Abstract
Granulocyte colony-stimulating factor (G-CSF) is the major regulator of granulopoiesis and acts through binding to its specific receptor (G-CSF-R) on neutrophilic granulocytes. Previous studies of signaling from the 4 G-CSF-R cytoplasmic tyrosine residues used model cell lines that may have idiosyncratic, nonphysiological responses. This study aimed to identify specific signals transmitted by the receptor tyrosine residues in primary myeloid cells. To bypass the presence of endogenous G-CSF-R, a chimeric receptor containing the extracellular domain of the epidermal growth factor receptor in place of the entire extracellular domain of the G-CSF-R was used. A series of chimeric receptors containing tyrosine mutations to phenylalanine, either individually or collectively, was constructed and expressed in primary bone marrow cells from G-CSF–deficient mice. Proliferation and differentiation responses of receptor-expressing bone marrow cells stimulated by epidermal growth factor were measured. An increased 50% effective concentration to stimulus of the receptor Ynullmutant indicated that specific signals from tyrosine residues were required for cell proliferation, particularly at low concentrations of stimulus. Impaired responses by mutant receptors implicated G-CSF-R Y764 in cell proliferation and Y729 in granulocyte differentiation signaling. In addition, different sensitivities to ligand stimulation between mutant receptors indicated that G-CSF-R Y744 and possibly Y729 have an inhibitory role in cell proliferation. STAT activation was not affected by tyrosine mutations, whereas ERK activation appeared to depend, at least in part, on Y764. These observations have suggested novel roles for the G-CSF-R tyrosine residues in primary cells that were not observed previously in studies in cell lines.
Introduction
Granulocyte colony-stimulating factor (G-CSF) plays a crucial role in proliferation, differentiation, and survival of the granulocytic lineage of myeloid cells exemplified by the neutropenia found in G-CSF–deficient mice.1 G-CSF binding to its receptor causes receptor homodimerization,2 resulting in tyrosine (Y) phosphorylation of the receptor itself and in phosphorylation of associated kinases such as those of the Janus kinase (JAK) family.3-6 Other important signaling molecules activated by the G-CSF receptor (G-CSF-R) are signal transducer and activator of transcription (STAT) proteins STAT1, STAT3, and STAT5,4,7-9 some members of mitogen-activated protein kinase (MAPK) pathways,5,10-12 and the tyrosine kinases Lyn, Syk, and Hck.13,14 Phosphorylated tyrosine residues of the hG-CSF-R in the cytoplasmic region (Y704, Y729, Y744, and Y764) potentially serve as docking sites for SH2 (src homology 2) and PTB (phospho-tyrosine binding) domain-containing proteins to initiate signaling pathways. Deletion studies have identified the membrane proximal 56 amino acids of the cytoplasmic domain as essential for cell proliferation. In contrast, the differentiation response has been reported to require the C-terminal region of the receptor containing the tyrosine residues.15-19
Some signaling pathways activated by G-CSF-R tyrosine phosphorylation have been analyzed by examining the effects of mutating cytoplasmic tyrosine residues in immortalized cell lines. However, the importance of each tyrosine residue appeared to differ in the cell lines, so that the role of tyrosine residues in signal transduction in primary cells remained unclear. The Y703 and Y728 residues of the murine G-CSF-R (analogous to human Y704 and Y729, respectively) transmitted differentiation signals in murine interleukin-3 (mIL-3)–dependent myeloid LGM-1 cells.20 Exogenous expression of mG-CSF-R in LGM-1 cells induced cell differentiation into neutrophils in response to G-CSF. However, transfectants expressing mG-CSF-R lacking Y703retained their blast cell morphology and showed reduced expression of the differentiation marker, myeloperoxidase (MPO), in response to G-CSF. Moreover, cells expressing mG-CSF-R lacking Y728became macrophagelike in their morphology and failed to express MPO in response to G-CSF.
In contrast, Nicholson et al8 reported the significance of Y744 and Y704 of the hG-CSF-R in myeloid cell line (M1) differentiation. Unlike LGM-1 cells, M1 cells expressing exogenous wild-type (WT) G-CSF-R differentiated into macrophages, becoming enlarged and vacuolated in response to G-CSF. Point mutation of the receptor Y744, and to a lesser degree Y704, diminished their differentiation responses. Mutation of Y729 had little effect. In these cells, STAT1, STAT3, and STAT5 were activated by G-CSF, but the activation was largely tyrosine independent. Multiple receptor tyrosine residues contributed to cell differentiation and survival at low concentrations of G-CSF in transfected myeloid 32D cells, with Y704 having the greatest effect followed by Y744 and Y729, respectively. STAT3 activation correlated with differentiation and survival in these cells.21 Moreover, Y764 of the receptor supported a strong proliferation response in 32D cells. Mutants lacking Y764 were unable to proliferate, but they still differentiated into morphologically mature neutrophils in response to G-CSF. Thus, mutation of Y764 resulted in fewer differentiated cells in culture.22 In Ba/F3 cells, however, the Y764 mutation did not affect cell proliferation.23 In summary, specific signals are initiated from phosphorylated receptor tyrosine residues, depending on the type of cultured cells used as a model for neutrophilic granulocyte development. Receptors Y704, Y729, and Y744 may activate signaling for cell differentiation, whereas Y764 may activate signaling for cell proliferation.
To resolve such discrepancies and to understand more about the function of receptor tyrosine residues in primary cells, we have attempted to identify the role of G-CSF-R tyrosine residues in bone marrow cells. To bypass endogenous G-CSF-R, a chimeric receptor substituting the whole extracellular domain of G-CSF-R with that of the epidermal growth factor receptor (EGF-R) was constructed and expressed in murine bone marrow cells from G-CSF–deficient mice by retroviral infection. In addition, chimeric receptors carrying cytoplasmic tyrosine mutations, either individually or collectively, were similarly expressed in G-CSF–deficient murine bone marrow cells. Infected bone marrow cells were tested for their ability to proliferate and differentiate in response to EGF and to signal through the STAT and MAPK pathways.
Materials and methods
Cell culture, cytokines, and antibodies
GP+E-86 cells were grown under conditions described before.24 Murine stem cell factor (mSCF) was provided by N. Nicola (Walter and Eliza Hall Institute of Medical Research, Parkville, Australia). Murine interleukin-6 (mIL-6) and murine EGF (mEGF) were provided by R. Simpson and E. Nice (Ludwig Institute for Cancer Research, Parkville, Australia), respectively. Recombinant hG-CSF was a gift from L. Souza (Amgen, Thousand Oaks, CA). WEHI-3B D−cell-conditioned medium was used as a source of mIL-3. Anti-EGF-R monoclonal antibody 52825 was provided by F. Walker (Ludwig Institute for Cancer Research). Antiphospho-p44/42 MAPK antibody was purchased from New England Biolabs (Beverly, MA), and the anti-ERK 1 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Construction of chimeric EGF–G-CSF receptor and its tyrosine mutants
To replace the extracellular domain of the G-CSF-R with that of the EGF-R, a KpnI site was introduced in G-CSF-R cDNA in pBluescript by substituting C2071CT with T2071AC, using a site-directed mutagenesis kit (QuickChange kit; Stratagene, La Jolla, CA). Similarly, a KpnI site was introduced in the EGF-R cDNA by replacing G2142GC with T2142AC, which resulted in the substitution of Ala629 to Thr629 in the encoded EGF-R protein. The 2 cDNAs were subcloned into pEF-BOS and were digested withKpnI and AatII. The 2KpnI/AatII fragments were swapped and ligated, resulting in a cDNA coding for a chimeric protein (Thr629of EGF-R was joined to Leu613 of G-CSF-R in the transmembrane region). In addition to the so-called WT EGF–G-CSF receptor (EG-R), a series of chimeric receptors containing tyrosine residues Y704, Y729, Y744, and Y764 mutated to phenylalanine (F), either individually or collectively, and a receptor containing 2 prolines (P640and P642) mutated to serine (S) were constructed in pEF-BOS from the corresponding G-CSF-R mutants.8 This was achieved by subcloning the cytoplasmic regions of each G-CSF-R mutant into the WT EG-R by a MscI digest. Junctions were confirmed by sequencing.
Generation of stable virus-producing packaging cells
All EG-R constructs were inserted into the XhoI site of the retroviral vector pMSCVpac,26 transfected into GP+E-86 packaging cells by electroporation with subsequent selection in puromycin (2 μg/mL) 36 to 48 hours after transfection. Supernatants of the puromycin-resistant transfectants were filtered (0.2 μm) and stored at −70°C. GP+E-86 cells (4 × 105/plate) were incubated in growth medium supplemented with tunicamycin (1 μg/mL) overnight. The medium was aspirated, and the supernatant from the transfections was added (2.5 mL/plate). Cells were incubated 4 hours before another aliquot of the supernatant was added. After 2 days, the original medium was replaced with fresh medium containing puromycin (2 μg/mL). Single clones of the infected cells were analyzed by flow cytometry for EG-R expression, and those with a high expression level were selected for these studies.
Identification of EG-R–expressing clones by flow cytometry
Receptor-expressing GP+E-86 clones were incubated with mouse antihuman EGF-R monoclonal antibody 528 (10 μg/mL) for 30 minutes, then incubated with fluorescein isothiocyanate-conjugated anti–mouse immunoglobulin antibody (Silenus Labs, Melbourne, Australia) for 30 minutes, followed by propidium iodide (1 μg/mL) staining with washes between each staining. The clones were analyzed using a FACScan flow cytometer (Becton Dickinson, Bedford, MA). At least 3 high EG-R–expressing clones of each construct were expanded and tested for infection efficiency on Ba/F3 cells.
Infection of bone marrow cells
G-CSF–deficient mice1 (6 to 7 weeks old) were injected intraperitoneally with 5-fluorouracil (5-FU) at 150 mg/kg body weight. On day 4 after injection, bone marrow cells were harvested and cocultured (3 × 106 cells/T25 flask) with adhered virus-producing GP+E-86 packaging clones (5 × 105/flask, irradiated at 30 Gy before seeding) for 5 days in Dulbecco modified Eagle medium supplemented with fetal calf serum (15%), WEHI-3B D− cell-conditioned medium (10%), mSCF (10 ng/mL), mIL-6 (10 ng/mL), 2-mercaptoethanol (0.1 mM), and polybrene (5 μg/mL). After infection, bone marrow cells were transferred to fresh flasks and incubated at 37°C for 2 hours to remove adherent cells. Nonadherent cells were then washed extensively and assayed. Infection efficiency of the bone marrow cells was determined by flow cytometry.
Detection of receptor-expressing bone marrow cells by flow cytometry
Bone marrow cells were blocked with mouse serum (1:500) and antimouse FcγIII/II receptor antibody (1:250) (Becton Dickinson) for 10 minutes on ice. Cells were then incubated with biotinylated 528 (10 μg/mL), washed, and incubated in streptavidin–phycoerythrin conjugate (PharMingen, San Diego, CA) for 30 minutes. Live cells were identified by propidium iodide (1 μg/mL) exclusion and were analyzed by flow cytometry.
Proliferation assays
The proliferation assay was performed as described previously,27 except that cell density was 20 000/well and cultures were pulsed with methyl–3H-thymidine for the last 20 hours of the 3-day culture.
Agar colony-forming unit assay
Infected bone marrow cells were seeded at a density of 30 000/plate in semisolid agar (0.3%) supplemented with a range of mEGF concentrations (0-60 ng/mL).28 As controls, cells were seeded in the presence of G-CSF (0-10 ng/mL) (15 000/plate) or without any growth factors (30 000/plate). Cultures were incubated in a humidified atmosphere of 37°C with 10% CO2 in air. On day 4, colonies of 50 or more cells were counted using a dissecting microscope at × 40 magnification.
Staining the whole-plate cultures
To analyze the type of stimulated colonies, glutaraldehyde (2.5%)–fixed agar cultures were transferred onto slides and stained with Luxol fast blue (1 hour) and hematoxylin (4 minutes). Stained colony counts tended to be higher than unstained colony counts. The mean ratio of stained colony counts to unstained colony counts (± SD) was 1.1 ± 0.6 at 20 ng/mL mEGF and 1.5 ± 0.9 at 0.7 ng/mL mEGF.
Myeloperoxidase staining
Six agar colonies stimulated by each receptor construct were picked using a 200-μL pipette tip under a dissecting microscope. Cells were washed and stained for MPO as described.29 This experiment was performed twice.
Isolation of RNA, reverse transcription–polymerase chain reaction analysis
Total RNA was extracted from G-CSF–deficient bone marrow cells or from a pool of 5 EGF-stimulated agar colonies expressing each receptor construct, using TRIzol reagent (Life Technologies-Gibco, Melbourne, Australia) according to the manufacturer's instructions. Total RNA was reverse transcribed using 500 ng random hexadeoxynucleotide primers (Promega, Sydney, Australia) and 200 U M-MuLV Reverse Transcriptase (New England Biolabs, Beverly, MA) in 20 μL total reaction volume. One tenth reaction volume was amplified (1 minute at 95°C, 1 minute at 52°C, 1 minute at 72°C) for 45 cycles followed by 10 minutes at 72°C in a Stratagene Robocycler. The sequence of the specific primers was as follows: β-actin,30 5′-ATGCCATCCTGCGTCTGGACCTGGC-3′, 5′-AGCATTTGCGGTGCACGATGGAGGG-3′; gelatinase,315′-ACGGTTGGTACTGGAAGTTCC-3′, 5′-CCAACTTATCCAGACTCCTGG-3′.
Electrophoretic mobility shift assay
Bone marrow cells were starved of growth factors for 6 hours, followed by 10 minutes stimulation in the presence of EGF (100 ng/mL), G-CSF (25 ng/mL), or no factors at a density of 1 × 106/mL. Electrophoretic mobility shift assay was performed essentially as described.32 The oligonucleotides used were β-cas,33 derived from the 5′ region of theβ-casein gene, which binds STAT5 and STAT1 and a high-affinity mutant of the sis-inducible element (SIE)34of the human c-fos gene, which binds STAT1 and STAT3.
Western blot analysis
Starved bone marrow cells (1 × 106/mL) were stimulated with EGF (100 ng/mL) for 10 minutes, followed by the addition of 10 vol ice-cold phosphate-buffered saline supplemented with 10 μM Na3VO4. Cells were pelleted and lysed in 50 mM Tris/HCl, 150 mM NaCl, 1% Triton-X-100, 1 mM EDTA, 0.1 mM Na3VO4, 1 mM dithiothreitol, 1 mM Pefablock, 50 μg/mL aprotinin, 50 μg/mL leupeptin, and 50 μg/mL bacitracin, followed by centrifugation at 13 000g for 15 minutes. Soluble proteins were mixed with sample buffer and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, using a 10% gel under nonreducing conditions. Proteins were electrophoretically transferred to a polyvinylidene fluoride membrane (Millipore, Sydney, Australia) and were blocked with TBST (10 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.05% (vol/vol) Tween 20) containing 3% bovine serum albumin for 1 hour. Membranes were incubated with antiphospho-p44/42 MAPK (ERK 1 and 2) antibody diluted in TBST containing 1% bovine serum albumin for 2 hours. After washing, the activated ERK 1 and 2 were detected with horseradish peroxidase–conjugated anti–rabbit immunoglobulin (Bio-Rad Laboratories, Sydney, Australia) and enhanced chemiluminescence (Amersham, Sydney, Australia). Membranes were stripped in 62.5 mM Tris-HCl, pH 6.7, 2% SDS, and 100 mM β-mercaptoethanol at 50°C for 30 minutes, washed, reblocked, and probed with anti-ERK 1 antibody as a loading control.
Statistics
All statistical analyses were performed using SPSS version 6.1 (SPSS, Sydney, Australia). EGF titration curves were analyzed using multiple regression analysis. Because there was far greater variability at high EGF concentrations, data transformation was necessary. Logarithmic transformation of agar colony numbers was not applicable because of a large number of zero values. Instead, square root transformation was performed. In methyl–3H-thymidine uptake assays, however, data were transformed logarithmically. In colony composition analysis, raw data were analyzed except for macrophage colony numbers, which were transformed to square root because of their low value. Differences were considered significant ifP < .01.
Results
Construction of EG-R chimeric mutants
To bypass endogenous G-CSF-R, this study used chimeric receptors in which the extracellular domain of EGF-R replaced that of G-CSF-R (Figure 1). All experiments were performed on bone marrow cells isolated from G-CSF–deficient mice to eliminate any effect of endogenous G-CSF in the functional assays. To confirm the integrity of the chimeric construct, so-called WT EG-R was expressed from pEF-BOS in murine myeloid leukemic (M1) cells, and the differentiation of the cells in response to EGF was examined. This mimicked the effect of G-CSF on G-CSF-R–transfected M1 cells, in which the cells became enlarged and vacuolated (data not shown). Similarly, proliferation of Ba/F3 cells expressing EG-R WT in response to EGF mimicked the proliferation of the cells expressing G-CSF-R in response to G-CSF (data not shown). In addition to WT EG-R, chimeric receptors containing Y→F mutations individually and collectively were constructed (Figure 1). A receptor with 2 P→S substitutions in the Box1 region, conserved in cytokine receptors, was also constructed for use as a negative control. This receptor is unable to activate JAK and, therefore, does not activate downstream signaling pathways.8 17
Schematic diagram of the retroviral vector pMSCVpac and a series of chimeric EG-R mutants.
EG-R (Y704→F), (Y729→F), (Y744→F), (Y764→F), and (Ynull) are mutant receptors with tyrosine substitution(s) as indicated; EG-R (P640, 642→S) is a nonfunctional receptor with 2 amino acid substitutions as indicated. LTR, long terminal repeat; pgk, phosphoglycerate kinase; pac, puromycin N-acetyltransferase; TM, transmembrane.
Schematic diagram of the retroviral vector pMSCVpac and a series of chimeric EG-R mutants.
EG-R (Y704→F), (Y729→F), (Y744→F), (Y764→F), and (Ynull) are mutant receptors with tyrosine substitution(s) as indicated; EG-R (P640, 642→S) is a nonfunctional receptor with 2 amino acid substitutions as indicated. LTR, long terminal repeat; pgk, phosphoglycerate kinase; pac, puromycin N-acetyltransferase; TM, transmembrane.
Selection of high-titer packaging clones
Receptor constructs were expressed from the retroviral vector pMSCVpac in GP+E-86 packaging cells. At least 3 individual packaging clones were selected on the basis of high receptor expression, and their infection efficiency was measured on Ba/F3 cells. These were cocultured with the virus-producing packaging clones and subsequently were used in an agar colony assay in which infection efficiency was measured as the proportion of Ba/F3 colonies formed in response to EGF to that in response to WEHI-3B D−cell-conditioned medium (data not shown). Single GP+E-86 packaging clones expressing each receptor construct at similar levels (Figure 2) with comparable infection efficiencies were selected for bone marrow cell infection.
Expression of EG-R mutants.
Expression of receptor constructs in GP+E-86 packaging clones detected by flow cytometry. Cells were incubated with mouse antihuman EGF-R antibody 528 or IgG2b (control), followed by fluorescein isothiocyanate–conjugated anti–mouse immunoglobulin. Receptor expression (unfilled histograms) is shown against the control (filled histograms).
Expression of EG-R mutants.
Expression of receptor constructs in GP+E-86 packaging clones detected by flow cytometry. Cells were incubated with mouse antihuman EGF-R antibody 528 or IgG2b (control), followed by fluorescein isothiocyanate–conjugated anti–mouse immunoglobulin. Receptor expression (unfilled histograms) is shown against the control (filled histograms).
Expression of EG-R in murine bone marrow cells
To understand the role of receptor tyrosine residues in signal transduction in bone marrow cells, all the receptor constructs were expressed by retroviral infection of bone marrow progenitor cells from 5-FU–treated mice. No evidence indicates that any EGF-R family member is expressed in mouse bone marrow cells35; therefore, chimeric receptors are expected to signal through homodimerization. Indeed, no colony growth or proliferation was observed in the presence of EGF. To prevent signaling from endogenous G-CSF production, G-CSF–deficient mice were used as a source of bone marrow cells. During the infection period, bone marrow cells were grown in a cocktail of growth factors that favored myeloid lineage development.36 Approximately 5% to 11% of the bone marrow cells expressed each receptor construct in each experiment (Table 1). Morphologic analysis of the infected bone marrow cells showed 93% of the receptor-expressing cells were of blast cell or myeloid lineage (data not shown). Infection efficiency was taken into account when calculating the EGF response in agar colony formation and in methyl–3H-thymidine uptake assays.
Infection efficiency in bone marrow cells
| Receptor . | Infection efficiency (%) . |
|---|---|
| EG-R WT | 6.9 ± 1.0 |
| EG-R (Y704 → F) | 6.2 ± 1.0 |
| EG-R (Y729 → F) | 6.2 ± 1.0 |
| EG-R (Y744 → F) | 6.1 ± 1.7 |
| EG-R (Y764 → F) | 9.2 ± 1.3 |
| EG-R (Ynull) | 8.0 ± 1.6 |
| EG-R (P640, 642→ S) | 6.1 |
| Receptor . | Infection efficiency (%) . |
|---|---|
| EG-R WT | 6.9 ± 1.0 |
| EG-R (Y704 → F) | 6.2 ± 1.0 |
| EG-R (Y729 → F) | 6.2 ± 1.0 |
| EG-R (Y744 → F) | 6.1 ± 1.7 |
| EG-R (Y764 → F) | 9.2 ± 1.3 |
| EG-R (Ynull) | 8.0 ± 1.6 |
| EG-R (P640, 642→ S) | 6.1 |
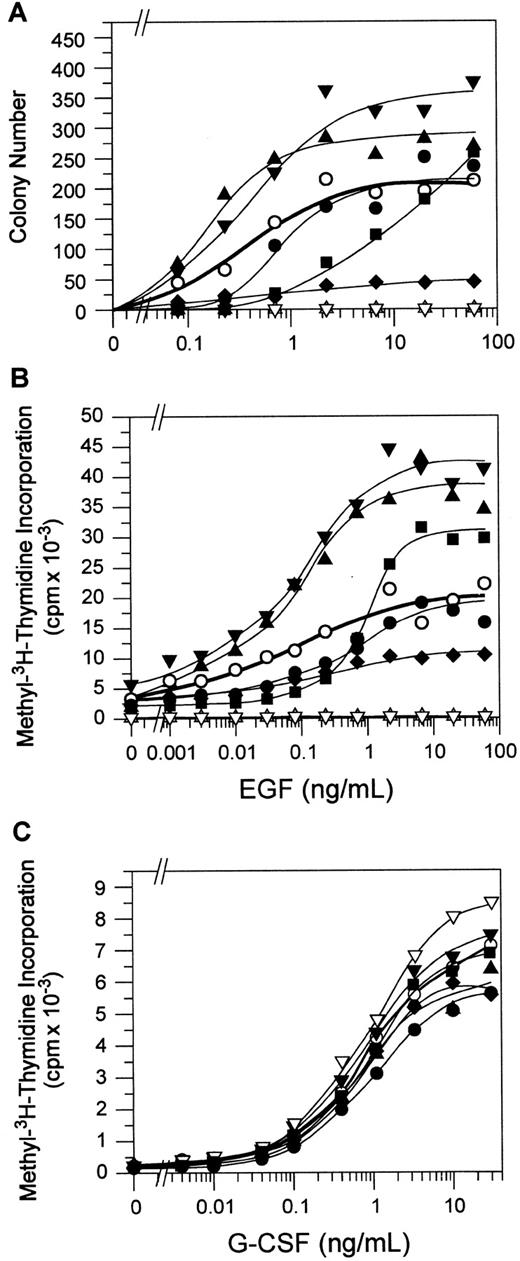
Receptor Y764 supports cell proliferation
Bone marrow cell proliferation was measured by agar colony formation and methyl–3H-thymidine uptake in response to EGF. In agar, the receptor-expressing bone marrow cells were stimulated by increasing concentrations of EGF, and the total number of colonies formed on day 4 was counted (Figure 3A). Colony formation stimulated by the WT receptor was characterized by a sigmoidal curve, and the effect of tyrosine mutations on variation from this curve was analyzed statistically (see “Materials and methods”). Colony formation by the receptor Y764 mutant was significantly reduced compared with that stimulated by the WT receptor (P < .01), although cells bearing the receptor Y744 mutant formed more colonies than bone marrow cells bearing the WT receptor (P < .01). Receptor Y704 and Y729 mutants stimulated similar numbers of colonies to the WT receptor. Receptor Ynullsupported maximal colony formation in response to EGF (the curve maximal response occurs at 60 ng/mL EGF; data at higher concentrations not shown), but it showed reduced sensitivity to EGF—the 50% effective concentration (EC50) of EGF was increased by more than 10-fold (9.0 vs 0.4 ng/mL) in cells expressing receptor Ynull compared with WT cells. Dose responsiveness was not altered in any of the single tyrosine mutants (EC50 = 0.8, 0.4, 0.3 and 0.4 ng/mL for Y704, Y729, Y744 , and Y764 mutants, respectively). As expected, the P640, 642 mutant did not form any colonies in response to EGF.
Proliferation and differentiation response of EG-R–expressing bone marrow cells.
(A) Total number of colonies per plate stimulated in response to increasing concentrations of EGF (0-60 ng/mL) in agar was counted on day 4, as described in “Materials and methods.” Colony numbers were corrected for infection efficiency. Data represent the average of 3 independent experiments. (B) Bone marrow cells were incubated in increasing concentrations of EGF (0-60 ng/mL) for 3 days, containing methyl–3H-thymidine for the last 20 hours of culture. Proliferation response was measured in counts per minute (cpm) and was corrected for infection efficiency. This figure is a representative of 2 independent experiments. (C) Proliferation response of bone marrow cells to G-CSF (0-30 ng/mL) as a control was also measured. This figure is representative of 2 independent experiments. WT, (○); Y704→F, (●); Y729→F, (▴); Y744→F, (▾); Y764→F, (♦); Ynull, (▪); P640, 642→S, (▵); and vector alone (▿).
Proliferation and differentiation response of EG-R–expressing bone marrow cells.
(A) Total number of colonies per plate stimulated in response to increasing concentrations of EGF (0-60 ng/mL) in agar was counted on day 4, as described in “Materials and methods.” Colony numbers were corrected for infection efficiency. Data represent the average of 3 independent experiments. (B) Bone marrow cells were incubated in increasing concentrations of EGF (0-60 ng/mL) for 3 days, containing methyl–3H-thymidine for the last 20 hours of culture. Proliferation response was measured in counts per minute (cpm) and was corrected for infection efficiency. This figure is a representative of 2 independent experiments. (C) Proliferation response of bone marrow cells to G-CSF (0-30 ng/mL) as a control was also measured. This figure is representative of 2 independent experiments. WT, (○); Y704→F, (●); Y729→F, (▴); Y744→F, (▾); Y764→F, (♦); Ynull, (▪); P640, 642→S, (▵); and vector alone (▿).
The requirement for the receptor Y764 for maximal bone marrow cell proliferation was evident in the methyl–3H-thymidine uptake assay, where the bone marrow cells stimulated by the receptor Y764 mutant showed significantly reduced proliferation compared with those stimulated by the WT receptor (Figure 3B) (P < .01). Other tyrosine mutations also showed some effect on cell proliferation. Receptor Y704 mutant supported a reduced proliferation of bone marrow cells, particularly at lower concentrations of EGF (P < .01). In contrast, receptor Y729 and Y744 mutants caused hyperproliferation of the bone marrow cells in response to EGF (P < .01). The P640, 642 mutant did not support bone marrow cell proliferation, and the receptor Ynull mutant caused an increase in EC50 compared with the WT receptor (0.8 vs 0.03). Overall, the results of the methyl–3H-thymidine uptake assay were similar to those of the colony assay. As an additional control, the G-CSF response of the bone marrow cells, expressing the WT receptor and mutants, was also measured. Their titration curves were similar to each other (Figure 3C), indicating that the different bone marrow infection cultures gave rise to similar progenitor populations.
Receptor Y729 supports granulocytic cell differentiation
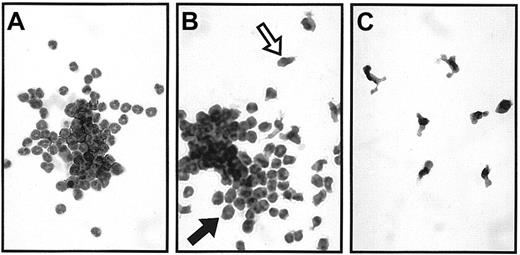
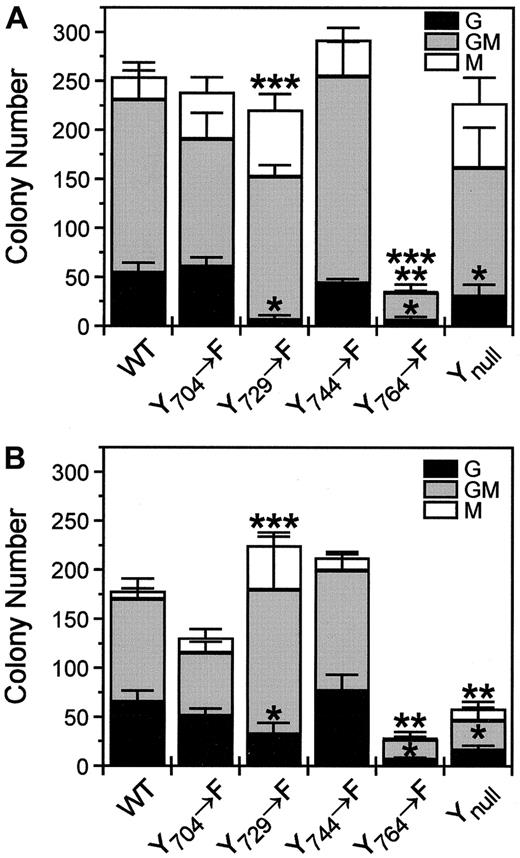
The WT receptor stimulated 3 types of bone marrow colonies in agar: granulocyte (G), macrophage (M), and granulocyte-macrophage (GM) colonies (Figure 4). Granulocyte colonies appeared morphologically normal, whereas macrophages were activated (with several projections). Receptor mutants stimulated the same types of colonies, but in different proportions. Although the total number of colonies stimulated by the receptor Y729 mutant was similar to that stimulated by the WT receptor, the number of granulocyte colonies stimulated by this mutant was significantly reduced (P < .01) (Figure 5A,B). Instead, this mutant supported more macrophage colony formation than the WT receptor (P < .01). Reduction in granulocyte colony formation was also apparent, but less marked, in the bone marrow cells stimulated by the receptor Ynull(P < .01). Receptor Y704 mutant stimulated similar numbers of G, GM, and M colonies compared with the WT receptor. Although the receptor Y744 mutant stimulated a higher number of colonies than the WT receptor, the increase was not significant for any individual colony type.
Typical examples of bone marrow colony types in agar in response to EGF.
EG-R WT receptor-stimulated (A) granulocyte, (B) granulocyte-macrophage, and (C) macrophage colonies. A macrophage and a granulocyte are indicated with an open and a closed arrow, respectively, in panel B (× 400).
Typical examples of bone marrow colony types in agar in response to EGF.
EG-R WT receptor-stimulated (A) granulocyte, (B) granulocyte-macrophage, and (C) macrophage colonies. A macrophage and a granulocyte are indicated with an open and a closed arrow, respectively, in panel B (× 400).
Absolute number of agar colony types stimulated with EGF.
Colony composition stimulated with (A) 20 ng/mL and (B) 0.7 ng/mL EGF by each receptor construct is shown. Differentially shaded bars represent the number of each colony type. Numbers of G (*), GM (**), or M (***) colonies stimulated by the receptor mutant are significantly different from those stimulated by the WT receptor. Data are presented as the mean ± SE of 3 independent experiments.
Absolute number of agar colony types stimulated with EGF.
Colony composition stimulated with (A) 20 ng/mL and (B) 0.7 ng/mL EGF by each receptor construct is shown. Differentially shaded bars represent the number of each colony type. Numbers of G (*), GM (**), or M (***) colonies stimulated by the receptor mutant are significantly different from those stimulated by the WT receptor. Data are presented as the mean ± SE of 3 independent experiments.
Proportions of G, GM, and M colonies were also compared between the WT and mutant receptors to determine whether the reduced colony number observed with the Y764 mutant resulted in the loss of a particular colony type (Table 2). Proportions of G, GM, and M colonies stimulated by the receptor Y704, Y744, or Y764 mutants were not significantly altered compared with the WT receptor at either stimulus concentration. As we saw in the absolute colony type counts, the receptor Y729 mutant stimulated a lower proportion of granulocyte colonies and a higher proportion of macrophage colonies than the WT receptor at either stimulus concentration. The increased proportion of stimulated macrophage colonies was also evident through the receptor Ynull. A higher proportion of granulocyte colonies and a lower proportion of macrophage colonies at lower concentrations of EGF were generated by all the receptor constructs (except for Y764 mutant, probably because of the small macrophage colony numbers), suggesting that granulocyte progenitors have higher sensitivity to stimuli than macrophage progenitors.
Proportion of bone marrow agar colony types stimulated by receptor constructs
| Receptor . | Colony formation (%)* . | |||||
|---|---|---|---|---|---|---|
| G . | GM . | M . | ||||
| High . | Low . | High . | Low . | High . | Low . | |
| EG-R WT | 23 | 37 | 69 | 59 | 9 | 4.2 |
| EG-R (Y704 → F) | 27 | 40 | 55 | 50 | 19 | 11 |
| EG-R (Y729 → F) | 3† | 15† | 67 | 64 | 30† | 21† |
| EG-R (Y744 → F) | 15 | 36 | 72 | 59 | 13 | 6 |
| EG-R (Y764 → F) | 19 | 33 | 80 | 62 | 2 | 6 |
| EG-R (Ynull) | 14 | 34 | 58 | 47 | 28† | 19† |
| Receptor . | Colony formation (%)* . | |||||
|---|---|---|---|---|---|---|
| G . | GM . | M . | ||||
| High . | Low . | High . | Low . | High . | Low . | |
| EG-R WT | 23 | 37 | 69 | 59 | 9 | 4.2 |
| EG-R (Y704 → F) | 27 | 40 | 55 | 50 | 19 | 11 |
| EG-R (Y729 → F) | 3† | 15† | 67 | 64 | 30† | 21† |
| EG-R (Y744 → F) | 15 | 36 | 72 | 59 | 13 | 6 |
| EG-R (Y764 → F) | 19 | 33 | 80 | 62 | 2 | 6 |
| EG-R (Ynull) | 14 | 34 | 58 | 47 | 28† | 19† |
SE < 25% in 90% of the colony numbers at high (20 ng/mL) and in 80% of the colony numbers at low (0.75 ng/mL) EGF concentrations.
Proportion of colonies stimulated by the receptor mutant is significantly different from those stimulated by the WT receptor at each particular EGF concentration.
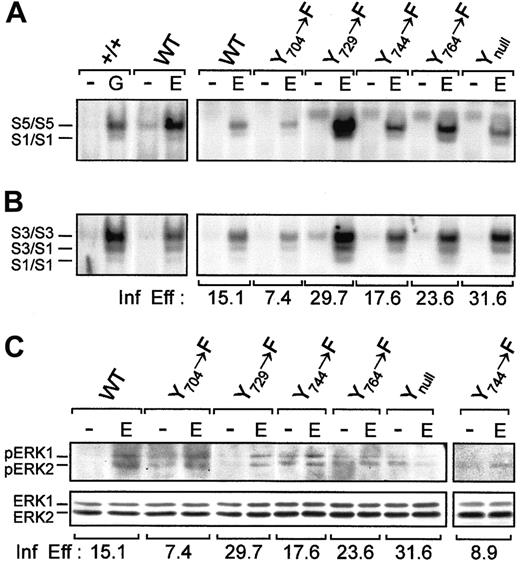
Activation of STAT and MAPK
To gain further insight into the signaling mechanisms used by the G-CSF-R to mediate the proliferation and differentiation responses observed, we examined activation of the STAT and MAPK pathways. First, we tested the ability of the WT receptor to activate STAT. This showed that the same complexes were activated as those we reported for +/+ bone marrow stimulated with G-CSF37(Figure 6A,B). Next, we examined the various mutants: all activated STAT1, STAT3, and STAT5 with only minor differences that appeared to be related to the relative infection efficiencies. Similar results were observed at 0.7 ng/mL EGF stimulation. Strong ERK 1/2 phosphorylation was observed through receptor WT, Y704, and Y729 mutants, whereas a modest activation was observed through receptor Y744mutant. In contrast, receptor Y764 and Ynullmutants failed to phosphorylate ERK1/2 (Figure 6C).
STAT and MAPK activation by receptor tyrosine mutants.
Nuclear extracts of 3 × 106 bone marrow cells were stimulated with or without EGF (100 ng/mL) and were incubated with 0.2 ng 32P-labeled double-stranded (A) β-cas (derived from the 5′ region of the β-casein gene) or (B) a high-affinity mutant of SIE. As a control, bone marrow cells of a normal mouse were stimulated with G-CSF (25 ng/mL), and STAT complexes were identified as described.37 S1, STAT1; S3, STAT3; S5, STAT5. These figures represent 1 of 3 independent experiments. (C) Bone marrow cells (3 × 106) expressing each receptor construct were stimulated with or without EGF (100 ng/mL), lysed, and electrophoresed on a 10% polyacrylamide gel. Proteins were transferred to a polyvinylidene difluoride membrane and were incubated with antiphospho-p44/42 MAPK antibody, followed by horseradish peroxidase-conjugated anti–rabbit immunoglobulin and enhanced chemiluminescence. The membrane was stripped and reprobed with anti-ERK 1 antibody. The main panel represents 1 of 2 independent experiments. The additional panel represents the repeated results for the Y744 mutant. Infection efficiency (Inf Eff) of bone marrow cells expressing each receptor construct for all experiments is also indicated.
STAT and MAPK activation by receptor tyrosine mutants.
Nuclear extracts of 3 × 106 bone marrow cells were stimulated with or without EGF (100 ng/mL) and were incubated with 0.2 ng 32P-labeled double-stranded (A) β-cas (derived from the 5′ region of the β-casein gene) or (B) a high-affinity mutant of SIE. As a control, bone marrow cells of a normal mouse were stimulated with G-CSF (25 ng/mL), and STAT complexes were identified as described.37 S1, STAT1; S3, STAT3; S5, STAT5. These figures represent 1 of 3 independent experiments. (C) Bone marrow cells (3 × 106) expressing each receptor construct were stimulated with or without EGF (100 ng/mL), lysed, and electrophoresed on a 10% polyacrylamide gel. Proteins were transferred to a polyvinylidene difluoride membrane and were incubated with antiphospho-p44/42 MAPK antibody, followed by horseradish peroxidase-conjugated anti–rabbit immunoglobulin and enhanced chemiluminescence. The membrane was stripped and reprobed with anti-ERK 1 antibody. The main panel represents 1 of 2 independent experiments. The additional panel represents the repeated results for the Y744 mutant. Infection efficiency (Inf Eff) of bone marrow cells expressing each receptor construct for all experiments is also indicated.
Expression of myeloperoxidase and gelatinase do not require tyrosine-mediated signaling
To measure the differentiation status of bone marrow granulocyte agar colonies stimulated by each receptor construct, granulocytes were isolated from agar colonies and stained for MPO. The WT receptor and all single- and multiple-tyrosine mutants supported MPO expression in granulocytes in a similar manner (Figure7A). Expression of neutrophil gelatinase (NG) in agar colonies was examined by reverse transcription–polymerase chain reaction (RT-PCR) (Figure 7B). Bone marrow granulocyte colonies expressing the WT receptor or its tyrosine mutants all expressed NG.
Expression of differentiation markers MPO and NG in bone marrow granulocyte colonies.
(A) Expression of MPO (black staining) is shown in the cytoplasm of granulocytes from agar colonies stimulated by EG-R (Ai) WT, (Aii) Y704→F, (Aiii) Y729→F, (Aiv) Y744→F, (Av) Y764→F, and (Avi) Ynull (× 1000). (B) Expression of NG mRNA (207 base pair [bp]) in granulocytes was detected by RT-PCR. As a control, bone marrow cells isolated from G-CSF–deficient mice (−/−) were also analyzed. (C) Negative control with no RNA. Expression of β-actin (605 bp) was shown as a control. This figure is representative of 2 independent experiments.
Expression of differentiation markers MPO and NG in bone marrow granulocyte colonies.
(A) Expression of MPO (black staining) is shown in the cytoplasm of granulocytes from agar colonies stimulated by EG-R (Ai) WT, (Aii) Y704→F, (Aiii) Y729→F, (Aiv) Y744→F, (Av) Y764→F, and (Avi) Ynull (× 1000). (B) Expression of NG mRNA (207 base pair [bp]) in granulocytes was detected by RT-PCR. As a control, bone marrow cells isolated from G-CSF–deficient mice (−/−) were also analyzed. (C) Negative control with no RNA. Expression of β-actin (605 bp) was shown as a control. This figure is representative of 2 independent experiments.
Discussion
The aim of this study was to improve our understanding of the role of G-CSF-R tyrosine residues in signal transduction in primary cells. Previous studies have been undertaken in cell lines that can show idiosyncratic responses that may not reflect the situation in normal, nonimmortalized cell types. We found that in primary bone marrow, receptor tyrosine residues are required for cell proliferation at lower concentrations of stimulus. Receptor Ynull showed less sensitivity to stimulation than the WT receptor in colony formation and methyl–3H-thymidine uptake assays. Multiple tyrosine residues contribute to signal transduction from the receptor. Y729 initiates granulocyte differentiation pathway(s), whereas Y764 transmits strong signals for cell proliferation in bone marrow cells, evident in agar colony formation and methyl–3H-thymidine uptake assays. This study confirms some of the findings in related cell lines and provides additional information about G-CSF-R signaling in primary cells.
Lack of receptor Y729 caused a defect in granulocyte differentiation in bone marrow cells. This finding was different from the results in M1 and 32D cells, where Y729 mutation had little effect on cell differentiation.8,22 However, mutation of Y728 of the mG-CSF-R caused a defect in granulocyte differentiation of LGM-1 cells, resulting in macrophage differentiation in these cells.20 It is likely that receptor Y729 initiates differentiation pathway(s) through recruitment of an as yet unidentified molecule(s) in bone marrow cells. Although the reduction of agar granulocyte colonies by the receptor Y729 mutant suggested that this tyrosine initiated neutrophil granulocyte differentiation pathways, we cannot rule out the possibility that the observed reduction in granulocyte colonies was caused by perturbed progenitor cell proliferation. If receptor Y729 sent granulocyte proliferation signals, in its absence there should have been fewer stimulated colonies; we did not observe this. The reduction in granulocyte colony formation was compensated for by increased macrophage colony formation by this mutant, suggesting no loss of total progenitors. Some evidence indicates that the differentiation of granulocytic and monocytic cells is inversely controlled by transcription factors.38 It is possible that the expression of specific transcription factor(s) is altered as a result of receptor Y729 mutation, resulting in a switch of the differentiation program. Another possibility is that in the absence of signals from receptor Y729, the sequence of neutrophil differentiation signals would be incomplete, allowing the cells to differentiate into macrophages instead as a default pathway. Receptor Y729 may have a dual function of promoting differentiation and suppressing cell proliferation as cell maturation coincides with cell cycle arrest in mature neutrophilic granulocytes.
In contrast to other studies, receptors Y704 and Y744 were found not to have major roles in bone marrow cell proliferation or differentiation. Unlike the effect in LGM-1 cells, mutation of Y704 or Y729 did not abrogate MPO expression in primary bone marrow cells. This was perhaps not surprising considering that MPO expression is detectable in CD34+, HLA-DR+ progenitor cells,39probably before G-CSF signaling is required. Although not apparently required for MPO expression, G-CSF signaling does enhance MPO expression in cell lines17 and in bone marrow cells.40 We found that granulocyte colonies, stimulated by WT EG-R or its tyrosine mutants, were terminally differentiated as measured by the expression of NG.41 Little is known about the role of G-CSF signaling in NG expression. However, its expression in G-CSF–deficient mice (this study) and its lack of expression in a G-CSF-R transduced myeloid cell line derived from PU.1 null mice42 suggests G-CSF signaling is redundant or is not involved in NG expression.
Strong proliferation signals were initiated from receptor Y764, and we have evidence from methyl–3H-thymidine uptake data that Y704 may also contribute to proliferation. These results are overall in agreement with studies in 32D cells.21,22 However, our results suggest that receptor Y744 and perhaps receptor Y729 engage inhibitory pathways for cell proliferation because bone marrow cells expressing receptors lacking these tyrosines were hyper-proliferative in terms of colony formation and3H incorporation. Such inhibitory effects have not been detected previously in studies of cell lines. In this study, simultaneous mutation of all 4 receptor tyrosine residues had a different effect from individual mutations of the tyrosine residues. For example, the observed reduction of cell proliferation caused by the mutation of receptor Y764 was not found in the receptor Ynull mutant. Clearly, the mutation of other tyrosine residues in receptor Ynull was compensated for by the mutation of Y764. It is possible that negative regulatory factors interact with tyrosine residues, so that when individual tyrosine residues are mutated, positive and negative signals are eliminated. Y764 binds to SHIP (SH2-containing inositol phosphatase) in Ba/F3 cells.43 SHIP may down-regulate the proliferation signals initiated from this tyrosine. Similarly, other factors may down-regulate signals from other tyrosine residues. The balance of signals transmitted from the whole receptor appears to determine the net outcome.
At high concentrations of EGF, signals from receptor Ynullwere sufficient for cell proliferation and differentiation, suggesting that there are receptor Y–independent mechanisms for activating cell proliferation and differentiation at optimal stimulus concentration. At lower concentrations of stimulus, however, tyrosine residues were required for signal transduction. Ward et al32 have shown that STAT3 activation is dependent on receptor tyrosine residues at low stimulus concentrations and that additional tyrosine-independent STAT3 activation signals occurred at high stimulus concentration. Together, these observations are consistent with the hypothesis that receptor tyrosine residues may signal under steady state conditions and that additional Y-independent pathways are activated in an emergency state.32 This hypothesis could also explain the decreased sensitivity to EGF stimulation of receptor Ynull that we observed in this study. By corollary, the sensitivity to erythropoietin (EPO) stimulation was reduced in Ba/F3 and DA-3 cells expressing EPO-R (Ynull) compared with cells expressing the WT receptor,44 suggesting that this may represent a more general phenomenon.
Because STAT and MAPK family members have been implicated in the control of G-CSF–mediated differentiation and proliferation responses,5,7-12,21 we sought to investigate whether they might play a role in this primary cell system. All receptor mutants were able to activate STAT1, STAT3, and STAT5 in a manner equivalent to that of the WT receptor (and indeed WT bone marrow), even at low ligand concentrations. This suggests that these signaling components are activated in a tyrosine-independent manner in these cells, and it equally suggests that they are not responsible for the differences observed among the various receptor forms. It does not, however, necessarily imply that they are not important for proliferation or differentiation. Given that all mutants do show some proliferation and differentiation, activation of STAT might be required. In contrast, ERK activation was predominantly dependent on Y764; it was absent from cells expressing Y764 and Ynullmutants. This is in agreement with other studies showing Y764-mediated activation of SHP-2, Grb2, and Shc (elements of MAPK pathway) in 32D cells45 and JNK-SAPK (a form of MAPK) in Ba/F3 cells.46
Chimeric growth factor receptors using the EGF-R extracellular domain have been shown to be functional.47-49 Here we analyzed the behavior of the EGF–G-CSF chimeric receptor in comparison with the intact G-CSF-R in cell lines. Although we cannot rule out the possibility that subtle differences in signaling occur from the chimeric receptor in comparison with the WT G-CSF-R, the chimeric receptor stimulated proliferation in Ba/F3 cells and differentiation in M1 cells in the same manner as the intact G-CSF-R. Furthermore, the chimeric receptor formed STAT complexes similar to those of the intact G-CSF-R in bone marrow cells. Therefore, we used chimeric receptors carrying tyrosine mutations to study the tyrosine-specific signals in bone marrow cells. Specific signals from the G-CSF-R were required to stimulate maximal granulocytic cell differentiation, supporting an instructive role for G-CSF-R in granulopoiesis. Further evidence for this was found in transgenic mice expressing G-CSF–EPO chimeric receptor, which did not undergo normal granulopoiesis.50Although myeloid cell lineage commitment was not altered, neutrophil terminal differentiation and function were impaired in these mice. These observations suggest that general signals are common to cytokine receptors and that, if they are placed in the correct cellular environment, they can mimic each other. However, certain signals specific to each cytokine receptor are essential for its full spectrum of functions.
We thank Prof Donald Metcalf and Dr George Hodgson for their assistance with agar colony typing, Dr Nicholas Wilson for his kind gift of antiphospho-ERK antibody, Dr Brendan Jenkins for the gift of anti-ERK antibody, and George Rennie for undertaking the statistical analysis. We also thank Fiona Connell for technical assistance.
Supported in part by a Research Scholarship of The University of Melbourne (S.A.), a grant from the National Health and Medical Research Council (no. 981133) (J.E.L.), a Sylvia and Charles Viertel Senior Medical Research Fellowship (A.C.W.), and a Wellcome Senior Research Fellowship in Medical Sciences in Australia (G.J.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Judith E. Layton, Ludwig Institute for Cancer Research, Royal Melbourne Hospital, PO Box 2008, Victoria 3050, Australia; e-mail: judy.layton@ludwig.edu.au.



![Fig. 7. Expression of differentiation markers MPO and NG in bone marrow granulocyte colonies. / (A) Expression of MPO (black staining) is shown in the cytoplasm of granulocytes from agar colonies stimulated by EG-R (Ai) WT, (Aii) Y704→F, (Aiii) Y729→F, (Aiv) Y744→F, (Av) Y764→F, and (Avi) Ynull (× 1000). (B) Expression of NG mRNA (207 base pair [bp]) in granulocytes was detected by RT-PCR. As a control, bone marrow cells isolated from G-CSF–deficient mice (−/−) were also analyzed. (C) Negative control with no RNA. Expression of β-actin (605 bp) was shown as a control. This figure is representative of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/3/10.1182_blood.v99.3.879/5/m_h80322091007.jpeg?Expires=1764960311&Signature=vIwGAjQoBd6SFCLnspATPhyJCxTRC~hQvOsUQWPAfmDW4AW1TJPU0y~MJN3sEXrdjVSJcyoQC~r7bwAVY60uX33uQvFP5H67PCgYTtTHbLheNRXb1RaRByTznmYzsZnYhuCOGbVoeKmxNqWyK6zzgui3JryqARAE3VT0RWQk4qeWo-OPsD275f3JyetVkhPl2-B181BZY41fGI9A48eBAuSDoYGPo6LSMh6DKlTWjgykqWPhuQFr9msnE1PQX0BCYxYRrDLegnVtbWmjTj8mCMAO6QMc~aSrtPangyzJ8zgOieu-HaZHGe6Lv1RJ02IYD0PPS4Ylal2z9Ct9wd-WpA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal