Abstract
The purpose of this study was to compare transplantation outcomes in patients with hematologic malignancies who received marrow grafts from either phenotypically matched unrelated, one- antigen–mismatched unrelated, or highly human leukocyte antigen (HLA)–disparate family donors. Between 1993 and 2000, 139 patients underwent transplantation from unrelated donors (81 matched and 58 mismatched) and 48 patients received marrow grafts from family donors that were mismatched at 2, 3, or 4 of 8 HLA loci. All patients received a standardized conditioning regimen and a graft-versus-host disease (GVHD) prophylaxis schedule with the exception of recipients of haploidentical marrow grafts, who received antithymocyte globulin after bone marrow transplantation as additional immunosuppression. There was no statistically significant difference in the rate of engraftment, or the cumulative incidences of acute and chronic GVHD between any of the 3 groups. The 2-year cumulative incidence of relapse was lower in matched unrelated patients (25%, P = .01) and mismatched unrelated patients (26%,P = .014) than in haploidentical patients (42%). Transplant-related mortality was significantly higher in recipients of mismatched unrelated grafts (45%, P = .01) and haploidentical grafts (42%, P = .001) compared with recipients of matched unrelated marrow grafts (23%). This resulted in a significantly higher probability of overall survival for matched unrelated patients (58%) versus either mismatched unrelated (34%,P = .01) or haploidentical (21%, P = .002) patients. There was no statistically significant difference in survival between patients who received mismatched unrelated grafts versus those who received haploidentical grafts. This study supports a donor selection algorithm whereby patients who lack a closely matched family donor be offered a phenotypically matched unrelated donor if available. There is no apparent advantage to using a mismatched unrelated versus a highly HLA-disparate family donor.
Introduction
Allogeneic bone marrow transplantation (BMT) is the only known curative therapy for many patients with hematologic malignancies. Although the majority of allogeneic marrow transplantations have been performed using either a human leukocyte antigen (HLA)–identical sibling or closely HLA-matched (ie, one-antigen–mismatched) family donor, alternative donor sources such as unrelated or more highly HLA-disparate family donors have emerged as viable alternatives.1-9 Whereas unrelated marrow transplantations are typically performed using either phenotypically matched or one-antigen–mismatched donors, related marrow transplantation has recently been successfully employed with donors that are mismatched at either 2, 3, or 4 HLA antigens.5-8Most centers employ phenotypically matched unrelated donors as the next best option for those patients who do not have a closely matched family donor. However, many patients, particularly those from ethnic or racial minority populations, do not have suitable unrelated marrow donors or require protracted donor searches that ultimately may be unsuccessful.10 Moreover, in other instances, the aggressive biology of the disease precludes a search for an unrelated donor, necessitating that a more highly HLA-disparate family donor be utilized if an allogeneic marrow transplantation is to be considered.
The ability to successfully perform a transplantation on some patients using haploidentical family donors that share only 4, 5, or 6 of 8 HLA antigens raises a question as to the optimal donor selection algorithm in those patients who have the potential to receive marrow grafts from either unrelated or highly HLA-disparate family donors. To date, this issue has not been addressed in a comparative fashion. Thus, whether transplantation outcome with matched or mismatched unrelated marrow grafts is superior or inferior to highly HLA-disparate family grafts is an unresolved question. For the past 7 years, we have performed both unrelated and highly HLA-disparate family donor transplantations for patients with hematologic malignancies using a standardized conditioning regimen and a graft-versus-host disease (GVHD) prophylaxis schedule. The purpose of this study was to determine whether donor selection affected transplantation outcomes of engraftment, GVHD, relapse, transplant-related mortality (TRM), and survival in these respective patient populations.
Patients, materials, and methods
Patient population
Between January 1, 1993, and March 1, 2000, 48 patients received haploidentical marrow grafts from family members who were HLA mismatched (in at least one direction) at 2, 3, or 4 of 8 HLA loci for the treatment of hematologic malignancies. Patients that received transplants of one-antigen–mismatched marrow grafts from family donors were not included in the haploidentical patient cohort. Over the same period of time, 139 patients received unrelated marrow grafts from phenotypically matched (n = 81) or one-antigen–mismatched donors (n = 58). This analysis excluded patients with myeloproliferative disorders other than chronic myelogenous leukemia (CML), relapsed leukemia after autologous BMT, and patients who received transplants of non T-cell–depleted marrow grafts. All transplantations were performed at the Medical College of Wisconsin at either Froedtert Memorial Lutheran Hospital or the Children's Hospital of Wisconsin. Informed consent was obtained from each patient (or his or her guardian) and all treatment was administered under protocols approved by the institutional review committees of the Medical College of Wisconsin.
The patient demographic data are shown in Table1. Disease status was classified into low-risk or high-risk categories. Low-risk disease was defined as acute leukemia or non-Hodgkin lymphoma in first or second complete remission, myelodysplasia (refractory anemia subtype), and CML in first chronic phase. High-risk disease included patients with acute leukemia or lymphoma in more than second remission, relapsed or refractory leukemia/lymphoma, secondary leukemia, myelodysplasia (RAEB or RAEB-T subtypes), and CML in accelerated, second chronic, or blast phase. Recipients of haploidentical marrow grafts were more likely to have received growth factor therapy with either granulocyte colony-stimulating factor (G-CSF) and/or granulocyte-macrophage colony-stimulating factor (GM-CSF) to accelerate myeloid recovery after transplantation and to be cytomegalovirus (CMV) seropositive. Patients who received transplants of matched unrelated marrow grafts received significantly higher doses of CD34 cells at BMT than recipients of either mismatched unrelated or haploidentical marrow grafts. There was no difference in any of the 3 groups with respect to patient age, gender, type of disease, risk status or method of T-cell depletion (TCD).
Preparative regimen, GVHD prophylaxis, and supportive care
All patients were treated in laminar air-flow or HEPA-filtered rooms. Pretransplantation conditioning consisted of high-dose cytosine arabinoside (3 gm/m2 × 6, day −7 to day −4), cyclophosphamide (45 mg/kg × 2, day −6 and day −5), and methylprednisolone (1 gm/m2 × 4, day −2 to day 0) followed by fractionated total body irradiation to a total dose of either 13.32 Gy or 14 Gy (day −2 to day 0).1,4 All patients had shielding of lungs, liver, and kidneys to reduce radiation exposure to these vital organs.11 GVHD prophylaxis from 1993 to 1998 consisted of ex vivo TCD with the αβ T-cell receptor antibody, T10B9, and baby rabbit complement12 plus posttransplantation cyclosporine. Due to unavailability of T10B9 thereafter,ex vivo TCD was performed with OKT3 antibody plus complement beginning in July 1998 in recipients of haploidentical marrow grafts, and in October 1998 in recipients of unrelated grafts. Patients who received transplants of haploidentical marrow grafts from family members also were treated with antithymocyte globulin (15 mg/kg) for either 7 or 14 days beginning on day 4 after BMT as additional GVHD prophylaxis. Six patients receiving transplants of one-antigen–mismatched unrelated grafts also received ATG on day 4 to day 10 after transplantation to prevent GVHD. G-CSF (5 μg/kg per day) and/or GM-CSF (250 μg/m2 per day) were administered to some patients beginning within the first week after transplantation to accelerate myeloid recovery (Table 1). CMV-seronegative patients received blood components from CMV-seronegative donors. All patients received prophylactic antibiotics according to institutional guidelines.
Donor selection and histocompatibility testing
The donor selection process for patients who did not have a suitable closely matched family donor was conducted with the goal of finding a matched unrelated donor as a first treatment option. If this was not possible, attempts were made to locate either a one-antigen–mismatched unrelated donor or a highly HLA-disparate family donor. If, in the opinion of the patient's physician, there was insufficient time to perform an unrelated search due to the patient's disease status or if the preliminary search indicated a very low likelihood of obtaining an unrelated donor, a family member was selected as the primary donor option in lieu of a matched or mismatched unrelated donor.
Serologic typing for class I antigens was done using standard microcytotoxicity assays to detect HLA-A and HLA-B antigens currently recognized by the World Health Organization (WHO).13 In some patients, HLA-A and HLA-B alleles were further resolved using one dimensional isoelectric focusing (IEF) as previously described14 and included the use in selected cases of carboxypeptidase to help resolve HLA-A and HLA-B locus bands that comigrated on the gels. High-resolution class I typing was performed by DNA sequence analysis. HLA-DR and DQ compatibility was assessed by either oligotyping or DNA sequence analysis using previously described techniques.15 In some instances, donor/recipient pairs were retrospectively analyzed with more discriminating HLA methodologies (ie, isoelectric focusing or DNA sequencing) so that the degree of HLA compatibility or lack thereof for the entire patient population could be determined with the highest accuracy. All assignments of HLA donor/recipient histocompatibility represent the optimal resolution of HLA disparities known at the time of latest clinical follow-up. Any HLA-A, HLA-B, HLA-DR, or HLA-DQ donor/recipient disparity that was detectable by serology, IEF, oligotyping, or DNA sequence analysis was considered a mismatch.
Assessment of engraftment, GVHD, and relapse
The date of engraftment was defined as the first of 3 consecutive days in which the absolute neutrophil count (ANC) was more than or equal to 500/μL. Acute GVHD was graded as 0 to IV according to criteria of Glucksberg and colleagues16 whereas chronic GVHD was defined as none, limited, or extensive.17Patients who had evidence of engraftment were evaluable for acute GVHD, whereas patients who engrafted and also survived more than 100 days were evaluable for chronic GVHD. TRM was defined as death resulting from any cause other than relapse. Relapse was defined by either morphologic evidence of disease in the peripheral blood, marrow, extramedullary sites, or by the recurrence and sustained presence of pretransplantation chromosomal abnormalities on cytogenetic analysis of bone marrow cells. Patients with CML whose sole evidence of disease was positivity for the bcr/abl RNA transcript by the polymerase chain reaction (PCR) were not classified as having relapsed. Patients who died from complications of a second transplantation or donor leukocyte infusion therapy given for recurrent disease were deemed to have died from disease relapse. Patients who died of complications from a second transplantation for graft rejection were classified as having died from graft rejection.
Graft characterization
CD34+ cells were measured in TCD marrow by flow cytometry using the International Society for Hematotherapy and Graft Engineering (ISHAGE) gating method and were analyzed as CD34+ cells/kg infused with the marrow graft.18
Immune reconstitution studies
Immunofluorescence was performed using a whole blood method consisting of the addition of appropriate amounts of undiluted conjugated antibody to 50 μL of whole blood followed by 30 minutes of incubation at 4°C. Red blood cells were lysed with FACS lysis buffer (Becton Dickinson, San Jose, CA), followed by 2 washes in phosphate-buffered saline (PBS) with 1% bovine serum albumin (BSA) and 0.02% sodium azide. Samples were analyzed on either an Epics Profile flow cytometer (Coulter Immunology, Hialeah, FL) or a FACSCalibur Flow Cytometer (Becton Dickinson). The lymphoid populations to be analyzed were gated using log 90° and forward-angle light scatter characteristics. CD45 and CD14 were used to gate out residual red blood cells and monocytes, respectively. Appropriate isotype controls were included with each panel. The 2-color immunophenotype panel included fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)–conjugated antibodies to T-cell, B-cell, and natural killer (NK)–cell subsets. Antibodies were obtained from Coulter Immunology or Becton Dickinson. Absolute cell content was determined by multiplying the percentage of lymphocyte gated cells expressing the antigen of interest by the absolute lymphocyte count determined from a white blood cell count and differential performed the same day as the immunophenotyping study. Normal values were established from the 95th percentile range of 42 healthy controls tested over the same time course as patients reported in this study.
The proliferative response to phytohemaglutinin (PHA) was determined using peripheral blood mononuclear cells (PBMCs) isolated from heparinized whole blood. PBMCs at 105 cells per well were stimulated with 2 predetermined optimal concentrations of phytohemaglutinin-P (PHA-P; DIFCO, Detroit, MI) in RPMI media supplemented with 10% pooled human serum, penicillin/streptomycin, and L-glutamine. The cultures were performed in flat-bottom microtiter wells in a final volume of 200 μL/well. Cultures were pulse labeled with 1 μCi/well (0.037 MBq) of 3H-thymidine for the last 24 hours of a 72-hour culture at 37°C in a humidified atmosphere of 5% CO2. The cultures were harvested onto glass-fiber filter paper, and radioactivity counted in a liquid scintillation counter. The absolute proliferative response was calculated as the mean counts per minute (cpm) of triplicate wells minus the unstimulated medium control. The result from the PHA concentration yielding the maximum proliferative response was reported. The normal response range was established from the 95th percentile range of a total of 370 healthy controls tested over the same time course as patients reported in this study.
Statistical analysis
For all 3 groups, we compared variables related to the transplant recipients, donors, underlying diseases, and transplantation procedures, using the chi-square statistic for categorical variables and the Mann-Whitney test for continuous variables. The probability of survival was estimated by the Kaplan-Meier method19; the log rank test was used for univariate comparisons. Survival and disease-free survival curves were adjusted for factors found to be significant in the multivariable analysis. Cumulative incidence curves were used to calculate the probability of acute and chronic GVHD, relapse, and TRM.20 Competing risks for GVHD were death without GVHD, relapse, and graft rejection. Relapse was a competing risk for TRM, and TRM was a competing risk for relapse. The associations of the type of graft with outcomes were evaluated in multivariable analyses, with the use of Cox proportional hazards regression to adjust for differences in potentially confounding variables between the cohorts. The variables considered were the recipient age, donor age, gender (recipient), gender match, donor/recipient CMV serostatus, therapy with growth factor, type of underlying disease, risk group, CD34 cell dose, and monoclonal antibody used for TCD. Each of the factors was checked for the assumption of proportional hazards by using a time-dependent covariate. First-order interactions with donor type (ie, matched unrelated, mismatched unrelated, and haploidentical) were assessed in a model that included only donor type and the variable under consideration. No interactions were found. A forward stepwise procedure was used to build the final multivariable model. The main effect of donor type was included in all models.21 Only factors significantly associated with an outcome (P < .05) were retained in the final models. AllP values are 2-sided. Endpoints were calculated at the date of last contact, with the date of latest follow-up being May 30, 2001. A 5% overall significance level was used to declare differences between any one of the 3 donor groups. To ensure the overall level, a Bonferonni correction was used so a P value of less than .017 ( = 0.05/3) was used to judge significance of pairwise comparisons. Parameters of immune reconstitution between groups within the time intervals measured were analyzed by ANOVA.
Results
Histocompatibility testing
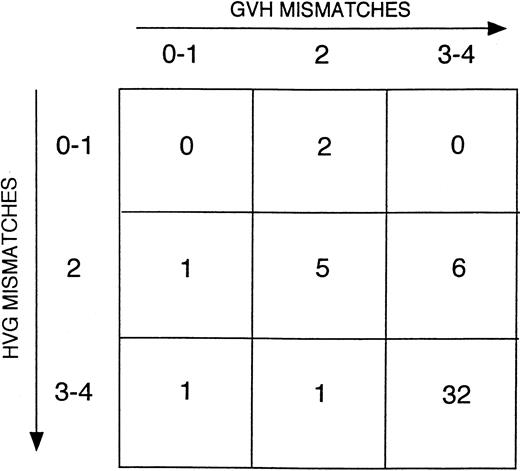
HLA class I typing for recipients of matched unrelated marrow grafts and their donors was performed by DNA sequencing in 70% of cases (57/81), IEF in 4% of cases (3/81), and serology in the remaining donor/recipient pairs (21/81, 26%). All of these donor/recipient pairs had class II typing performed by either oligonucleotide genotyping (27%), DNA sequencing (14%), or a combination of the 2 (59%). Typically, in the latter instance, DNA sequencing was used to determine HLA-DRB1 identity and oligotyping employed for determination of HLA-DQB1 identity. Class I typing for recipients of one-antigen–mismatched marrow grafts and their donors was performed by DNA sequencing in 50% of cases, IEF in 14%, and serology in 36% of donor/recipient pairs. As for matched unrelated transplants, all donor/recipient pairs of mismatched marrow grafts had class II typing done by either oligonucleotide genotyping (41%), DNA sequencing (7%), or both (52%). HLA class I typing was performed by serology in the majority of donors and the recipients of haploidentical marrow grafts (75%). All but 3 of these patients had their class II typing done by oligotyping and/or DNA sequencing. Approximately 75% of patients who received transplants of haploidentical grafts were mismatched at either 3 or 4 HLA loci with their donor. The directionality of HLA responses in these patients is shown in Figure1.
Directionality of HLA disparities in haploidentical marrow transplant recipients.
The number of patients in each category is noted in the respective boxes. HVG indicates host versus graft.
Directionality of HLA disparities in haploidentical marrow transplant recipients.
The number of patients in each category is noted in the respective boxes. HVG indicates host versus graft.
Engraftment
All 81 patients receiving transplants of phenotypically matched unrelated marrow grafts and 55 of 58 (95%) patients receiving one-antigen–mismatched marrow grafts were evaluable for engraftment. Forty-six of 48 (96%) patients who received transplants of haploidentical grafts were evaluable for engraftment. Nonevaluable patients died before engraftment due to transplant-related complications. The median time to an ANC greater than or equal to 500/μL for 3 consecutive days was 16 (range, 8-34), 15 (range, 9-48), and 14 (range, 5-34) days for matched unrelated, mismatched unrelated, and haploidentical transplant recipients, respectively. There were no statistically significant differences in the rate of engraftment between any of the 3 patient groups when assessed in pairwise comparisons. Graft rejection occurred in 2 recipients of haploidentical marrow grafts, 3 recipients of mismatched unrelated grafts, and 2 recipients of matched unrelated grafts. The patient who received a transplant of a matched unrelated graft received a cryopreserved chronic-phase CML marrow resulting in autologous recovery of blood counts and is currently alive with disease more than 6.5 years after transplantation. The remaining 6 patients died of graft rejection.
Graft-versus-host disease
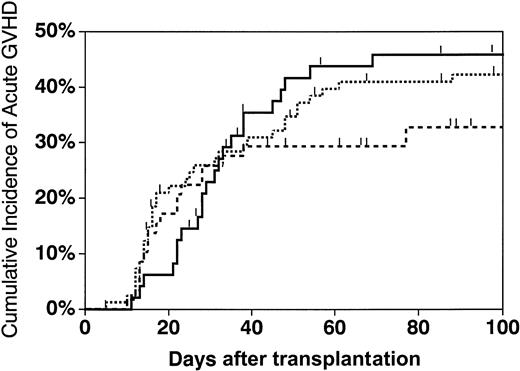
The cumulative incidence of grades II-IV acute GVHD at 100 days was 42% in patients who received transplants of phenotypically matched unrelated marrow grafts (95% confidence interval [CI], 31%-53%), 33% in patients receiving one-antigen–mismatched grafts (95% CI, 21%-45%), and 46% for those who received haploidentical grafts (95% CI, 31%-61%) (Figure 2). There were no statistically significant differences in acute GVHD rates between any of these 3 groups (P = .46). There was also no significant difference in the grade III-IV acute GVHD rates in these 3 groups (P = .18) (data not shown). The cumulative incidence of chronic GVHD at 1.5 years (either limited or extensive) in patients receiving matched versus one-antigen–mismatched unrelated marrow grafts was 57% (95% CI, 44%-71%) and 51% (95% CI, 35%-68%), respectively. The incidence of chronic GVHD in recipients of haploidentical marrow grafts was 50% (95% CI, 31%-69%). There were no statistically significant differences between any of the groups (P = .69). The cumulative incidence of extensive chronic GVHD for matched unrelated, mismatched unrelated, and haploidentical transplant recipients was 17% (95% CI, 8%-26%), 21% (95% CI, 9%-33%), and 20% (95% CI, 6%-34%), respectively. None of these groups differed from each other (P = .44). From the final Cox models, we found that TCD with OKT3 versus T10B9 monoclonal antibody was associated with a higher risk of grades II-IV (P = .007) and III-IV (P = .001) acute GVHD, but not chronic GVHD. Age was a risk factor for the development of extensive chronic GVHD only (RR = 1.05, P < .001).
Cumulative incidence of grades II-IV acute GVHD in recipients of matched unrelated, mismatched unrelated, and haploidentical marrow grafts.
The cumulative incidence of acute GVHD at 100 days in matched unrelated (dotted line), mismatched unrelated (dashed line), and haploidentical (solid line) recipients was 42% (95% CI, 31%-53%), 33% (95% CI, 21%-45%), and 46% (95% CI, 31%-61%), respectively.
Cumulative incidence of grades II-IV acute GVHD in recipients of matched unrelated, mismatched unrelated, and haploidentical marrow grafts.
The cumulative incidence of acute GVHD at 100 days in matched unrelated (dotted line), mismatched unrelated (dashed line), and haploidentical (solid line) recipients was 42% (95% CI, 31%-53%), 33% (95% CI, 21%-45%), and 46% (95% CI, 31%-61%), respectively.
Relapse
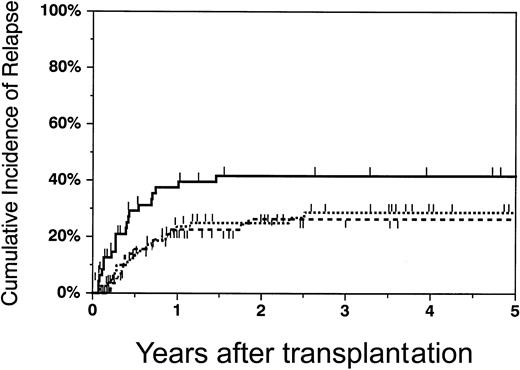
The cumulative incidence of relapse at 2 years was 25% (95% CI, 15%-35%) in patients who received transplants of phenotypically matched unrelated marrow grafts, 26% (95% CI, 14%-38%) in patients who received one-antigen–mismatched grafts, and 42% (95% CI, 26%-57%) for those who received transplants of haploidentical grafts (Figure 3). There was a statistically significant lower risk of relapse in patients who received transplants of matched unrelated grafts versus haploidentical grafts (RR = 0.45,P = .01), and mismatched unrelated grafts versus haploidentical marrow grafts (RR = 0.43, P = .014) (Table 2). There were no significant differences in the relapse rate between recipients of matched grafts versus mismatched unrelated grafts (RR = 1.04, P = .90). Relapse was significantly higher for patients with high-risk disease after adjustment for donor type (RR = 3.74 versus low-risk patients,P < .0001).
Cumulative incidence of relapse in recipients of matched unrelated, mismatched unrelated, and haploidentical marrow grafts.
The cumulative incidence of relapse at 2 years in matched unrelated (dotted line), mismatched unrelated (dashed line), and haploidentical (solid line) recipients was 25% (95% CI, 15%-35%), 26% (95% CI, 14%-38%), and 42% (95% CI, 26%-57%), respectively.
Cumulative incidence of relapse in recipients of matched unrelated, mismatched unrelated, and haploidentical marrow grafts.
The cumulative incidence of relapse at 2 years in matched unrelated (dotted line), mismatched unrelated (dashed line), and haploidentical (solid line) recipients was 25% (95% CI, 15%-35%), 26% (95% CI, 14%-38%), and 42% (95% CI, 26%-57%), respectively.
One relapsed patient with CML who received a transplant of a matched unrelated graft and one who received a transplant of a haploidentical marrow graft are both alive in cytogenetic relapse. Four patients who received transplants of matched unrelated, 4 who received mismatched unrelated grafts, and 3 who received haploidentical marrow grafts were treated with donor leukocyte infusions (DLI) for relapsed disease. Two matched unrelated patients with CML and myelodysplasia (MDS) and one mismatched unrelated patient with secondary acute myelogenous leukemia (AML) are in remission after DLI. All remaining relapsed patients failed to respond and eventually died of their disease. Nine patients (4 matched unrelated, 2 mismatched unrelated, and 3 haploidentical) received second marrow transplants for treatment of recurrent disease. All but one recipient of a matched unrelated graft who is alive in remission died due to disease recurrence or transplant-related toxicities.
Transplant-related mortality
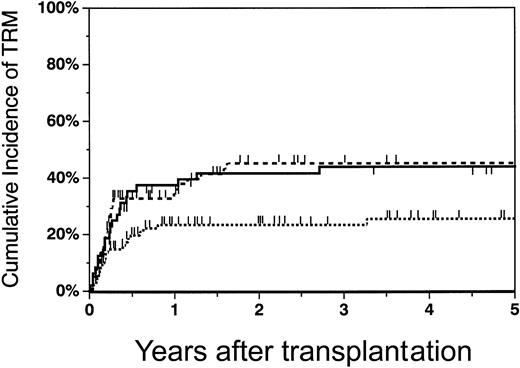
The cumulative incidence of TRM at 2 years was 23% (95% CI, 14%-33%) in patients who received transplants of phenotypically matched marrow grafts, 42% (95% CI, 27%-56%) in recipients of haploidentical grafts, and 45% (95% CI, 32%-59%) in recipients of mismatched unrelated marrow grafts (Figure4). The TRM rate was significantly lower in recipients of matched unrelated grafts versus either mismatched unrelated (RR = 0.47, P = .01) or haploidentical marrow grafts (RR = 0.35, P = .001) (Table 2). There was no statistically significant difference in TRM rates between recipients of mismatched unrelated grafts versus haploidentical grafts (RR = 0.76,P = .35). Increasing age (P = .0002) was associated with a higher risk of TRM in all patient groups.
Cumulative incidence of transplant-related mortality in recipients of matched unrelated, mismatched unrelated, and haploidentical marrow grafts.
The cumulative incidence of TRM at 2 years in matched unrelated (dotted line), mismatched unrelated (dashed line), and haploidentical (solid line) recipients was 23% (95% CI, 14%-33%), 45% (95% CI, 32%-59%), and 42% (95% CI, 27%-56%), respectively.
Cumulative incidence of transplant-related mortality in recipients of matched unrelated, mismatched unrelated, and haploidentical marrow grafts.
The cumulative incidence of TRM at 2 years in matched unrelated (dotted line), mismatched unrelated (dashed line), and haploidentical (solid line) recipients was 23% (95% CI, 14%-33%), 45% (95% CI, 32%-59%), and 42% (95% CI, 27%-56%), respectively.
Causes of death
The causes of death for all patients are shown in Table3. The primary cause of death in all groups was disease relapse that accounted for 30% to 45% of all fatalities. Infection was the next major cause of death in all patient groups, with 10/58 (17%) mismatched unrelated, 7/48 (15%) haploidentical, and 7/81 (9%) matched unrelated transplant recipients dying from infectious complications. GVHD was the proximate cause of death in a minority of patients in all 3 groups, although nearly all patients who died from infectious complications had ongoing GVHD or were receiving immunosuppressive therapy for treatment or prevention of GVHD. Thus GVHD could not be excluded as a contributory factor in these deaths. Death due to Epstein-Barr virus (EBV) lymphoproliferative disorders was observed in 4 patients, 2 each who received transplants of mismatched unrelated and haploidentical marrow grafts.
Survival
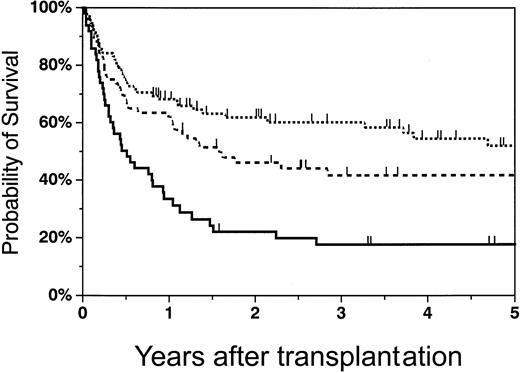
The Kaplan-Meier probability of overall survival at 2 years was 58% (95% CI, 47%-69%) for recipients of matched unrelated marrow grafts, 34% (95% CI, 22%-46%) for recipients of one-antigen–mismatched grafts, and 21% (95% CI, 9%-32%) for recipients of haploidentical marrow grafts. Figure5 shows the survival curves adjusted for growth factor and risk group, which were found to be significant factors in the multivariable analysis. Survival was significantly higher in patients who received transplants of matched unrelated grafts versus haploidentical marrow grafts (RR = 0.48,P = .002), and between recipients of matched grafts versus one-antigen–mismatched unrelated marrow grafts (RR = 0.54,P = .01). There was no statistically significant difference in survival between recipients of one-antigen–mismatched unrelated grafts versus haploidentical marrow grafts (RR = 0.87,P = .67). Survival was significantly worse in high-risk versus low-risk patients (RR = 2.4, P < .0001) and in patients who received growth factor to accelerate myeloid reconstitution (RR = 1.72, P = .014).
Adjusted probability of survival in recipients of matched unrelated, mismatched unrelated, and haploidentical marrow grafts.
The probability of 2-year survival in matched unrelated (dotted line), mismatched unrelated (dashed line), and haploidentical (solid line) recipients was 58% (95% CI, 47%-69%), 34% (95% CI, 22%-46%), and 21% (95% CI, 9%-32%), respectively.
Adjusted probability of survival in recipients of matched unrelated, mismatched unrelated, and haploidentical marrow grafts.
The probability of 2-year survival in matched unrelated (dotted line), mismatched unrelated (dashed line), and haploidentical (solid line) recipients was 58% (95% CI, 47%-69%), 34% (95% CI, 22%-46%), and 21% (95% CI, 9%-32%), respectively.
The 2-year probability of disease-free survival (DFS) was 52% (95% CI, 41%-63%) for recipients of unrelated grafts, 29% (95% CI, 17%-40%) for patients who received transplants of one-antigen–mismatched grafts, and 17% (95% CI, 6%-27%) for recipients of haploidentical grafts. DFS was significantly higher in matched unrelated patients versus haploidentical patients (RR = 0.50,P = .003). There was no statistically significant difference in DFS between recipients of mismatched unrelated grafts versus haploidentical graft transplant recipients (RR = 0.84,P = .48), or between matched and mismatched unrelated patients (RR = 0.60, P = .029). DFS was significantly worse in high-risk versus low-risk patients (RR = 2.3,P < .0001) and in patients receiving growth factor therapy (RR = 1.67, P = .018) (Table 3).
Immune reconstitution
Immune reconstitution in each of these patient groups was examined to determine if there were any differences in the recovery of T and B cells after transplantation. Patients were tested at intervals by immunophenotyping of lymphocyte subsets and proliferative response to a T-cell mitogen. Recovery of lymphocyte subsets was similar among the 3 patient groups. There were no significant differences in recovery of CD3+, CD4+, or CD8+ T cells, CD56+ NK cells, or CD19+ B cells between groups (Figure 6). The total number of T cells was below the lower level of the healthy controls in each of the 3 patient groups until approximately 18 months after transplantation (Figure 6A). This was primarily due to low CD4 counts (Figure 6B), since CD8 T-cell counts were in the low normal range for most patients by one year (Figure 6C). NK cells normalized early and remained in the normal range (Figure 6D). In fact, during the first 60 days, NK cells made up the majority of the peripheral blood lymphocytes in each of the patient groups. B-cell numbers did not return to normal until the one-year assessment (Figure 6E). The proliferative response to PHA was consistent with T-cell numbers in that PHA responses did not enter the normal range until the one-year assessment (Figure 6F). The PHA responses were comparable among all 3 groups.
Immune reconstitution in recipients of matched unrelated, mismatched unrelated, and haploidentical marrow grafts.
Data are shown as box plots including the median and 1st to 3rd quartile of absolute cell number (A-E) or PHA response (F) at the indicated testing interval after transplantation. The 95th percentile range of absolute lymphocyte counts (per μL) from 42 healthy controls are shown as the shaded area in each graph. The 95th percentile range of PHA response of 370 healthy controls is shown as the shaded area in panel F. Data are presented in triplicate groupings. Matched unrelated patients are represented by the thin lined boxes, mismatched unrelated patients by the thick lined boxes, and haploidentical patients by the shaded boxes. The number of patients tested at each time interval for absolute cell counts ranged from 4 to 26 and for PHA response ranged from 5 to 18. Insufficient data points were available for intervals beyond 1 year for the PHA response.
Immune reconstitution in recipients of matched unrelated, mismatched unrelated, and haploidentical marrow grafts.
Data are shown as box plots including the median and 1st to 3rd quartile of absolute cell number (A-E) or PHA response (F) at the indicated testing interval after transplantation. The 95th percentile range of absolute lymphocyte counts (per μL) from 42 healthy controls are shown as the shaded area in each graph. The 95th percentile range of PHA response of 370 healthy controls is shown as the shaded area in panel F. Data are presented in triplicate groupings. Matched unrelated patients are represented by the thin lined boxes, mismatched unrelated patients by the thick lined boxes, and haploidentical patients by the shaded boxes. The number of patients tested at each time interval for absolute cell counts ranged from 4 to 26 and for PHA response ranged from 5 to 18. Insufficient data points were available for intervals beyond 1 year for the PHA response.
Discussion
Most patients who require an allogeneic marrow transplantation lack an HLA-identical sibling or closely matched family donor, necessitating that alternative donor options be considered if a transplantation is to be performed. For many patients, acceptable donors may be available from both within the family and from volunteer registries, raising the question as to the optimal donor when more than one option is available. The purpose of this study was to comparatively analyze transplantation outcomes in a consecutive series of patients who underwent allogeneic marrow transplantation from either unrelated or highly HLA-disparate family donors. Our results demonstrate that patients who received transplants of phenotypically matched unrelated marrow grafts had superior overall survival than did recipients of either mismatched unrelated or haploidentical marrow grafts. Superior survival in matched unrelated versus haploidentical transplant recipients was attributable to both a decrease in relapse and TRM. In contrast, the survival advantage conferred by transplantation of a matched versus a mismatched unrelated marrow graft was attributable exclusively to significantly lower TRM. DFS was also significantly higher in matched versus haploidentical transplant recipients, but did not reach statistical significance in matched versus mismatched patients. Notably, there were no differences in either DFS or overall survival among recipients of one-antigen–mismatched grafts versus recipients of haploidentical grafts. Thus, these data support the use of a phenotypically matched unrelated donor as the preferred option for patients who lack closely matched family donors, but do not indicate any advantage between one-antigen–mismatched unrelated donors versus haploidentical donors.
Comparison of transplantation outcomes in the various patient populations in this study was dependent upon patients being treated in a uniform fashion. To that end, all patients were treated with a standardized conditioning regimen over the 8 years of this study period. Additionally, the TCD methodology was consistently applied to all groups and resulted in similar percentages of patients whose grafts were depleted with T10B9 versus OKT3 antibodies. The one disparity in the GVHD prophylaxis schedule was that haploidentical patients received ATG as additional immunosuppression. Although this is a distinguishing feature between the groups, we do not believe that this affects the interpretation of the data or the conclusions of the study. All currently described haploidentical transplantation regimens5-8 employ more intense immune suppression than optimized unrelated transplantation regimens1-4,9 22 in order to prevent GVHD and facilitate engraftment. It is therefore highly unlikely that, even in a randomized setting, patients would be enrolled in a study where the degree of immunosuppression was equivalent between recipients of matched unrelated grafts versus recipients of highly HLA-disparate marrow grafts. Rather, the requirement for more intense immunosuppression in the haploidentical group is an integral aspect of the current treatment “package” for these patients.
With respect to unrelated marrow transplant recipients, an important aspect of the analysis is the precision with which HLA typing in both the donor and recipient is determined. A number of studies have shown that molecular typing methods are superior to serology in detecting HLA disparities.23-26 Moreover, the degree of HLA matching between donor and recipient has been shown to affect the incidence and severity of GVHD.27-31 In that regard, it is noteworthy that in the current study the vast majority of patients were typed using molecular techniques. All recipients of unrelated marrow grafts had class II typing determined by either oligonucleotide genotyping or DNA sequencing. Moreover, more than 60% of unrelated donor/recipient pairs had class I typing performed using DNA sequencing or isoelectric focusing. For those unrelated patients not class I typed using molecular techniques, our experience is that molecular typing would uncover additional class I disparities in only 15% to 20% of these patients (C.A.K.-T., unpublished data, March 2000). Therefore, we do not believe that the lack of complete molecular typing on all unrelated patients is a significant confounding factor in this analysis.
All patients in this study received TCD marrow grafts. Thus, these results may or may not be generalizable to demographically similar recipients of unrelated non-TCD marrow grafts. We would emphasize, however, that, with rare exceptions,32 TCD is the primary approach to prevent GVHD in recipients of highly HLA-disparate haploidentical family marrow grafts5-8 due to concerns about toxicity from GVHD with unmodified marrow grafts. Given that the vast majority of haploidentical marrow transplantations performed to date have employed some form of TCD, a comparison between recipients of TCD unrelated versus TCD haploidentical marrow grafts can be justified on these grounds as well as on the need for standardization of the GVHD prophylaxis regimens. Moreover, the fact that the current analysis was restricted to only recipients of TCD grafts may not necessarily limit its generalizability, given a recent analysis indicating no significant differences in transplantation outcomes among recipients of TCD versus unmodified unrelated marrow grafts.22
Although the purpose of this study was to correlate donor type with transplantation outcomes, we observed 2 other significant findings in the multivariable analysis. The first was that growth factor administration was associated with lower DFS and overall survival. The effect of growth factor administration on transplantation outcome has previously been examined primarily in HLA-identical sibling transplantations. A number of randomized studies have observed no effect of growth factor on survival in this patient population.33-35 Results in alternative donor transplantations have been more difficult to interpret due to small patient numbers and lack of randomization,36 37 but have also shown no statistically significant effect of growth factor administration on overall survival. This study is therefore significant in indicating that growth factor administration has a deleterious effect on survival and additional studies are warranted to confirm this observation. We were unable to determine how growth factor therapy specifically worsened survival since there was no adverse effect on either relapse or TRM. This may have been due to the limited sample size of the study.
A second observation was that the antibody used for TCD affected the incidence of acute GVHD. TCD with OKT3 as opposed to T10B9 resulted in a higher incidence of acute GVHD. A potential explanation for this observation is that T-cell depletion with T10B9 versus OKT3 differs in 2 aspects. First, the overall degree of TCD using OKT3 is less than that attained with T10B9 (C.A.K.-T., unpublished data, March 2001). Second, when T10B9 is used to deplete T cells by complement-mediated lysis, the TCRγδ+ T-cell subset is selectively spared (0.5 log depletion versus 1.9 log depletion of the TCRαβ+ T-cell subset).38,39 This selective sparing of TCRγδ+ T cells was not seen for marrow TCD with OKT3 in our preclinical studies38 or in assessment of the grafts received by the patients in this study (median 1.1 log depletion of TCRγδ+ T cells). Additionally, OKT3 is mitogenic and may stimulate and promote survival of residual T cells whereas T cells surviving after TCD with T10B9 may be more likely to undergo apoptosis.40
Patients who received transplants of haploidentical marrow grafts received more intense GVHD prophylaxis with the addition of ATG as immunosuppression. Thus, we questioned whether this would hinder immune reconstitution relative to recipients of unrelated marrow grafts, particularly with respect to T-cell recovery. Examination of immune reconstitution in all 3 groups, however, revealed no significant differences in recovery of CD4+ or CD8+ T cells, B cells, NK cells, or in T-cell responses to a mitogenic stimulus. It should be emphasized, though, that these data do not measure functional immune responses against specific pathogens. We also did not assess the rates of specific infections in all 3 groups and therefore cannot draw any conclusions as to whether patients in any of these groups were more susceptible to opportunistic infections.
We would point out several limitations to the study. First, this was not a randomized study. The decision to utilize a haploidentical family donor was typically made after failure to ascertain a suitable unrelated donor or after recognition that the aggressiveness of the underlying disease precluded an unrelated search. Although the statistical analysis accounted for a number of patient, treatment, and disease-related variables, we cannot exclude that this approach may have introduced other confounding variables into the analysis. It is noteworthy, however, that the survival outcomes of the haploidentical patient population in this study are not dissimilar to those reported by other centers.5-8,41 For example, Henslee-Downey and colleagues6 reported a 2-year probability of survival of 33% in 72 recipients of 2 to 3 antigen mismatched grafts transplanted primarily for acute and chronic leukemia. A recent updated study from this same institution on 220 patients who received transplants of haploidentical marrow grafts for treatment of AML or acute lymphoblastic leukemia (ALL) reported a 3-year probability of survival of 20%.41 Aversa and coworkers,7 using a different preparative regimen and GVHD prophylaxis schedule, performed transplantations on 43 patients using one-haplotype–mismatched marrow grafts for treatment of either AML or ALL. The probability of DFS for patients with AML was 36% at 1.5 years and 17% at 2.5 years for patients with ALL. Thus, our haploidentical transplantation results are not inconsistent with these published studies and suggest that a center effect was not operative with respect to survival outcome.
Second, this study did not compare transplantation outcomes in recipients of cord blood, which is the other major source of alternative donor transplants.42-45 A recent study in pediatric patients has shown no difference in survival between those who received transplants of 0 to 3 HLA-mismatched unrelated cord blood versus phenotypically matched unrelated marrow grafts for the treatment of either malignant or nonmalignant diseases.46 Similar results have been reported by Rocha and colleagues,47although in this publication a significant number of children in the unrelated bone marrow group were HLA-mismatched with their donors and the analysis was restricted to patients with leukemia. Thus, these results indicate that cord blood is a viable stem cell alternative for some patients with hematologic malignancies. The role of cord blood in the alternative donor algorithm for adults has yet to be similarly analyzed in a comparative fashion.
In summary, our results demonstrate that patients who received transplants of marrow grafts from phenotypically matched unrelated donors had superior survival to those patients who received transplants of grafts from either mismatched unrelated or haploidentical donors. This study, therefore, supports a donor selection algorithm whereby patients with hematologic malignancies who lack a closely matched family donor be offered a phenotypically matched unrelated as opposed to a highly HLA-disparate family or one-antigen–mismatched unrelated donor. In those instances where the selection is between a mismatched unrelated versus a haploidentical marrow donor, our data do not support superiority of one donor option over the other. In those cases, other relevant issues (eg, timing of transplantation, available marrow cell dose, disease status) may be determinative in the selection of a donor.
We would like to thank Claudia Kabler-Babbitt and Diane Bauer for assistance with the data collection. The authors also thank the nursing staff, physician assistants, and nurse practitioners at the Froedert Memorial Lutheran Hospital and Children's Hospital of Wisconsin for excellent clinical care of the patients.
Supported by grants from the Midwest Athletes against Children's Cancer (MACC) Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
William R. Drobyski, Bone Marrow Transplant Program, Froedtert Memorial Lutheran Hospital, 9200 W Wisconsin Ave, Milwaukee, WI 53226; e-mail: bill@bmt.mcw.edu.