, but only VAD embryos show extensive apoptosis in the primitive erythroid (PE) clusters and in at least some of the surrounding endothelial (EN) cells. Original magnification × 100.
Inappropriate apoptosis is present in blood island regions of VAD embryos.
Sections derived from normal (A, C) or VAD (B, D) embryos isolated at stage 8 (A, B) or stage 11 (C, D) were analyzed by TUNEL staining to identify cells undergoing apoptosis. Large clusters of dying cells are evident in the VAD blood islands, indicated by bright yellow. A few positive cells are found in the yolky endoderm (YE) of normal and VAD embryos, but only VAD embryos show extensive apoptosis in the primitive erythroid (PE) clusters and in at least some of the surrounding endothelial (EN) cells. Original magnification × 100.
Discussion
Before this study, a function for retinoids during primitive hematopoiesis had not been described. Characterization of the VAD embryo focused on the cardiovascular phenotype and on the failure to connect embryonic and extra-embryonic circulatory systems.16 On closer inspection, we found a significant defect in primitive erythropoiesis. The anemic phenotype goes beyond an indirect defect caused by vascular failure because there are qualitative differences in benzidine-staining patterns throughout early embryogenesis when comparing VAD and normal embryos and a quantitative defect for globin production in the VAD quail. The finding that GATA-2 fails to be expressed normally in the VAD embryo provides a molecular explanation for the phenotype.
However, the basis for the blood defect is complicated—erythropoiesis is reduced but not absent, and blood cells appear to form initially and to express GATA-1, a specific marker for the erythroid lineage. TheGATA-2 gene is expressed relatively widely in the ectoderm, endothelium, and blood cells. Therefore, the lack of GATA-2 could reflect a primary defect in an early progenitor (hemangioblast), a stromal component (ectoderm or endothelial), or the hematopoietic lineage proper. The first possibility, that retinoids regulate hemangioblast development, seems the least likely. The requirement for retinoids occurs no earlier than stage 8 of development because the addition of exogenous RA at or before the 5-somite stage rescues completely the VAD phenotype (cardiac, neural, vascular, and hematopoietic). The fact that primitive blood islands are present at stage 8 with relatively normal morphology suggests that the defect lay beyond the development of a hemangioblast. Because GATA-1 expression is activated, retinoids are also not required for establishing an erythroid program, which suggests an extrinsic defect in blood cell development for VAD embryos. The failure in GATA-2 expression indicates a more general developmental defect than would be explained by a problem in hemoglobin synthesis related to iron metabolism. Therefore, the most likely explanation is that RA contributes to the yolk sac stromal environment during primitive erythropoiesis.
The early literature investigating a connection between retinoids and hematopoiesis concerned definitive lineage blood cells. Interpretations were limited by a lack of molecular markers, though hemoglobin production and immune functions were modulated by vitamin A, presumably by defective hematopoiesis. RARα null mice do have a normal granulocyte population, though precursors from these mice differentiate early in culture.33 Furthermore, VAD mouse embryos have a significant increase in myeloid cells from bone marrow, spleen, and peripheral blood, correlating with impaired apoptosis,11 supporting the concept that retinoid signaling normally regulates hematopoiesis. Our studies using the VAD embryo extend this observation to the primitive yolk sac compartment.
The components of the yolk sac stromal environment that regulate primitive hematopoiesis are unclear. Erythropoietin (EPO) is a major regulator of definitive hematopoiesis, and EPO and its receptor are also expressed during normal yolk sac development. Excess RA given to pregnant mice results in increased EPO expression from avascular yolk sacs in anemic embryos,34 indicating that RA is capable of mediating EPO-dependent yolk sac hematopoiesis. Recently, it was shown that RA directly activates the EPO gene through binding of RARs to a hypoxia-response enhancer element that also can bind HNF-4.7,35 It is physiologically sensible for theEPO gene to be regulated through an alternative, hypoxia-independent mechanism for primitive hematopoiesis. Although EPO is not essential for murine yolk sac hematopoiesis, the EPO mutation does cause reduced primitive erythropoiesis,3 indicating that the EPO gene may be a target relevant to the VAD phenotype.
Other potential targets include growth factors implicated in early embryonic patterning. Fibroblast growth factor (FGF) is required for specification of ventral mesoderm, including the blood-forming tissues.36 Epiblast explant assays using exogenous FGF-2 or blocking antibodies support a role for this factor as an inductive signal from hypoblast required for primitive erythropoiesis.37 Angioblast induction is also dependent on FGF signaling.38 Therefore, vascular and hematopoietic defects might be explained by defects in the FGF signaling pathway caused by VAD. Indeed, RA has been shown to activate FGF-2 expression through RARα and thereby to induce angiogenesis.39
BMPs regulate another signaling pathway downstream of RA. There is a well-studied connection between RA and BMP signaling required for normal limb development. The BMP2 gene is induced by RA in F9 embryonal carcinoma cells40 and avian limb buds.25 At least some of this regulation may be direct because an RA-response element has been identified in the promoter of the BMP2 gene.41 In the developing limb of a normal avian embryo, induction of BMPs by exogenous RA promotes apoptosis and, therefore, has been proposed to regulate interdigital cell death.42 We find that BMP4 expression declines in the VAD embryo, correlating with defects in cell survival—that is, cell death increases in the absence of RA. This is in agreement with our earlier observations of increased apoptosis in the foregut endoderm of the VAD quail embryo20 and of increased apoptosis in the developing neural pathways of the VAD quail embryos.43,44Similarly, vascular networks in the cardiac inflow tract region of the VAD embryo are significantly reduced.4 It is likely that an RA–BMP pathway controlling apoptosis is a conserved mechanism in a variety of embryonic contexts.
Previous experiments in the frog implicated BMP signaling as a regulator of blood island development,28,29 whereas BMP4 and FGF or activin were shown using explant assays to synergize for blood cell induction.45Xenopus explant studies indicate that a blood-inducing signal comes from ectoderm. Mesoderm cultured alone activates globin RNA transcription, but the progenitors only mature to make hemoglobin protein when exposed to ventral ectoderm or animal cap tissue stimulated by previous injection of BMP4 or GATA-2.28 This signal could be related to the RA-dependent factor missing in the VAD embryo. Activin is another transforming growth factor-β family member with erythropoietic-inducing activity that can be modulated by RA.46 Finally, signaling from vascular endothelial growth factor receptor (VEGFR) tyrosine kinases is required for angiogenesis, and Flk1 (VEGFR2) is required for the development of endothelial and primitive blood cells.47The requirement of Flk-1 for normal hematopoiesis may reflect an early role in hemangioblast development,48 yet the primary blood defect in the VAD embryo may be attributed to the lack of an endothelial-derived stromal factor of the VEGF family.49
However, the retinoid link to BMP signaling is particularly attractive because this pathway has been shown to activate GATA-2,28,29 which clearly fails in the VAD embryo. Our results are consistent with the phenotype of mice with a targeted mutation of the GATA-2 gene.26 Similar to the VAD quail embryos, these mice have primitive erythroid cells with the total number reduced several-fold. The extension of the RA–BMP pathway to activation of downstream GATA transcription factors has been described before in the context of heart development. We found that VAD embryos fail to activate GATA-4 in the cardiogenic region,50 resulting in apoptosis of the foregut endoderm and a morphogenetic defect in heart tube development.20Therefore, we propose that an RA–BMP–GATA pathway is conserved for regulating cell survival of blood islands and foregut endoderm. The downstream target genes of GATA factors that control cell survival are mostly unknown, but they include bcl-xL with relevance to erythropoiesis.51
We thank Cliff Tabin for providing the BMP2 and BMP4 cDNA, Claudio Stern for the cGATA-2 cDNA, and Richard Harland for CHO cells expressing recombinant noggin. Recombinant purified BMP4 was a gift from Research Genetics Institute.
Supported by National Institutes of Health grants HL56182 and HL64282 (T.E.), by United States Department of Agriculture grant 0035200-9062 (M.H.Z.), and by the Michigan Agricultural Experiment Station (M.H.Z.). T.E. is also supported by the Irma T. Hirschl Trust.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Todd Evans, Department of Developmental and Molecular Biology, Chanin 501, Albert Einstein College of Medicine, 1300 Morris Park Ave, Bronx, NY 10461; e-mail: tevans@aecom.yu.edu.

![Fig. 2. Quantification of hemoglobin content and morphology of erythroid cells in VAD and normal embryos. / Erythroid cells were isolated from normal or VAD embryos at approximately stage 15 (55-hour incubation), and the total number of benzidine-staining cells was counted using a hemacytometer (inset, n = 3 embryos each). Alternatively, total hemoglobin was extracted and measured as shown in the graph (n = 21 [normal] or 33 [VAD]). As indicated by the representative cell in the inset (arrow), there is a high incidence from VAD embryos of cells with abnormal membrane morphology (17.6% compared to 2.2% for normal embryos; n = 1000 cells). Original magnification × 1000.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/7/10.1182_blood.v99.7.2379/6/m_h80722343002.jpeg?Expires=1765899474&Signature=XOexuaO92oKIfNsbBfG5dTFmgW4sKdVqzG6KzEd3pWWz5kHnzS6GWWMvGYLiqHVc9ceJTsHSoKLETCshCsmQfnL3UHeVDetqPEHMYnWqhqdAkRYnbOrIq9jORv1oGwnu9gGH-qxrznWyUkm1bUwQmy-VQyQUOrmyTZ1rJOyilb~9ERIUUuH9aLyqMsGFZ0WN3dz9xMpthWFv1Z4~vKt-laVtKqAJmwdTkrCNaBpOspWX6tZ-D7VHSdp0gvTwOHkH0n6LP4R9eXKLna7aPoo2iuNhrRUl3BSZ0edWrLNGliag6FGCF0u~Nczud249lXwvAZJZfevtEM0AjMY74JzdCA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal