Abstract
All genetic types of severe combined immunodeficiency (SCID) can be cured by stem cell transplantation from related donors. The survival rate approaches 80%, and most deaths result from opportunistic infections acquired before transplantation. It was hypothesized that the survival rate and kinetics of immune reconstitution would be improved for infants receiving transplants in the neonatal period (first 28 days of life), prior to the development of infections. A 19.2-year retrospective/prospective analysis compared immune function in 21 SCID infants receiving transplants in the neonatal period with that in 70 SCID infants receiving transplants later. Lymphocyte phenotypes, proliferative responses to mitogens, immunoglobulin levels, and T-cell antigen receptor excision circles (TRECs) were measured before transplantation and sequentially after transplantation. Of 21 SCID infants with transplantations in the neonatal period, 20 (95%) survive. Neonates were lymphopenic at birth (1118 ± 128 lymphocytes per cubic millimeter). Infants receiving transplants early developed higher lymphocyte responses to phytohemagglutinin and higher numbers of CD3+ and CD45RA+ T cells in the first 3 years of life than those receiving transplants late (P < .05). TRECs peaked earlier and with higher values (P < .01) in the neonatal transplantations (181 days to 1 year) than in the late transplantations (1 to 3 years). SCID recipients of allogeneic, related hematopoietic stem cells in the neonatal period had higher levels of T-cell reconstitution and thymic output and a higher survival rate than those receiving transplants after 28 days of life. An improved outcome for this otherwise fatal syndrome could be achieved with newborn screening for lymphopenia so that transplantation could be performed under favorable thymopoietic conditions.
Introduction
Severe combined immunodeficiency (SCID) is a rare congenital syndrome characterized by profound deficiencies of cellular and humoral immunity.1 Recently, several molecular defects responsible for SCID have been identified. X-linked SCID accounts for 46% of all cases and is caused by defects in the chain (γc) common to the interleukin-2 (IL-2), IL-4, IL-7, IL-9, IL-15, and IL-21 receptors.1-5 Mutations in the genes encoding adenosine deaminase (ADA),6 Janus kinase 3 (Jak3),7 the alpha chain of the IL-7 receptor,8 recombinase activating genes 1 or 2,9 CD45,10 or the Artemis gene11also result in SCID and are inherited in an autosomal recessive pattern. Despite different genetic causes, infants with SCID exhibit consistent laboratory, pathologic, and clinical features. Characteristic laboratory features of SCID include lymphopenia, hypogammaglobulinemia, and absent lymphocyte proliferation to mitogens, antigens, or allogeneic blood mononuclear cells.1 4 Infants with SCID have no tonsils, rarely have detectable peripheral lymph nodes, and have a small, poorly differentiated thymus gland.
The mean age of diagnosis for SCID is 6.6 months, but most patients experience recurrent infection prior to this as their transplacentally acquired maternal antibody levels decline.1 Initial infections may be those frequently encountered in infancy, such as oral candidiasis, otitis media, or bronchiolitis. However, infants with SCID have illnesses unresponsive to conventional therapies, and most eventually develop chronic diarrhea, failure to thrive, or an opportunistic infection that leads to their diagnosis or demise.12 Without recognition and treatment, this syndrome is uniformly fatal, usually in the first year of life.
Bone marrow transplantation, either with HLA-identical marrow or T-cell–depleted haploidentical parental marrow, is the standard of care for SCID. Currently, 78% of all SCID patients and 77% of those receiving haploidentical, T-cell–depleted bone marrow at Duke University Medical Center (Durham, NC) survive at varying times up to more than 19 years after transplantation.4 However, opportunistic infections and malnutrition remain major contributors to the morbidity and mortality of these infants as well as to the health care costs associated with thir care. It is our hypothesis that earlier diagnosis and transplantation, before the onset of recurrent infection and failure to thrive, could further increase survival rates and decrease morbidity. To address this hypothesis, we performed a retrospective/prospective study of the development of immune function in 21 infants who were given bone marrow transplants in the first 28 days of life and compared the findings with those of 70 infants successfully receiving transplants after that time.
Patients, materials, and methods
Patient characteristics
Twenty-one of 117 consecutive infants with SCID receiving transplants at Duke University Medical Center during the past 19.2 years were given allogeneic bone marrow cells in their first 28 days of life. Nine received diagnoses in utero and 12 at birth, all because of a positive family history. Eighteen patients were born at term after uncomplicated pregnancies. Two pregnancies were complicated by twin gestation. The first produced 2 full-term male infants with SCID. The second twin pregnancy was further complicated by preterm labor leading to delivery at 31 weeks' gestation. Only one of the latter twins had SCID; he was born with respiratory distress syndrome requiring surfactant therapy and mechanical ventilation for fewer than 12 hours. Nineteen neonates were male, 2 were female, 19 were white, 1 was black, and 1 was Hispanic. The molecular basis of SCID was γc deficiency in 15 (71%), ADA deficiency in 2 (10%), and Jak3 deficiency in 1 (5%). Three (14%) had SCID of an unknown molecular defect inherited in an autosomal recessive pattern.
None of the neonates received either pretransplantation chemotherapeutic conditioning or posttransplantation graft-versus-host disease (GVHD) prophylaxis. The median age at transplantation was 10 days (range, 7-24 days). Two male neonates with γcdeficiency received T-cell–depleted HLA-identical sibling marrow, and the remaining 19 neonates received T-cell–depleted haploidentical parental marrow. Donors were mothers in 16 cases, fathers in 3, and sisters in 2. The mean number of nucleated marrow cells given was 4.07 × 108/kg body weight. Four donors were cytomegalovirus (CMV) IgG positive; 13 were negative; and the status of the remaining 4 was not determined. Six neonates were breastfed exclusively; 6 were formula fed; and 9 were breastfed but also received supplementary formula.
Of the remaining 96 patients who received allogeneic bone marrow cells after the first 28 days of life, 71 (74%) survive and are included in this analysis. Of these, 55 were male, 16 were female, 58 were white, 7 were Hispanic, 5 were black, and 1 was Native American. The molecular basis of SCID was γc deficiency in 29 (41%), ADA deficiency in 14 (20%), and Jak3 deficiency in 4 (6%). Eighteen (25%) had SCID of an unknown molecular defect. The remaining patients had mutations in recombinase-activating genes (3 patients), alpha chain of the IL-7 receptor (2 patients), and cartilage hair hypoplasia (1 patient). The median age at transplantation for the late group was 190 days (range, 45-516 days).
Laboratory studies
Three-color flow cytometry was performed on blood lymphocytes with the use of murine monoclonal antibodies to CD3, CD4, CD8, CD45RA, and CD45RO purchased from Pharmingen (San Diego, CA), AMAC (Westbrook, ME), Coulter, (Hialeah, FL), DAKO (Carpinteria, CA), and Becton Dickinson (San Jose, CA). Lymphocyte proliferation was determined by the amount of 3Hthymidine incorporation after the patients' peripheral blood mononuclear cells (PBMCs) were stimulated with phytohemagglutinin (PHA), concanavalin A, and pokeweed mitogens. Each cellular study was run in parallel with PBMCs from healthy adult volunteers. Serum concentrations of IgG, IgM, and IgA were determined by nephelometry or by single radial diffusion and of IgE by double-antibody radioimmunoassay or enzyme-linked radioimmunosorbent assay. HLA typing was determined by microcytotoxicity assays, flow cytometry, or polymerase chain reaction assays. Bone marrow was purged of mature T cells by soybean lectin agglutination and 2 cycles of sheep erythrocyte rosetting, as previously described.13 T-cell antigen–receptor excision circles (TRECs) were measured from frozen PBMC samples by polymerase chain reaction assays, as previously described.14 The studies were performed with the approval of the Duke University Committee on Human Investigations, and written, informed consent was obtained from the parents.
Statistics
The kinetics of immune reconstitution for those patients receiving transplants in the neonatal period (early) were compared with those receiving transplants after the first 28 days of life (late).4 Lymphocyte phenotype and proliferation were analyzed at the following intervals: before transplantation, 1 through 30 days, 31 through 60 days, 61 through 90 days, 91 through 120 days, 121 through 180 days, 181 through 270 days, 271 through 365 days, 1 to 2 years, 2 to 3 years, 3 to 4 years, 4 to 5 years, 5 to 6 years, 6 to 9 years, and more than 9 years after transplantation. TRECs were analyzed before transplantation, 0 through 90 days, 91 through 181 days, 181 days through 1 year, 1 to 3 years, and 3 to 7 years after transplantation. Only one measurement for each test was analyzed for an individual patient in any given interval. If more than one measurement for an individual patient was available in the same interval, then the mean of the measurements was analyzed. The Wilcoxon rank-sum test was used to compare differences in immunologic parameters between the early and late transplantations. Statistical analyses were also performed with Statistica software (StatSoft, Tulsa, OK). All ± values are reported as SEM.
Results
Survival
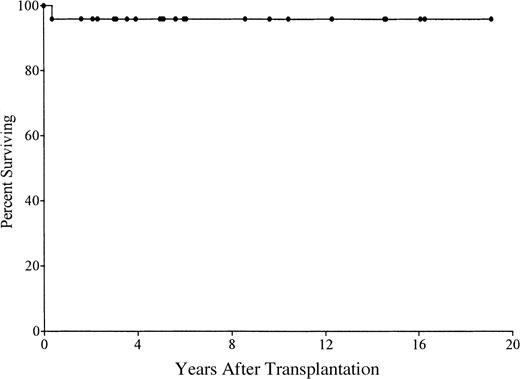
Of 21 patients receiving transplants in the first 28 days of life, 20 (95%) are alive (Figure 1). They range from 8 months to 19 years in age. Of the survivors, 3 are less than 1 year after transplantation; 8 are 1 to 5 years; 4 are 5 to 10 years; and 5 are 10 to 19 years. One ADA-deficient SCID patient died of CMV encephalitis at 4 months after transplantation despite successful engraftment. No other patients developed an opportunistic infection. Most have had normal growth and development and have had few, if any, restrictions placed on their activities.
Kaplan-Meier survival curve for 21 patients with SCID who received stem cell transplants in the first 28 days of life.
One patient died at 4 months of age from CMV encephalitis.
Kaplan-Meier survival curve for 21 patients with SCID who received stem cell transplants in the first 28 days of life.
One patient died at 4 months of age from CMV encephalitis.
Lymphocyte phenotypes at birth
SCID neonates were lymphopenic at birth. The mean absolute lymphocyte count (ALC) of all neonates was 1118 ± 128/mm3, with the normal range at birth being 2000 to 11 000/mm3.15 The ADA-deficient neonates had the most profound lymphopenia, with a mean ALC of 368/mm3. One neonate with γc deficiency and one with Jak3 deficiency had ALCs that fell within the normal range, and the cells were predominantly B lymphocytes. Neonatal lymphocyte phenotypes varied according to genotype, as previously reported (Figure2A).1 4 Regardless of the genotype, all neonates had few T cells. The mean number of B cells was normal in patients with γc and Jak3 deficiency but below normal in those with ADA deficiency and autosomal recessive SCID of unknown molecular cause. The mean number of natural killer (NK) cells was normal in those with autosomal recessive SCID and low in the remaining genotypes.
Lymphocyte subsets and proliferation to mitogens before transplantation and after transplantation in SCID infants given stem cell transplants in the neonatal period.
(A-B) The mean (± SEM) numbers of T, B, and NK cells at birth (A) and at the latest posttransplantation evaluation (B) for surviving patients according to genotype. ▪, CD3+ T cells; ■, CD20+ B cells; ░, CD16+ NK cells. Normal range represents the 95% confidence interval for healthy infants at birth (A) or for healthy children at 6 years of age (B). (C) The mean (± SEM) counts per minute of3Hthymidine incorporation by proliferating PBMCs stimulated with PHA (▪), concanavalin A (■), and pokeweed (░) mitogens before transplantation and at the most recent evaluation compared with healthy controls.
Lymphocyte subsets and proliferation to mitogens before transplantation and after transplantation in SCID infants given stem cell transplants in the neonatal period.
(A-B) The mean (± SEM) numbers of T, B, and NK cells at birth (A) and at the latest posttransplantation evaluation (B) for surviving patients according to genotype. ▪, CD3+ T cells; ■, CD20+ B cells; ░, CD16+ NK cells. Normal range represents the 95% confidence interval for healthy infants at birth (A) or for healthy children at 6 years of age (B). (C) The mean (± SEM) counts per minute of3Hthymidine incorporation by proliferating PBMCs stimulated with PHA (▪), concanavalin A (■), and pokeweed (░) mitogens before transplantation and at the most recent evaluation compared with healthy controls.
Posttransplantation lymphocyte phenotypes
At the most recent posttransplantation evaluation, the mean numbers of T, B, and NK cells for all neonates were within normal ranges (Figure 2B). Variations were observed according to genotype. Mean numbers of T cells were within the normal range for all genotypes. The mean number of B cells was increased in the Jak3-deficient patient and decreased in the surviving ADA-deficient patient. The mean number of NK cells was decreased in the Jak3-deficient patient.
T-cell function
Figure 2C shows in vitro PBMC proliferation to PHA, concanavalin A, and pokeweed mitogen before and at the latest posttransplantation evaluation as compared with healthy adults. Responses to mitogens were absent in all SCID neonates at birth. The mean counts per minute of 3Hthymidine incorporation was normal to each mitogen at the patient's most recent evaluation. Significant and normal T-cell functions were defined as in vitro stimulation indices of at least 10 and 100, respectively, to 1 or more mitogens. Mean time to significant T-cell function in all neonates was 33 ± 4 days and to normal T-cell function was 103 ± 15 days. Once normal T-cell function was achieved, no patients receiving transplants in the neonatal period developed impaired in vitro responses. One γc–deficient SCID who had GVHD required booster peripheral blood and unfractionated bone marrow transplants from his successfully engrafted identical twin.
Effects of age at transplantation on lymphocyte subsets and proliferation
Results of studies in SCID infants receiving transplants in the first 28 days of life (early) were compared with those in patients receiving transplants after the neonatal period (late). Figure3 shows the kinetics of proliferation to the T-cell mitogen PHA. Before transplantation, cells from neither group proliferated to PHA. Incorporation of 3Hthymidine increased in both groups at all time intervals after tranplantation. However, the mean count per minute was higher in the early group at times occurring between 91 and 270 days after transplantation (P < .05). T-cell proliferation to PHA remained stable in both groups at all time intervals greater than 1 year after transplantation. Before transplantation, the late group had a higher mean number of CD3+ cells (P < .05) (Figure4). However, infants receiving transplants early developed higher circulating numbers of CD3+ and CD45RA+ (naive) T cells for the first 3 years after transplantation (P < .05). There were similar trends for both CD4+ and CD8+ T-cell subsets (data not shown). The numbers of CD3+ and CD45RA+ T cells gradually declined in patients given the transplants early so that they became similar to those of the late recipients by 6 years after transplantation. There were no differences in mean numbers of CD45RO+ T cells at any time interval after transplantation.
Comparison of proliferative responses to PHA in the early and late transplantation groups.
Shown are the mean (± SEM) counts per minute of3Hthymidine incorporation. Comparisons were made at the following intervals: before transplantation, 1 through 30 days, 31 through 60 days, 61 through 90 days, 91 through 120 days, 121 through 180 days, 181 through 270 days, 271 through 365 days, 1 to 2 years, 2 to 3 years, 3 to 4 years, 4 to 5 years, 5 to 6 years, 6 to 9 years, and more than 9 years after transplantation. Mean values were plotted at the midpoint of each interval. Infants receiving transplants within the first 28 days of life (n = 20) had increased T-cell proliferation to PHA at 91 through 120 days, 121 through 180 days, and 181 through 270 days after transplantation compared with those receiving transplants late (n = 69) (P < .05); n = total number of individuals analyzed in each group over the last 19.2 years.
Comparison of proliferative responses to PHA in the early and late transplantation groups.
Shown are the mean (± SEM) counts per minute of3Hthymidine incorporation. Comparisons were made at the following intervals: before transplantation, 1 through 30 days, 31 through 60 days, 61 through 90 days, 91 through 120 days, 121 through 180 days, 181 through 270 days, 271 through 365 days, 1 to 2 years, 2 to 3 years, 3 to 4 years, 4 to 5 years, 5 to 6 years, 6 to 9 years, and more than 9 years after transplantation. Mean values were plotted at the midpoint of each interval. Infants receiving transplants within the first 28 days of life (n = 20) had increased T-cell proliferation to PHA at 91 through 120 days, 121 through 180 days, and 181 through 270 days after transplantation compared with those receiving transplants late (n = 69) (P < .05); n = total number of individuals analyzed in each group over the last 19.2 years.
Increased numbers of circulating naive T cells in the early versus late transplantation groups.
Shown are the mean (± SEM) numbers of CD3+, CD45RA+, and CD45RO+ cells in patients receiving transplants early (n = 20) compared with those receiving transplants late (n = 66). Data were analyzed at the following intervals: before transplantation, 1 through 30 days, 31 through 60 days, 61 through 90 days, 91 through 120 days, 121 through 180 days, 181 through 270 days, 271 through 365 days, 1 to 2 years, 2 to 3 years, 3 to 4 years, 4 to 5 years, 5 to 6 years, 6 to 9 years, and more than 9 years after transplantation. Mean values were plotted at the midpoint of each interval; n = total number of individuals analyzed in each group over the last 19.2 years. The early group had increased numbers of CD3+ cells at 271 days to 1 year, 1 to 2 years, and 2 to 3 years after transplantation (P < .05). The early group had increased numbers of CD45RA+ T cells at 91 through 120 days, 1 to 2 years, and 2 to 3 years after transplantation (P < .05). These numbers gradually declined and were comparable to the late group by 6 years after transplantation.
Increased numbers of circulating naive T cells in the early versus late transplantation groups.
Shown are the mean (± SEM) numbers of CD3+, CD45RA+, and CD45RO+ cells in patients receiving transplants early (n = 20) compared with those receiving transplants late (n = 66). Data were analyzed at the following intervals: before transplantation, 1 through 30 days, 31 through 60 days, 61 through 90 days, 91 through 120 days, 121 through 180 days, 181 through 270 days, 271 through 365 days, 1 to 2 years, 2 to 3 years, 3 to 4 years, 4 to 5 years, 5 to 6 years, 6 to 9 years, and more than 9 years after transplantation. Mean values were plotted at the midpoint of each interval; n = total number of individuals analyzed in each group over the last 19.2 years. The early group had increased numbers of CD3+ cells at 271 days to 1 year, 1 to 2 years, and 2 to 3 years after transplantation (P < .05). The early group had increased numbers of CD45RA+ T cells at 91 through 120 days, 1 to 2 years, and 2 to 3 years after transplantation (P < .05). These numbers gradually declined and were comparable to the late group by 6 years after transplantation.
Effects of age at transplantation on thymus function
TRECs are currently one of the best available measures of thymic function. They have been used to determine the kinetics of immune reconstitution and thymic output in SCID.14 In this study, TRECs were undetectable before transplantation and gradually increased by 91 through 180 days after transplantation in both groups (Figure5). The patients receiving transplants early had higher mean TREC values at time intervals 91 through 180 days and 181 days to 1 year after transplantation (P < .01). The mean TREC value peaked earlier in those receiving transplants in the first 28 days of life than in those receiving transplants later (181 days to 1 year versus 1 to 3 years). Mean TREC values reached a plateau at 2 years after transplantation in the patients who received transplants late and gradually decreased in the patients who received transplants early so that the groups were indistinguishable by 5 years after transplantation.
Faster and quantitatively higher thymic output in the early- versus late-transplantation groups.
Shown are the mean (± SEM) number of TRECs for patients receiving early (n = 19) and late (n = 55) transplantations. TRECs were measured before transplantation, 0 through 90 days, 91 through 181 days, 181 days to 1 year, 1 to 3 years, and 3 to 7 years after transplantation. Mean values were plotted at the midpoint of each interval; n = total number of individuals analyzed in each group over the last 19.2 years. Patients receiving transplants early had higher TREC values at 91 through 180 days and 181 days to 1 year after transplantation (P < .01). The mean TREC value peaked at 181 days to 1 year in those receiving transplants early and at 1 to 3 years in those receiving transplants late.
Faster and quantitatively higher thymic output in the early- versus late-transplantation groups.
Shown are the mean (± SEM) number of TRECs for patients receiving early (n = 19) and late (n = 55) transplantations. TRECs were measured before transplantation, 0 through 90 days, 91 through 181 days, 181 days to 1 year, 1 to 3 years, and 3 to 7 years after transplantation. Mean values were plotted at the midpoint of each interval; n = total number of individuals analyzed in each group over the last 19.2 years. Patients receiving transplants early had higher TREC values at 91 through 180 days and 181 days to 1 year after transplantation (P < .01). The mean TREC value peaked at 181 days to 1 year in those receiving transplants early and at 1 to 3 years in those receiving transplants late.
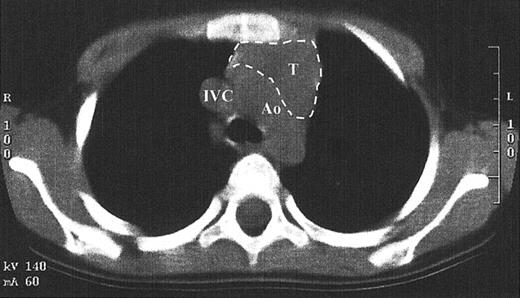
While TRECs are an excellent surrogate marker for thymic function, little direct data exist that show thymopoiesis. This is in part due to the inappropriateness of performing a thymic biopsy in children who have developed normal function. Since the pretransplantation SCID thymus is vestigial, weighing less than a gram, it is difficult to find by radiography.1,14 A computed tomography scan of the anterior mediastinum of the Jak3-deficient SCID patient at 4 years after transplantation shown in Figure 6demonstrates a thymus with a transverse dimension of 3.8 cm, which is normal for age.16 The thymus expansion seen here provides further evidence for thymopoiesis after hematopoietic stem cell transplantation in SCID.
Computed tomography scan of the anterior mediastinum of a Jak3-deficient SCID patient 4 years after transplantation.
Thymic tissue is indicated by the dashed lines. It is in the normal position and measures 3.8 cm in its greatest transverse dimension, which is normal for age. Ao indicates aortic arch; IVC, inferior vena cava; T, thymus.
Computed tomography scan of the anterior mediastinum of a Jak3-deficient SCID patient 4 years after transplantation.
Thymic tissue is indicated by the dashed lines. It is in the normal position and measures 3.8 cm in its greatest transverse dimension, which is normal for age. Ao indicates aortic arch; IVC, inferior vena cava; T, thymus.
B-cell function
Table 1 shows the current extent of B-cell engraftment and function. Of the 20 surviving neonates, 9 have donor B-cell engraftment, defined as 5% to 100% of peripheral B cells determined to be of donor origin by karyotype analysis or restriction fragment length polymorphism. Of these 9 neonates, 7 are γc-deficient; 1 is ADA-deficient; and 1 is autosomal recessive. Forty-five percent have normal IgA concentrations for age, and 70% have normal IgM. This is comparable to our previous report of B-cell function in 89 SCID patients after stem cell transplantation, where 50% and 80% had normal IgA and IgM concentrations, respectively.4 Thirteen of the 20 (65%) surviving neonates are receiving intravenous immunoglobulin (IVIG). The 7 remaining neonates have normal antibody-forming capacity to diphtheria and tetanus toxoids or to bacteriophage φX-174. Donor B-cell engraftment did not seem to correlate with B-cell function. For instance, 2 autosomal recessive SCID neonates have only host B cells but have normal immunoglobulin concentrations and antibody synthesis and do not require IVIG. On the other hand, 7 of 15 γc-deficient SCID patients have donor B cells, but 11 of 15 are receiving IVIG.
Graft-versus-host disease
For 13 of 21 neonates, the posttransplantation period was uneventful and free of GVHD even though only 2 patients had HLA-identical donors (Table 2). The pretransplantation mixed lymphocyte reaction between donor's and recipient's mononuclear cells was positive in all of the mismatched transplants, but only 8 of 21 (38%) neonates developed GVHD and 5 of the 8 had grade 1 GVHD. Nine patients received a 2-antigen–mismatched transplant and 3, all with γc deficiency, developed grade 1 GVHD. One of these patients had benign pneumatosis intestinalis, which resolved with supportive therapy. The other 2 patients had peripheral blood eosinophilia, which responded to corticosteroids. Nine neonates received a 3-antigen–mismatched transplant and 5 developed GVHD. Two of these 5 patients did not require treatment for transient grade 1 GVHD. The identical twin γc-deficient neonates developed grade 3 GVHD, manifested by fever, rash, and severe diarrhea. Both recovered after treatment with cyclosporine and corticosteroids. One neonate with autosomal recessive SCID who received a 3-antigen–mismatched transplant developed grade 4 GVHD with autoimmune hemolytic anemia, bone marrow suppression, diarrhea, and cholestatic liver disease. He subsequently received a liver transplant from a related living donor and remains on a low dose of tacrolimus 1.5 years later. His mother was the donor for both the bone marrow and the liver segment. He is a complete hematopoietic chimera and now has normal liver function.
Discussion
The studies presented here support our hypothesis that infants whose SCID is diagnosed at an early age and who receive transplants of normal related bone marrow stem cells before acquiring opportunistic infections will have a higher survival rate and lower morbidity than those whose SCID is diagnosed later when untreatable infections have developed. All but one (95%) of these SCID infants who received T-cell–depleted identical or haploidentical stem cell transplants in the first 28 days of life currently survive, with the period of survival ranging from 8 months to more than 19.2 years after transplantation. This compares favorably with a 74% survival rate of 96 infants receiving transplants after the neonatal period.
In addition to having a higher survival rate, SCID patients receiving transplants in the neonatal period demonstrated increased lymphocyte proliferation to PHA and higher numbers of CD3+ and CD45RA+ T cells when compared with those receiving transplants after the first 28 days of life. Superior T-cell reconstitution was most evident from 3 months to 3 years after transplantation. This is a critical period for the development and maturation of host defenses in response to new pathogens that are frequently encountered at a young age. The observed differences in T-cell function can be attributed to higher thymic output after transplantation in the SCID neonates than in the older SCID infants. This conclusion is supported by the finding of higher TREC levels, higher numbers of circulating CD3+ T cells, higher numbers of CD45RA+(naive) T cells, and more vigorous early T-cell proliferation to mitogens in the neonates than in the older group. This supposition is also confirmed by the finding of a normal-sized thymus at 4 years after transplantation in one patient in whom computerized tomography of the thymus was obtained. In aggregate, these findings confirm our previous conclusion that the vestigial SCID thymus is able to support T-cell development from donor stem cells.14 One explanation for a favorable effect of early stem cell transplantation for SCID may be that those SCID infants receiving transplants after the neonatal period may have already suffered from recurrent or opportunistic infections, malnutrition, and failure to thrive. Stress from these comorbid factors could have had a deleterious effect on thymic function.
Early transplantation does not appear to improve B-cell function. The majority (65%) of our SCID patients receiving transplants in the neonatal period receive monthly IVIG infusions. This percentage is comparable to our previous report of 89 SCID patients, 63% of whom were receiving IVIG. The high percentage of γc-deficient SCID patients in the present report (71%) is undoubtedly contributory. Infants with γc-deficient SCID have poor B-cell function after transplantation because they continue to have host B cells with abnormal cytokine receptors. One patient with autosomal recessive SCID has never required IVIG, even in the immediate posttransplantation period. Five of the 13 patients receiving IVIG are less than 3 years after transplantation. Previous studies have reported that B-cell function can take up to 3 years to develop after T-cell–depleted haploidentical stem cell transplantations.13 Thus, some of the patients who have received transplants more recently may eventually be able to discontinue IVIG treatment. Management of these infants after transplantation includes frequent measurement of immunoglobulin concentrations. If IgA and IgM are increasing, this suggests the ability to isotype switch. The patient may then be immunized with bacteriophage φX-174 to determine his or her ability to produce specific antibody. If the patient can mount a normal response to this neoantigen, IVIG may be discontinued.
Prenatal diagnosis can identify affected fetuses in the first trimester of pregnancy if there is a family history of SCID from a known molecular defect. Prenatal diagnosis is recommended for families who are considering terminating an affected pregnancy, to alleviate anxiety, or to allow families time to plan for postnatal treatment of affected infants.17 Alternatively, all infants could be screened for SCID at birth even when there is no family history; this could be done by routine performance of a white blood cell count and a manual differential count and then flow cytometry if lymphopenia is present.1 4 Each patient in this analysis was initially evaluated for SCID because one or more family members was affected by the disease. For 9 of 21 patients, SCID was diagnosed prenatally. However, the majority of patients were identified at birth from a white blood cell count and a manual differential count that demonstrated lymphopenia. Two of our neonates had ALCs just over 2000/mm3, and the cells were predominantly B cells. Therefore, for those with a family history of SCID, lymphocyte phenotyping and T-cell functional studies should also be performed. Regardless of the genotype, nearly all SCID neonates were lymphopenic, and the lymphocyte phenotypes at birth were typical for the particular molecular defect.
The ability to diagnose SCID in the first trimester of pregnancy led to attempts to treat this syndrome in utero with the hope that the infant would then be born with intact immunity. There are 4 literature reports of successful in utero bone marrow transplantation for SCID.18-24 These reports suggest that, although lymphoid reconstitution of SCID can be achieved with prenatal stem cell transplantation, T-cell development was not complete at birth. Thus, the infant may still be at risk for opportunistic infections. Our results of stem cell transplantation for SCID in the neonatal period show enhanced, stable T-cell reconstitution and 95% survival over a period of 19 years. It is difficult to justify in utero stem cell transplantation when such excellent results can be achieved by stem cell transplantation in the neonatal period. The kinetics of T-cell reconstitution with in utero transplantation demonstrate no benefit over postnatal transplantation. In addition, previous reports of prenatal stem cell transplantation for a variety of inherited diseases reveal an 18% rate of fetal demise.20
The majority of neonates in this study did not require prolonged hospitalizations. Most were admitted to the hospital for a 23-hour period of observation for the transplant procedure, discharged to local apartments, and evaluated frequently in an outpatient clinic. This method of protective isolation is preferable to reverse isolation in a hospital where nosocomial infections present great risks to the immunocompromised patient. Furthermore, outpatient care decreases the expense of caring for infants with SCID. Few neonates developed GVHD, and in most cases it was mild. Only one neonate developed an opportunistic infection. The mean time to significant T-cell function in all neonates was 33 ± 4 days and to normal T-cell function was 103 ± 15 days. Once T-cell engraftment and normal function were achieved, they were sustained. Of the 20 surviving patients, none have developed an opportunistic infection, a malignancy, or loss of T-cell immunity.
The recent report of successful gene therapy for γc-deficient SCID provides hope for another treatment option without posing additional risks to the recipient.25However, the high success rate for transplantation in the neonatal period indicates that future directives for the care of these infants should also focus on early diagnosis before the onset of opportunistic infections and failure to thrive. The results of T-cell reconstitution observed with early transplantation should not detract from the success of T-cell–depleted haploidentical stem cell transplantations performed for SCID patients of all ages. The overall survival rate for these infants at our institution is 79%. These transplantations are done without the use of pretransplantation chemotherapeutic conditioning or posttransplantation GVHD prophylaxis.
The findings reported here suggest that the outcomes of postnatal stem cell transplantation for SCID could be further improved if routine testing for this syndrome were included in newborn screening. A cord-blood white blood cell count and a manual differential count demonstrating lymphopenia could detect nearly all infants born with SCID. This test, while relatively expensive compared with the cost of current perinatal testing for genetic defects, poses no risks to the mother or fetus. Until newborn screening becomes an accepted practice, close attention should be paid to the ALC in all infants presenting with fever, chronic diarrhea, or failure to thrive. Physicians caring for infants also need to be reminded that the lower limit of a normal ALC in the first few months of life is higher (2000 to 4500/mm3) than in older children and adults. Furthermore, an infant with a normal ALC but clinical manifestations suggestive of SCID should undergo lymphocyte phenotyping and studies of T-cell function, because transplacental transfer of maternal T cells in utero could obscure lymphopenia. In these rare cases, the T-cell number is low but not absent, and lymphocyte proliferation is profoundly depressed. Early diagnosis of SCID would allow for transplantation to be performed not only before a potentially fatal opportunistic infection develops but under conditions ideal for thymopoiesis.
The authors gratefully acknowledge the contributions of the many referring physicians, postdoctoral fellows, nurses, and other personnel who participated in the care of these infants. We thank Drs Joanne Kurtzberg, Paul Szabolcs, Paul Martin, Richard Howrey, and Timothy Driscoll and Mr Gilbert Ciocci for harvesting the marrow. We are grateful to Mrs Roberta Parrott, Mrs Carol Koch, Mrs Ruby Johnson, Ms Kim Curtis, Mrs Sherrie Schiff, and Mrs Katherine Coyne for their assistance with the T-cell depletions of donor marrow and for their expert technical assistance. We thank Ms Maria Gooding and Dr Greg Sempowski for their expert technical assistance in the TREC analyses. We thank Dr Michael Hershfield for performing adenosine deaminase and deoxyadenine nucleotide studies. We are also grateful to Genetic Counselor Joie Davis for her advice to the families of these patients.
Supported by National Institutes of Health grants AI42951, AI47604, and AI47605 and by grant MO1-RR-30 from the National Center for Research Resources, General Clinical Research Centers Program, National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Laurie A. Myers, Box 3559, Duke University Medical Center, Durham, NC 27710; e-mail: myers019@mc.duke.edu.