Abstract
De novo CD5+ diffuse large B-cell lymphoma (CD5+ DLBCL) is known to have phenotypically and genotypically different characteristics than CD5− DLBCL and mantle cell lymphoma (MCL). To further characterize CD5+ DLBCL, 109 patients with CD5+ DLBCL were reviewed, and the results were compared with those of 384 CD5− DLBCL and 128 cyclin D1+ MCL patients. Patients with CD5+ DLBCL showed a higher age distribution (median, 66 years; P = .0083) and a female predominance (male-female ratio, 49:60, P = .011) compared with those with CD5− DLBCL. CD5+DLBCL was more closely associated with many aggressive clinical features or parameters than CD5− DLBCL: 69% older than 60 years (P = .039), 34% with performance status greater than 1 (P = .0016), 69% with serum lactate dehydrogenase level higher than normal (P < .0001), 62% with stage III/IV disease at diagnosis (P = .0023), 35% with more than one extranodal site (P = .023), and 40% with B symptoms (P = .0031). The overall International Prognostic Index score was thus significantly higher for the patients with CD5+ DLBCL than for those with CD5− DLBCL (P = .00005). The most frequent site of extranodal involvement was bone marrow (28%), a higher frequency than that for CD5− DLBCL (P < .0001) but lower than that for cyclin D1+ MCL (P = .0015). Histopathologically, CD5+ DLBCL showed centroblastic morphology except for 3 patients with immunoblastic disease, and interfollicular growth pattern (7%) and intravascular or intrasinusoidal infiltration (19%) were observed. Immunophenotypically, CD5+ DLBCL was characterized by a CD5+CD10−CD19+ CD20+CD21−CD23−cyclin D1− phenotype and a predominance of surface IgMκ. Of particular interest is that CD5+ DLBCL was characterized by a survival curve significantly inferior to that for patients with CD5− DLBCL (P = .0026). These findings suggest that CD5+ DLBCL may constitute a unique subgroup of DLBCL.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the largest category of aggressive lymphomas; it is regarded as a heterogeneous group of lymphomas in terms of surface markers, histology, and clinical features.1 Some patients with DLBCL can be cured by combination chemotherapy, but more than half of them die of their disease.2 Therefore, identification of a high-risk group or a specific subtype of DLBCL is particularly important and long overdue.
The CD5 molecule is a 67-kd glycoprotein that is expressed by most T cells and a subset of B cells.3 CD5+ B cells are the predominant B-cell population in human fetal spleen and cord blood, but they represent only 10% to 20% of adult peripheral B cells.3-5 They are distinct from CD5−conventional B cells with respect to anatomic localization, gene usage, and function.3,5-10 CD5+ B cells also synthesize low-affinity polyreactive immunoglobulins, and increased numbers of these cells have been reported in many nonneoplastic diseases, including certain types of autoimmune disorders.3,10 11
In mature B-cell neoplasms, CD5 is expressed in most patients with chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL), but less frequently in patients with DLBCL12 and in only few patients with marginal zone B-cell lymphoma13,14 and Burkitt lymphoma.15 Of special interest is that CD5+ DLBCL not preceded by any other lymphoproliferative disease have been reported,16,17 though they are often identified as arising secondarily in a CLL (Richter syndrome). Matolcsy et al16 have highlighted the phenomenon of CD5 expression in DLBCLs evolving de novo, not as a result of transformation, thus suggesting that such DLBCL is genotypically distinct from Richter syndrome–associated DLBCL.
We have also provided evidence that de novo CD5+ DLBCL is phenotypically and genotypically distinct from MCL.17-22CD5+ cases account for approximately 10% of DLBCL, which is usually negative for CD10, CD21, and CD23.17-22Immunohistochemical examinations have demonstrated that cyclin D1 is not overexpressed in CD5+ cases18 and that immunoglobulin heavy chain genes are somatically mutated.19,20,22 These findings support our hypothesis that de novo CD5+ DLBCL constitutes a distinct subtype, but previous reports from a single institution include only a small number of patients.21,23 24 Therefore, the clinicopathologic features of de novo CD5+ DLBCL remain to be thoroughly identified. This prompted us to investigate these features in a large patient population. To further characterize the de novo CD5+ DLBCL, we performed a collaborative study on 109 patients.
Patients, materials, and methods
Patient selection
We selected 109 patients with de novo CD5+ DLBCL from 12 collaborating institutions. All patients were diagnosed between 1984 and 2000 as having DLBCL according to the REAL classification.1 They had no history of other lymphoproliferative disorders. All specimens for histologic and immunophenotypic studies were obtained at the initial presentation of the patients. CD5 antigen expression was examined by means of fluorescence-activated cell sorter analysis or immunohistochemistry. All patients were immunohistochemically confirmed to be cyclin D1−.
For the control group, 384 patients with CD5− DLBCL were selected. They were diagnosed consecutively between 1984 and 2000 at 3 major institutions (Mie University School of Medicine, Aichi Cancer Center, and Fujita Health University School of Medicine). Moreover, the clinical data of 128 of our patients with cyclin D1+ MCL25 were simultaneously reviewed for comparison with de novo CD5+ DLBCL.
Histopathology
Tissue was fixed in 10% formalin and embedded in paraffin. Sections (5-μm thick) were stained with hematoxylin and eosin, periodic acid-Schiff, Giemsa, and Gomori silver impregnation. Histologic sections were reviewed by 2 independent pathologists.
Immunophenotypic study
Immunohistochemical and flow cytometric analyses were performed as described previously.18 26 Monoclonal antibodies used were Leu4 (CD3), Leu1 (CD5), and CALLA (CD10) (Becton Dickinson, Mountain View, CA); J5 (CD10) and B1 (CD20) (Coulter, Hialeah, FL); H107 (CD23) (Nichirei, Tokyo, Japan); MHM6 (CD23), UCHL1 (CD45RO), HM57 (CD79a), anti-IgG, anti-IgA, anti-IgM, anti-IgD, anti-κ, and anti-λ (DAKO, Carpinteria, CA); 4C7 (CD5) and NCL-CD10 (CD10) (Novocastra, Newcastle, United Kingdom), and cyclin D1 (IBL, Gunma, Japan). More than 20% positivity of the tumor cells was judged as indicating positivity for the purposes of this study. In fact, most neoplastic cells were confirmed to be positive for CD5 in most patients. In 99 patients with de novo CD5+ DLBCL, CD5 expression was examined by means of flow cytometric analysis or immunohistochemistry in frozen sections and by immunohistochemically in paraffin sections in the remaining 10 patients.
Statistical analysis
Correlations between the 2 groups were examined by chi-square analysis, the Fisher exact test, the Student ttest, and the Mann-Whitney U test. Patient survival data were analyzed by the Kaplan-Meier method and were compared by means of the log-rank test. Univariate and multivariate analyses were performed with the Cox proportional hazard regression model. Data were analyzed with the SAS system (SAS Institute, Cary, NC).
Results
Patient characteristics for de novo CD5+ DLBCL, CD5− DLBCL, and cyclin D1+ MCL
Table 1 summarizes clinical features of the patients at presentation. In comparison with CD5− DLBCL, patients with de novo CD5+ DLBCL showed a higher age distribution (median, 66 vs 63 years;P = .0083, Student t test) and a female predominance (female-male ratio, 60:49 vs 159:225;P = .011). Especially noteworthy is that the patients with de novo CD5+ DLBCL showed a closer association with the aggressive clinical features or parameters: 75 patients older than 60 (69%, P = .039), 37 with performance status (PS) greater than 1 (34%, P = .0016), 75 with serum lactate dehydrogenase (LDH) level higher than normal (69%,P < .0001), 68 with stage III/IV disease at diagnosis (62%, P = .0023), 38 with more than one extranodal site (35%, P = .023), and 44 with B symptoms (40%,P = .0031). As a result, the International Prognostic Index (IPI) score27 for the patients with de novo CD5+ DLBCL was significantly higher than that for patients with CD5− DLBCL (P = .000 05), with 40 (36%) of the CD5+ group categorized in the IPI high-risk group.
Clinical features
| . | De novo CD5+ DLBCL (n = 109) . | CD5− DLBCL (n = 384) . | Cyclin D1+ MCL (n = 128) . | P* . | P† . |
|---|---|---|---|---|---|
| No. (%) . | No. (%) . | No. (%) . | |||
| Age at diagnosis, y | |||||
| Median | 66 | 63 | 65 | .0083 | .21 |
| Range | 22-91 | 17-92 | 36-81 | — | — |
| Older than 60 | 75 (69) | 222 (58) | 81 (64) | .039 | .42 |
| Sex (male/female) | 49/60 | 225/159 | 89/39 | .011 | .0001 |
| Performance status higher than 1 | 37 (34) | 76 (20) | 23 (20) | .0016 | .020 |
| Serum LDH level higher than normal | 75 (69) | 174 (45) | 37 (32) | < .0001 | < .0001 |
| Stage | .00004 | .011 | |||
| I | 16 (15) | 81 (21) | 6 (5) | — | — |
| II | 25 (23) | 127 (33) | 13 (10) | — | — |
| III | 18 (16) | 96 (25) | 32 (26) | — | — |
| IV | 50 (46) | 80 (21) | 74 (59) | — | — |
| III/IV | 68 (62) | 176 (46) | 106 (85) | .0023 | .0001 |
| Extranodal involvement | 84 (77) | 266 (69) | 87 (71) | .11 | .20 |
| More than 1 site | 38 (35) | 92 (24) | 43 (36) | .023 | .94 |
| IPI | .00005 | .10 | |||
| Low | 29 (27) | 176 (45) | 27 (23) | — | — |
| Low intermediate | 23 (21) | 75 (20) | 40 (34) | — | — |
| High intermediate | 17 (16) | 64 (17) | 31 (27) | — | — |
| High | 40 (36) | 68 (18) | 19 (16) | — | — |
| B symptoms present | 44 (40) | 100 (27) | 31 (39) | .0031 | .72 |
| . | De novo CD5+ DLBCL (n = 109) . | CD5− DLBCL (n = 384) . | Cyclin D1+ MCL (n = 128) . | P* . | P† . |
|---|---|---|---|---|---|
| No. (%) . | No. (%) . | No. (%) . | |||
| Age at diagnosis, y | |||||
| Median | 66 | 63 | 65 | .0083 | .21 |
| Range | 22-91 | 17-92 | 36-81 | — | — |
| Older than 60 | 75 (69) | 222 (58) | 81 (64) | .039 | .42 |
| Sex (male/female) | 49/60 | 225/159 | 89/39 | .011 | .0001 |
| Performance status higher than 1 | 37 (34) | 76 (20) | 23 (20) | .0016 | .020 |
| Serum LDH level higher than normal | 75 (69) | 174 (45) | 37 (32) | < .0001 | < .0001 |
| Stage | .00004 | .011 | |||
| I | 16 (15) | 81 (21) | 6 (5) | — | — |
| II | 25 (23) | 127 (33) | 13 (10) | — | — |
| III | 18 (16) | 96 (25) | 32 (26) | — | — |
| IV | 50 (46) | 80 (21) | 74 (59) | — | — |
| III/IV | 68 (62) | 176 (46) | 106 (85) | .0023 | .0001 |
| Extranodal involvement | 84 (77) | 266 (69) | 87 (71) | .11 | .20 |
| More than 1 site | 38 (35) | 92 (24) | 43 (36) | .023 | .94 |
| IPI | .00005 | .10 | |||
| Low | 29 (27) | 176 (45) | 27 (23) | — | — |
| Low intermediate | 23 (21) | 75 (20) | 40 (34) | — | — |
| High intermediate | 17 (16) | 64 (17) | 31 (27) | — | — |
| High | 40 (36) | 68 (18) | 19 (16) | — | — |
| B symptoms present | 44 (40) | 100 (27) | 31 (39) | .0031 | .72 |
De novo CD5+ DLBCL versus CD5− DLBCL.
De novo CD5+ DLBCL versus MCL.
A comparison with cyclin D1+ MCL (Table 1) showed that the patients with de novo CD5+ DLBCL were characterized by a female predominance (P = .0001), worse PS (P = .020), higher serum LDH level (P < .0001), and lower disease stage (P = .011). The IPI score for the patients with de novo CD5+ DLBCL had the tendency to be higher than that for cyclin D1+ MCL (P = .10).
Table 2 summarizes anatomic sites of extranodal involvement in de novo CD5+ DLBCL. In 31 (28%) patients of the current series, the disease was limited to extranodal sites, 25 (23%) had only lymphadenopathies without extranodal involvement, and the remaining 53 had lymphadenopathies with extranodal involvement.
Sites of extranodal involvement in de novo CD5+DLBCL
| Extranodal only . | Nodal only . | Nodal and extranodal . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | Age/Sex . | Extranodal involvement . | No. . | Age/Sex . | Extranodal involvement . | No. . | Age/Sex . | Extranodal involvement . | No. . | Age/Sex . | Extranodal involvement . |
| 1 | 61/M | S | 1 | 59/F | None | 1 | 54/M | L | 31 | 53/F | Nasal cavity |
| 2 | 52/M | BM | 2 | 69/F | None | 2 | 78/F | L | 32 | 75/M | Nasal cavity |
| 3 | 70/F | BM | 3 | 74/F | None | 3 | 50/M | S | 33 | 46/M | Thyroid gland |
| 4 | 65/M | BM | 4 | 51/M | None | 4 | 77/F | S | 34 | 57/F | W |
| 5 | 61/M | S, BM/PB | 5 | 71/F | None | 5 | 38/F | BM | 35 | 39/M | W |
| 6 | 78/M | L, BM/PB | 6 | 82/F | None | 6 | 61/F | BM | 36 | 63/M | W |
| 7 | 63/M | L, S, BM | 7 | 70/M | None | 7 | 67/M | BM | 37 | 56/F | W |
| 8 | 71/F | L, S, BM | 8 | 81/M | None | 8 | 86/M | S, BM/PB | 38 | 64/M | W |
| 9 | 82/F | L, S, BM | 9 | *66/F | None | 9 | 80/M | S, BM | 39 | 36/F | W |
| 10 | 73/F | L, S, BM | 10 | 56/M | None | 10 | 61/F | S, BM | 40 | 63/M | W |
| 11 | 57/F | L, S, BM | 11 | 85/F | None | 11 | 58/M | S, BM | 41 | 81/F | W |
| 12 | 73/F | L, S, BM, lung | 12 | 67/F | None | 12 | 77/M | L, S | 42 | 71/F | W |
| 13 | 84/F | L, S, BM, lung | 13 | 60/M | None | 13 | 59/M | L, S, BM | 43 | 65/F | Small intestine |
| 14 | 50/F | L, S, BM, lung | 14 | 61/M | None | 14 | 72/F | L, S, BM | 44 | 63/M | W, small intestine |
| 15 | 70/M | S, BM/PB, lung, brain | 15 | 79/M | None | 15 | 68/F | S, subcutaneous tissue | 45 | 91/M | Pleural effusion |
| 16 | 61/M | L, BM, CNS | 16 | 70/F | None | 16 | 67/F | BM, W | 46 | 73/F | Pleural effusion |
| 17 | 74/F | BM, bone | 17 | 82/M | None | 17 | 91/F | BM/PB, stomach | 47 | 56/M | Ascites |
| 18 | 69/M | Bone, muscle, peritoneum | 18 | 72/F | None | 18 | 78/M | L, S, BM, stomach | 48 | 59/M | Cerebrospinal fluid |
| 19 | 45/F | Subcutaneous tissue | 19 | 71/M | None | 19* | 73/F | L, S, BM, pleural effusion | 49 | 60/M | Testis |
| 20 | 56/M | Skin | 20 | 22/M | None | 20 | 44/M | L, S, lung, pleural effusion | 50 | 62/M | Testis |
| 21 | 66/F | Skin/subcutaneous tissue | 21 | 62/F | None | 21* | 64/F | L, lung, breast, kidney, adrenal gland | 51 | 70/F | Bone |
| 22 | 58/F | Skin/subcutaneous tissue | 22 | 76/F | None | 22 | 72/M | S, BM, testis | 52 | 66/F | Bone, uterus |
| 23 | 89/M | Skin/subcutaneous tissue | 23 | 65/M | None | 23 | 36/F | L, S, ovary | 53* | 62/F | Brain, thorax |
| 24 | 62/F | Stomach | 24 | 69/F | None | 24 | 77/F | S, BM/PB, muscle | — | —/— | — |
| 25 | 57/F | Stomach | 25 | 65/F | None | 25 | 74/M | Stomach | — | —/— | — |
| 26 | 54/F | Breast | — | —/— | — | 26 | 51/F | Stomach, breast | — | —/— | — |
| 27 | 63/F | Breast | — | —/— | — | 27 | 51/F | Breast | — | —/— | — |
| 28 | 74/M | W | — | —/— | — | 28 | 43/F | Breast | — | —/— | — |
| 29 | 77/M | W | — | —/— | — | 29 | 40/F | Breast | — | —/— | — |
| 30 | 81/F | Orbit | — | —/— | — | 30 | 28/F | Breast, W | — | —/— | — |
| 31 | 79/M | Pleural effusion | — | —/— | — | — | —/— | — | — | —/— | — |
| Extranodal only . | Nodal only . | Nodal and extranodal . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | Age/Sex . | Extranodal involvement . | No. . | Age/Sex . | Extranodal involvement . | No. . | Age/Sex . | Extranodal involvement . | No. . | Age/Sex . | Extranodal involvement . |
| 1 | 61/M | S | 1 | 59/F | None | 1 | 54/M | L | 31 | 53/F | Nasal cavity |
| 2 | 52/M | BM | 2 | 69/F | None | 2 | 78/F | L | 32 | 75/M | Nasal cavity |
| 3 | 70/F | BM | 3 | 74/F | None | 3 | 50/M | S | 33 | 46/M | Thyroid gland |
| 4 | 65/M | BM | 4 | 51/M | None | 4 | 77/F | S | 34 | 57/F | W |
| 5 | 61/M | S, BM/PB | 5 | 71/F | None | 5 | 38/F | BM | 35 | 39/M | W |
| 6 | 78/M | L, BM/PB | 6 | 82/F | None | 6 | 61/F | BM | 36 | 63/M | W |
| 7 | 63/M | L, S, BM | 7 | 70/M | None | 7 | 67/M | BM | 37 | 56/F | W |
| 8 | 71/F | L, S, BM | 8 | 81/M | None | 8 | 86/M | S, BM/PB | 38 | 64/M | W |
| 9 | 82/F | L, S, BM | 9 | *66/F | None | 9 | 80/M | S, BM | 39 | 36/F | W |
| 10 | 73/F | L, S, BM | 10 | 56/M | None | 10 | 61/F | S, BM | 40 | 63/M | W |
| 11 | 57/F | L, S, BM | 11 | 85/F | None | 11 | 58/M | S, BM | 41 | 81/F | W |
| 12 | 73/F | L, S, BM, lung | 12 | 67/F | None | 12 | 77/M | L, S | 42 | 71/F | W |
| 13 | 84/F | L, S, BM, lung | 13 | 60/M | None | 13 | 59/M | L, S, BM | 43 | 65/F | Small intestine |
| 14 | 50/F | L, S, BM, lung | 14 | 61/M | None | 14 | 72/F | L, S, BM | 44 | 63/M | W, small intestine |
| 15 | 70/M | S, BM/PB, lung, brain | 15 | 79/M | None | 15 | 68/F | S, subcutaneous tissue | 45 | 91/M | Pleural effusion |
| 16 | 61/M | L, BM, CNS | 16 | 70/F | None | 16 | 67/F | BM, W | 46 | 73/F | Pleural effusion |
| 17 | 74/F | BM, bone | 17 | 82/M | None | 17 | 91/F | BM/PB, stomach | 47 | 56/M | Ascites |
| 18 | 69/M | Bone, muscle, peritoneum | 18 | 72/F | None | 18 | 78/M | L, S, BM, stomach | 48 | 59/M | Cerebrospinal fluid |
| 19 | 45/F | Subcutaneous tissue | 19 | 71/M | None | 19* | 73/F | L, S, BM, pleural effusion | 49 | 60/M | Testis |
| 20 | 56/M | Skin | 20 | 22/M | None | 20 | 44/M | L, S, lung, pleural effusion | 50 | 62/M | Testis |
| 21 | 66/F | Skin/subcutaneous tissue | 21 | 62/F | None | 21* | 64/F | L, lung, breast, kidney, adrenal gland | 51 | 70/F | Bone |
| 22 | 58/F | Skin/subcutaneous tissue | 22 | 76/F | None | 22 | 72/M | S, BM, testis | 52 | 66/F | Bone, uterus |
| 23 | 89/M | Skin/subcutaneous tissue | 23 | 65/M | None | 23 | 36/F | L, S, ovary | 53* | 62/F | Brain, thorax |
| 24 | 62/F | Stomach | 24 | 69/F | None | 24 | 77/F | S, BM/PB, muscle | — | —/— | — |
| 25 | 57/F | Stomach | 25 | 65/F | None | 25 | 74/M | Stomach | — | —/— | — |
| 26 | 54/F | Breast | — | —/— | — | 26 | 51/F | Stomach, breast | — | —/— | — |
| 27 | 63/F | Breast | — | —/— | — | 27 | 51/F | Breast | — | —/— | — |
| 28 | 74/M | W | — | —/— | — | 28 | 43/F | Breast | — | —/— | — |
| 29 | 77/M | W | — | —/— | — | 29 | 40/F | Breast | — | —/— | — |
| 30 | 81/F | Orbit | — | —/— | — | 30 | 28/F | Breast, W | — | —/— | — |
| 31 | 79/M | Pleural effusion | — | —/— | — | — | —/— | — | — | —/— | — |
S indicates spleen; BM, bone marrow; PB, peripheral blood; L, liver; CNS, central nervous system; W, Waldeyer ring.
This patient had a history of autoimmune disease.
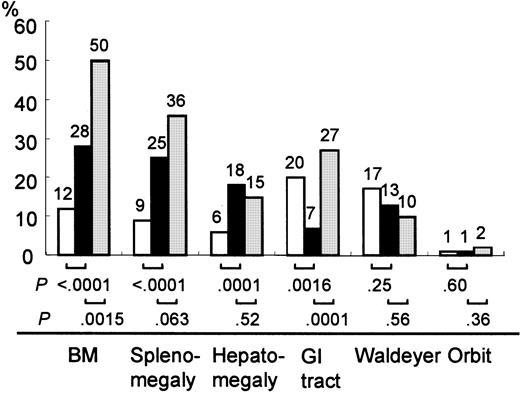
The most frequent site of extranodal involvement in de novo CD5+ DLBCL was bone marrow (n = 31, 28% of patients; Table 2; Figure 1). Atypical lymphocyte contents (range, 3%-53%) were noted at presentation in the peripheral blood smears of 6 patients, whose white blood cell counts ranged from 3700 to 15 100/μL. Twenty-seven (25%) of the patients had splenomegaly and 20 (18%) had hepatomegaly at presentation (Table 2; Figure 1). Sites of extranodal involvement in the patients without lymphadenopathies were relatively limited. Twenty-six of 31 patients had extranodal involvement in at least one in the following sites: bone marrow, liver, spleen, lung, skin, stomach, and breast. Nodal and extranodal disease presented a greater variety of extranodal involvement when compared to extranodal-only disease.
Extranodal involvement of de novo CD5+DLBCL, CD5− DLBCL, and cyclin D1+ MCL.
In de novo CD5+ DLBCL (▪), the incidence of bone marrow involvement was higher than that of CD5− DLBCL (■) and lower than that of cyclin D1+ MCL (░). Hepatomegaly and splenomegaly occurred more frequently in de novo CD5+DLBCL than in CD5− DLBCL. Gastrointestinal involvement occurred less frequently than in either CD5− DLBCL or cyclin D1+ MCL. BM, bone marrow; GI, gastrointestinal.
Extranodal involvement of de novo CD5+DLBCL, CD5− DLBCL, and cyclin D1+ MCL.
In de novo CD5+ DLBCL (▪), the incidence of bone marrow involvement was higher than that of CD5− DLBCL (■) and lower than that of cyclin D1+ MCL (░). Hepatomegaly and splenomegaly occurred more frequently in de novo CD5+DLBCL than in CD5− DLBCL. Gastrointestinal involvement occurred less frequently than in either CD5− DLBCL or cyclin D1+ MCL. BM, bone marrow; GI, gastrointestinal.
A comparison with CD5− DLBCL and cyclin D1+MCL demonstrated that the incidence of bone marrow involvement in de novo CD5+ DLBCL was higher than that of CD5−DLBCL (P < .0001) and lower than that of cyclin D1+ MCL (P = .0015). Hepatomegaly and splenomegaly occurred more frequently in patients with de novo CD5+ DLBCL than in patients with CD5− DLBCL (P = .0001 and P < .0001, respectively). Gastrointestinal involvement occurred less frequently in de novo CD5+ DLBCL than in CD5− DLBCL (P = .0016) or cyclin D1+ MCL (P = .0001). There were no significant differences in the incidence of involvement in Waldeyer ring or the orbit among these 3 groups.
Four (4%) of our patients had a history of autoimmune disease; 2 with rheumatoid arthritis, and one each had a history of rheumatoid arthritis with Sjögren syndrome and of primary biliary cirrhosis. Three patients had nodal and extranodal involvement (Table 2).
Histologic features
De novo CD5+ DLBCL showed a diffuse and monomorphic proliferation of large lymphoid cells (Figure2). These tumor cells were usually morphologically centroblastic and seldom immunoblastic (3 of 109, 3%; Table 3). In many patients, the tumor cells had a moderate rim of pale basophilic or amphophilic cytoplasm. Nuclei were round or sometimes irregular, indented or multilobated, and they contained vesicular chromatin and small distinct nucleoli. In 8 (7%) of the patients, these tumor cells were distributed throughout the interfollicular area while sparing the follicles, which retained their mantle cuffs (Figure 2). Although they were the subject of special attention, the typical mantle zone pattern or naked germinal centers characteristic of MCL were not observed. Moreover, intravascular or intrasinusoidal infiltration was identified in 21 (19%) patients with de novo CD5+ DLBCL. Focal necrosis and infiltration of macrophages were occasionally seen.
Histopathologic features of de novo CD5+DLBCL.
(A) Lymphoma cells spare a follicle retaining a lymphocyte cuff. (B) Lymphoma cells are large and show a centroblastic feature.
Histopathologic features of de novo CD5+DLBCL.
(A) Lymphoma cells spare a follicle retaining a lymphocyte cuff. (B) Lymphoma cells are large and show a centroblastic feature.
Summary of morphologic features of de novo CD5+DLBCL
| . | No. patients (%) . |
|---|---|
| Morphologic variant | |
| Centroblastic | 106 (97) |
| Immunoblastic | 3 (3) |
| Residual follicular pattern | |
| No residual follicles | 101 (93) |
| Sparing of follicles with a mantle cuff | 8 (7) |
| Naked germinal centers | 0 (0) |
| Intravascular or intrasinusoidal infiltration | 21 (19) |
| Focal necrosis | 5 (5) |
| Infiltration of macrophages | 2 (2) |
| . | No. patients (%) . |
|---|---|
| Morphologic variant | |
| Centroblastic | 106 (97) |
| Immunoblastic | 3 (3) |
| Residual follicular pattern | |
| No residual follicles | 101 (93) |
| Sparing of follicles with a mantle cuff | 8 (7) |
| Naked germinal centers | 0 (0) |
| Intravascular or intrasinusoidal infiltration | 21 (19) |
| Focal necrosis | 5 (5) |
| Infiltration of macrophages | 2 (2) |
Phenotypic features
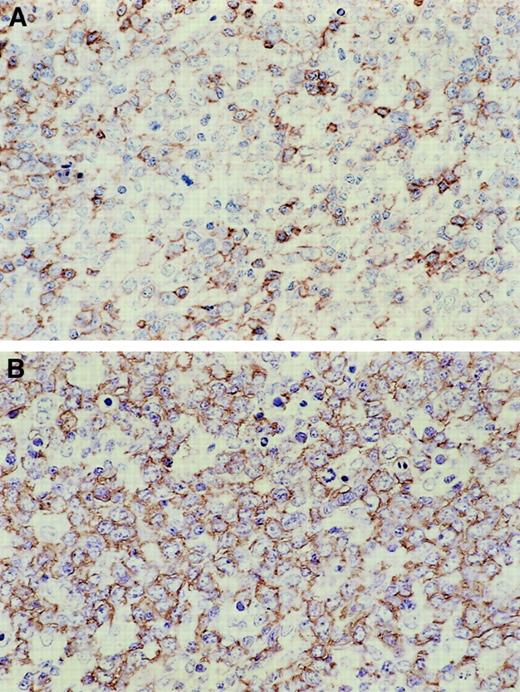
Immunophenotypic features are summarized in Table4. According to the definition adopted for this study, all patients tested positive for CD5 and B-cell markers (CD19 or CD20) (Figure 3) and negative for cyclin D1. Only 5 (5%) of the patients tested positive for CD10, and even fewer (3 [4%]) tested positive for CD23. This immunophenotype also accentuated the follicular dendritic cells on the paraffin sections and showed that few follicular dendritic cells were interspersed among the tumor cells. The immunoglobulin isotype was most commonly IgM (71 patients, 85%). Fifty-one of the patients examined were also positive for κ light chain and 25 for λ light chain. A comparison with CD5− DLBCL demonstrated that de novo CD5+ DLBCL was characterized by a CD5+CD10− CD19+CD20+CD21−CD23−phenotype and a predominance of surface IgMκ (Table 4).
Immunophenotypic features
| . | De novo CD5+ DLBCL4-150(%) . | CD5− DLBCL4-151 (%) . | P . |
|---|---|---|---|
| CD5 | 109/109‡ (100) | 0/384 (0) | |
| CD10 | 5/109 (5) | 46/360 (13) | .016 |
| CD19 | 90/92 (98) | 334/363 (92) | .031 |
| CD20 | 104/105 (99) | 370/376 (98) | .53 |
| CD21 | 8/35 (23) | 144/316 (46) | .010 |
| CD23 | 3/77 (4) | 13/78 (17) | .0082 |
| IgG | 8/50 (16) | 67/158 (42) | .0007 |
| IgA | 2/49 (4) | 16/159 (10) | .16 |
| IgM | 71/84 (85) | 200/358 (56) | < .0001 |
| IgD | 8/66 (12) | 65/320 (20) | .12 |
| κ chain | 51/81 (63) | 168/349 (48) | .016 |
| λ chain | 25/81 (31) | 100/350 (29) | .68 |
| . | De novo CD5+ DLBCL4-150(%) . | CD5− DLBCL4-151 (%) . | P . |
|---|---|---|---|
| CD5 | 109/109‡ (100) | 0/384 (0) | |
| CD10 | 5/109 (5) | 46/360 (13) | .016 |
| CD19 | 90/92 (98) | 334/363 (92) | .031 |
| CD20 | 104/105 (99) | 370/376 (98) | .53 |
| CD21 | 8/35 (23) | 144/316 (46) | .010 |
| CD23 | 3/77 (4) | 13/78 (17) | .0082 |
| IgG | 8/50 (16) | 67/158 (42) | .0007 |
| IgA | 2/49 (4) | 16/159 (10) | .16 |
| IgM | 71/84 (85) | 200/358 (56) | < .0001 |
| IgD | 8/66 (12) | 65/320 (20) | .12 |
| κ chain | 51/81 (63) | 168/349 (48) | .016 |
| λ chain | 25/81 (31) | 100/350 (29) | .68 |
n = 109.
n = 384.
Positive/examined patients.
Immunohistochemical features of de novo CD5+DLBCL.
Lymphoma cells are positive for CD5 (A) and CD20 (B).
Immunohistochemical features of de novo CD5+DLBCL.
Lymphoma cells are positive for CD5 (A) and CD20 (B).
Therapeutic response and prognosis
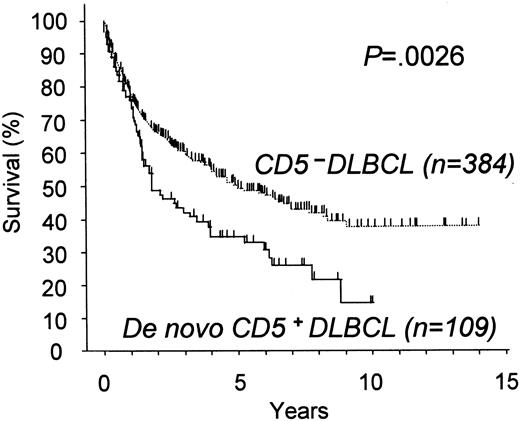
Treatment consisted of chemotherapeutic regimens containing anthracycline for 91 patients and without anthracycline for 4 patients. Eight patients with stage I disease did not undergo chemotherapy but were treated with radiotherapy or surgical resection alone. Finally, the 6 patients who had not received any therapy because of their poor PS died of the disease. In total, 63% (69 of 109) of the patients with de novo CD5+ DLBCL achieved complete remission with the initial therapy. De novo CD5+ DLBCL thus showed a survival curve significantly inferior to that for the CD5− (P = .0026, Figure4), with a 5-year survival rate of 34% for the former.
Overall survival for patients with de novo CD5+ DLBCL and with CD5− DLBCL.
De novo CD5+ DLBCL showed significantly worse survival than CD5− DLBCL.
Overall survival for patients with de novo CD5+ DLBCL and with CD5− DLBCL.
De novo CD5+ DLBCL showed significantly worse survival than CD5− DLBCL.
Univariate Cox analysis identified the following prognostic factors for the 493 patients with CD5+ and CD5− DLBCL: CD5 expression, age, PS, serum LDH level, clinical stage, extranodal involvement of more than one site, IPI category, and presence of B symptoms (Table5). Multivariate analysis, including IPI categories, showed age older than 60 years, PS greater than one, high LDH level, and advanced stage (III or IV), but not extranodal involvement of more than one site or CD5 positivity, to be significant and prognostic factors (Table 6). When multivariate analysis was performed for CD5 positivity and IPI categories, CD5 positivity was found to be an almost significant and independent prognostic factor (Table 6).
Prognostic factors affecting overall survival, univariate analysis
| Variables . | Unfavorable factor . | Univariate . | |
|---|---|---|---|
| Relative risk (95% CI) . | P . | ||
| CD5 status | Positive | 1.60 (1.20-2.13) | .0012 |
| Age | > 60 y | 2.00 (1.52-2.63) | .0000007 |
| Performance status | 2-4 | 3.54 (2.72-4.61) | < .0000001 |
| LDH | > Normal | 3.14 (2.41-4.10) | < .0000001 |
| Stage | III/IV | 2.90 (2.23-3.77) | < .0000001 |
| Extranodal disease | > 1 site | 2.58 (1.99-3.35) | < .0000001 |
| IPI | HI/H | 3.95 (3.06-5.10) | < .0000001 |
| B symptoms | Present | 2.98 (2.30-3.85) | < .0000001 |
| Variables . | Unfavorable factor . | Univariate . | |
|---|---|---|---|
| Relative risk (95% CI) . | P . | ||
| CD5 status | Positive | 1.60 (1.20-2.13) | .0012 |
| Age | > 60 y | 2.00 (1.52-2.63) | .0000007 |
| Performance status | 2-4 | 3.54 (2.72-4.61) | < .0000001 |
| LDH | > Normal | 3.14 (2.41-4.10) | < .0000001 |
| Stage | III/IV | 2.90 (2.23-3.77) | < .0000001 |
| Extranodal disease | > 1 site | 2.58 (1.99-3.35) | < .0000001 |
| IPI | HI/H | 3.95 (3.06-5.10) | < .0000001 |
| B symptoms | Present | 2.98 (2.30-3.85) | < .0000001 |
CI indicates confidence interval.
Prognostic factors affecting overall survival, multivariate analysis including IPI categories
| Variables . | Multivariate . | Multivariate (final model) . | ||
|---|---|---|---|---|
| Relative risk (95% CI) . | P . | Relative risk (95% CI) . | P . | |
| Comparison with risk factors | ||||
| CD5 status | 1.11 (0.83-1.49) | .49 | — | — |
| Age | 1.85 (1.40-2.45) | .000016 | 1.89 (1.43-2.50) | .0000082 |
| Performance status | 1.82 (1.34-2.46) | .00012 | 1.90 (1.40-2.57) | .000034 |
| LDH | 1.86 (1.38-2.53) | .000057 | 1.90 (1.41-2.57) | .000029 |
| Stage | 1.73 (1.24-2.39) | .0011 | 1.92 (1.42-2.58) | .000019 |
| Extranodal disease | 1.27 (0.94-1.72) | .12 | — | — |
| Comparison with IPI category | ||||
| CD5 status | — | — | 1.31 (0.98-1.77) | .068 |
| IPI | — | — | 3.84 (2.96-4.97) | < .0000001 |
| Variables . | Multivariate . | Multivariate (final model) . | ||
|---|---|---|---|---|
| Relative risk (95% CI) . | P . | Relative risk (95% CI) . | P . | |
| Comparison with risk factors | ||||
| CD5 status | 1.11 (0.83-1.49) | .49 | — | — |
| Age | 1.85 (1.40-2.45) | .000016 | 1.89 (1.43-2.50) | .0000082 |
| Performance status | 1.82 (1.34-2.46) | .00012 | 1.90 (1.40-2.57) | .000034 |
| LDH | 1.86 (1.38-2.53) | .000057 | 1.90 (1.41-2.57) | .000029 |
| Stage | 1.73 (1.24-2.39) | .0011 | 1.92 (1.42-2.58) | .000019 |
| Extranodal disease | 1.27 (0.94-1.72) | .12 | — | — |
| Comparison with IPI category | ||||
| CD5 status | — | — | 1.31 (0.98-1.77) | .068 |
| IPI | — | — | 3.84 (2.96-4.97) | < .0000001 |
CI indicates confidence interval.
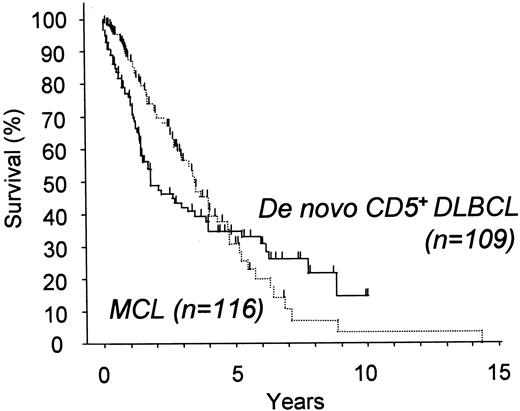
We also compared the survival of patients with de novo CD5+ DLBCL with that of patients with cyclin D1+ MCL (Figure 5). Although both groups had poor outcomes, several details of the survival curves were different. The curve for cyclin D1+ MCL gradually but steadily declined without any plateau, suggesting that MCL is a generally incurable disease. In contrast, de novo CD5+DLBCL initially followed a more aggressive clinical course than MCL, but the survival curve crosses that of MCL 5 years after diagnosis and shows superior survival thereafter.
Overall survival for patients with de novo CD5+ DLBCL and with cyclin D1+ MCL.
De novo CD5+ DLBCL showed an initially more aggressive clinical course than MCL, but the survival curve crossed that of MCL 5 years after diagnosis and showed superior survival thereafter.
Overall survival for patients with de novo CD5+ DLBCL and with cyclin D1+ MCL.
De novo CD5+ DLBCL showed an initially more aggressive clinical course than MCL, but the survival curve crossed that of MCL 5 years after diagnosis and showed superior survival thereafter.
Discussion
An analysis of 109 patients with de novo CD5+ DLBCL highlighted previously unrecognized features of this disease—high age at onset, female predominance, frequent association with poor prognostic components of IPI, and aggressive clinical course. These features were significantly different from those of CD5−DLBCL and MCL. Although it cannot be definitively concluded, on the basis of our results alone, whether CD5+ DLBCL constitutes a distinct subtype of B-cell lymphoma or represents merely a prognostic factor, de novo CD5+ DLBCL seems to constitute a unique subgroup of DLBCL.
The prognosis of de novo CD5+ DLBCL was significantly poorer than that of CD5− tumors. CD5 expression as a biologic marker appeared to be closely associated with the IPI index because 36% (40 of 109) of patients with de novo CD5+DLBCL were categorized in the high-risk IPI group. Comparison with the data reported by the Non-Hodgkin's Lymphoma Classification Project shows that this frequency is also higher than that for the other lymphomas in the high-risk IPI category—for example, 27% for peripheral T-cell lymphoma and 19% for DLBCL.2 These findings indicate that de novo CD5+ DLBCL is a highly aggressive subtype. However, a multivariate analysis of CD5 status and the IPI or components did not identify CD5 expression as an independent prognostic factor. Given that CD5 expression is closely correlated with each of the factors making up the IPI, CD5 expression in DLBCL may represent a biologic feature of aggressiveness also detectable by means of these clinical parameters. Interestingly, the other CD5+B-cell malignancies, CLL and MCL, represent a high incidence of bone marrow involvement and advanced disease, though the clinical behavior of the CLL patients is generally indolent. This finding suggests that CD5+ B cells have a tendency to spread widely within the tissues. Some authors have recently reported that the CD5 molecule on B cells interacts as a ligand with heavy-chain variable framework regions of surface immunoglobulins, which implies a possible role in the maintenance, selection, or expansion of normal, autoimmune, or transformed B cells.28 29 The molecular bases for these phenomena mediated by CD5 on B cells deserved to be clarified in the future.
De novo CD5+ DLBCL showed a female predominance, which has also been noted in 3 subtypes of malignant lymphoma, ie, follicular lymphoma, marginal zone B-cell lymphoma, and primary mediastinal large B-cell lymphoma; the other types of non-Hodgkin lymphoma usually tend to show male predominance.2 Indeed, in our series of DLBCL, the male-to-female ratio was significantly different for the CD5+ and CD5− groups, suggesting that female predominance can be regarded as one of the biologic features of de novo CD5+ DLBCL. This is consistent with the female predominance among autoimmune disorders, in which CD5+ B cells are shown to play, at least in part, an important pathogenetic role.3,7 8 In our study, however, only 4 patients showed evidence of immune function disorders, including rheumatoid factors, the significance of which deserves further clarification.
Morphologically, 8 patients with de novo CD5+ DLBCL showed an interfollicular growth pattern not seen in CD5−. Low-grade components were not identified in the lesions, nor were other morphologic findings suggestive of MCL, such as mantle zone patterns and naked germinal centers. Biopsy specimens of another 20 patients with de novo CD5+ DLBCL showed the localization of tumor cells within vessels or sinuses, which may be regarded as intravascular lymphoma (IVL).30 In addition, frequent involvement in bone marrow, liver, spleen, and lung is shared by both de novo CD5+ DLBCL and IVL. Recently, several patients with CD5+ IVL have been described.31 32 These clinical and morphologic similarities may indicate that the 2 types might overlap or lie in a continuing spectrum. Further investigation is needed to clarify this issue.
In conclusion, the current study sheds further light on the clinicopathologic features of de novo CD5+ DLBCL, which may constitute a unique subtype of DLBCL with an aggressive clinical course. Innovative therapeutic strategies must be established by a prospective study.
We thank the members of the research project study group supported by the Ministry of Health, Labour, and Welfare, which is aimed for delineation of molecular biological profile of the refractory lymphoid malignancy and the development of its tumor type–specific management. We also thank the members of the Adult Lymphoma Treatment Study Group. We thank collaborators who provided the patient data and specimens; the names of their institutions are listed in the .
Patient data and specimens were received from collaborators at the following institutions: Akita University School of Medicine, Akita Kumiai General Hospital, National Miyagi Hospital, Saka General Hospital, Tohoku University School of Medicine, Sendai City Hospital, Furukawa City Hospital, Fukushima Medical College, Iwaki General Hospital, Ohta Nishinouchi General Hospital, Takeda General Hospital, Tokyo Women's Medical University Daini Hospital, Matsudo Municipal Hospital, Higashi Matsudo Hospital, Niigata University, Toyama Prefectural Central Hospital, Kanazawa Medical University, Inazawa Municipal Hospital, Aichi Prefectural Hospital, Toyota Memorial Hospital, Fujita Health University School of Medicine, Okazaki Municipal Hospital, Kasugai Municipal Hospital, Japanese Red Cross Nagoya First Hospital, Nagoya Memorial Hospital, Aichi Cancer Center, Suzuka Chuo General Hospital, Suzuka Kaisei General Hospital, Mie University School of Medicine, Matsusaka Municipal Hospital, Matsusaka Chuo General Hospital, Matsusaka Saiseikai General Hospital, Yamada Red Cross Hospital, Ise City General Hospital, Kyoto University, Kyoto Prefectural University School of Medicine, Okayama University Medical School, Okayama Saiseikai General Hospital, Chugoku Central Hospital of the Mutual Aid Association of Public School Teachers, Okayama Red Cross General Hospital, Fukuoka University School of Medicine, Kyushu Cancer Center, and Kyusyu University.
Supported in part by a Grant-in-Aid for the 2nd-Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health, Labour, and Welfare, a Grant-in-Aid for Science on Primary Areas (Cancer Research), a Grant-in-Aid for the Encouragement of Young Scientists from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and a Grant-in-Aid from the Bristol-Myers Squibb Unrestricted Biomedical Research Grants Program.
M.S. and S.N. share senior authorship and should both be regarded as corresponding authors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Masao Seto, Division of Molecular Medicine, and Shigeo Nakamura, Department of Pathology and Molecular Diagnostics, Aichi Cancer Center, 1-1 Kanokoden, Chikusa-ku, Nagoya 464-8681; e-mail: mseto@aichi-cc.jp and snakamur@aichi-cc.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal