Abstract
Although the presence or absence of somatic mutations in the immunoglobulin variable region (IgVH) genes in chronic lymphocytic leukemia (B-CLL) identifies subtypes with very different prognoses, the assay is technically complex and unavailable to most laboratories. CD38 expression has been suggested as a surrogate marker for the 2 subtypes. IgVHmutations and CD38 expression in 145 patients with B-CLL with a long follow-up were compared. The 2 assays gave discordant results in 41 patients (28.3%). Multivariate analysis demonstrated that Binet stage,IgVH mutations and CD38 were independent prognostic indicators. Median survival time in patients whose cells had unmutated IgVH genes and expressed CD38 was 8 years; in those with mutated IgVHgenes not expressing CD38, it was 26 years. For those with discordant results, median survival time was 15 years. Thus, although CD38 expression does not identify the same 2 subsets as IgVHmutations in CLL, it is an independent risk factor that can be used with IgVH mutations and clinical stage to select patients with B-CLL with the worst prognoses. Using cryopreserved cells taken at intervals during the course of the disease, however, changes of CD38 expression over time were demonstrated in 10 of 41 patients. Causes of the variation of CD38 expression require further study. Additional prospective studies are required for comparing CD38 expression with other prognostic factors and for taking sequential measurements during the course of the disease.
Introduction
In 1999 our group1 and Damle et al2 documented and mutually corroborated the finding that the mutational status of immunoglobulin (Ig) heavy chain variable region (VH) genes identified 2 subsets of patients with chronic lymphocytic leukemia (CLL). One, with unmutatedIgVH genes, has a median survival of approximately 8 years, whereas the other, with mutatedIgVH genes, has a median survival of approximately 25 years. Others have since confirmed these findings.3,4 Damle et al2 suggested that the expression of surface CD38 on the tumor cells gave the same information and might be a surrogate assay. Initial analysis of 50 of our patients, however, failed to show a significant correlation betweenIgVH gene mutation status and CD38 expression.5
CD38 is a type 2 trans-membrane glycoprotein that acts as a complex ecto-enzyme with adenosine diphosphate–ribosyl cyclase and cyclic adenosine diphosphate–ribose hydrolase activities.6 In the B-cell compartment CD38 is not a lineage marker, but it is expressed at times during B-cell development when cell-to-cell interactions are crucial to development.7 Examples include an early bone marrow precursor cell, cells in the germinal center, and plasma cells.8 All the factors that signal its up-regulation are as yet unknown, but they include α- and γ-interferon.9
Standard management for stage A B-CLL is to delay therapy until there is evidence of progression.10 Meta-analysis of 6 trials involving 2048 patients showed no benefit of immediate treatment over delayed treatment with alkylating agents.11 Nevertheless, approximately half the patients allocated to the watch-and-wait option eventually require treatment, and one fourth die of a cause related to B-CLL.12 If those whose disease is destined to progress are identifiable at diagnosis, either by knowledge of the degree of CD38 expression or the status of IgVH mutations, it might be feasible to treat the disease more effectively with newer agents while the tumor burden is low.
We have detailed clinical information on more than 600 patients with B-CLL who have been treated and followed up by this department for the past 28 years. This long follow-up has enabled us to appreciate fully the natural history of B-CLL and has allowed us to take death, rather than simply time to progression, as the end-point for our studies. For the past 10 years we have been cryopreserving sequential blood samples from many of these patients. This has given us a rare opportunity to examine IgVH mutations and CD38 expression as prognostic indicators for B-CLL. Moreover, we have the opportunity to establish whether CD38 expression is a reproducible and stable marker in the disease.
Patients, materials, and methods
One hundred forty-five patients were chosen from more than 600 patients with B-CLL who have attended the hematology clinics of the Royal Bournemouth Hospital in the past 28 years. An attempt was made to include a representative selection of karyotypic disorders, but most patients were chosen simply because they happened to be attending the outpatient clinic for routine follow-up or because we had cryopreserved previous samples. Details of IgVH mutational status have been reported for 61 patients,1 and details ofIgVH mutational status and CD38 expression have been reported for 50.5 All patients came from the local area and included many whose diagnoses were made incidentally from a blood count ordered for another purpose and whose B-CLL has remained entirely asymptomatic. It has been our practice, nevertheless, to continue to observe such asymptomatic patients once or twice a year. They were staged at diagnosis according to the Binet classification.13 Immunophenotyping was performed afresh during the course of the current investigation. All patients scored 4 or 5 according to the Royal Marsden scoring system for CLL.14
Patients have been followed up for at least a year since diagnosis; those who died within that year have been included. The longest length of follow-up was 28 years 4 months. Median follow-up was 98 months. Patients were designated as having progressive disease on the basis of the following criteria: lymphocyte count doubling time of less than 1 year; progression to a more advanced Binet stage; development of systemic symptoms; development of Richter syndrome; downward trend of hemoglobin or platelet count because of bone marrow suppression to below the normal range (Hb less than 13.5 g/dL for males and less than 11.5 g/dL for females. platelet count less than 150 × 109/L) even when not meeting the criteria for stage C disease (Hb less than 10 g/dL, platelet count less than 100 × 109/L). The presence of one of these characteristics was sufficient to signify progressive disease. Patients without these features were designated as having stable disease.
Generally, patients with progressive disease have been treated conventionally with chlorambucil as first-line therapy and with fludarabine for resistant disease. Patients with stable disease were not offered chemotherapy. Because they had all been followed up locally, it was possible to assess whether patients died because of their leukemia or of an unrelated cause. Causes of death that were regarded as unrelated to CLL included myocardial infarction, stroke in the absence of thrombocytopenia, accident, and cancer. Two investigators (T.J.H. and D.G.O.) made this assessment independently, and they resolved any discrepancy by discussion and careful examination of the case notes.
Blood samples
Blood samples for testing have been taken at various times during the past 10 years. Some patients were tested immediately; for others, the lymphocytes were cryopreserved and tested later. We have previously demonstrated in 3 patients that theIgVH gene signature does not change during the clinical course of CLL,1 and we have subsequently confirmed this in another 6. It was thus deemed satisfactory to determine this at any stage of the disease. There is little information as to whether CD38 expression remains constant throughout the course of the disease or whether the process of freezing and thawing affects its expression. Because of this, several samples were tested before and after freezing and thawing, and for 41 patients, several samples that had been cryopreserved for up to 9 years were examined.
VHgene analysis
Immunoglobulin variable region genes were sequenced as previously described.1 The preferred source material was RNA. Complementary DNA was synthesized by reverse transcription using an oligo(dT) primer, and amplified by polymerase chain reaction using a mixture of oligonucleotide 5′ primers specific for each leader sequence of the VH1 to theVH6 families, together with mixed 3′ primers complementary to the germ line JH regions or 3′ primers complementary to the constant region. Clonal sequences were determined by sequencing amplicons from at least 2 independent polymerase chain reactions. Most samples were sequenced directly using an automated DNA sequencer (Applied Biosystems, Foster City, CA). Nucleotide sequences were aligned to EMBL/GenBank and current databases (V-BASE sequence directory,15 using MacVector 4.0 sequence analysis software; International Biotechnologies, New Haven, CT).
Analysis of CD38 expression
Whole blood or 106 cells from cryopreserved specimens were incubated for 15 minutes with 5μL each of the following antibodies: fluorescein isothiocyanate (FITC)–labeled anti-CD5 (clone DK23; DAKO, Glostrup, Denmark), phycoerythrin (PE)–labeled anti-CD38 (clone HB7; Becton Dickinson, San Jose, CA); and RPE-Cy5 labeled anti-CD19 (clone HD37; DAKO). Red cells were lysed with Facslyse (Becton Dickinson), and at least 10 000 cells were acquired in the Cellquest program on a FACSCalibur flow cytometer (Becton Dickinson). Each sample was run with an appropriate isotype control (FITC-labeled mouse IgG1 and PE-labeled mouse IgG1, both from DAKO), and this was used to define the negatively stained cells. In each case the dot-plot was gated on the lymphoid gate on the side scatter–forward scatter (SCC-FSC) plot. Within this gate the markers were set on the isotype control to define the negative population. A single cell was regarded as CD38+ if its position lay outside this marker. The tumor population was defined by gating of the lymphoid population on the SCC-FSC plot, followed by gating of the CD5+CD19+ population. The percentage of CD38+ cells in this gate was then determined.
To use CD38 positivity to screen for patients likely to die of B-CLL within 10 years, we chose a cut-off point for CD38 that give the highest possible Youden index.16 Cut-off points of 20% and 30% gave the highest, but similar, Youden values—58% and 60%, respectively. To allow comparison with Damle et al,2 we used 30%.
Statistical analysis
The significance of associations between characteristics was determined using the Fisher exact test. Survival curves from date of diagnosis and date of the sample used to determine CD38 expression were plotted using GraphPad Prism software (version 2; GraphPad Software, San Diego, CA). This program calculates survival fractions using the Kaplan-Meier method and compares survival curves using the log-rank test. Multivariate analysis to determine the interdependence of prognostic factors was carried out by Cox proportional hazards regression using SPSS for Windows, version 8. The type 1 error rate was set at 5%.
Youden index
We examined the validity of the 30% cut-off level for CD38 expression by calculating a Youden index on our own data. A larger group of 184 of our own patients, all of whom had CD38 estimations but 39 of whose IgVH genes had not been analyzed, was studied. Sensitivity and specificity were calculated for a number of different cut-off points. Sensitivity is the probability of having a CD38 level above the cut-off point among those dying, and specificity is the probability of having a CD38 level below the cut-off point among survivors. Specificity and sensitivity were calculated using 10-year survival rates (Kaplan-Meier method) separately for patients below and above the cut-off point, together with the percentage of patients above the cut-off point. The Youden index combines information on sensitivity and specificity (giving equal weight to each) to give an overall measure of the percentage gain in certainty of predicting death. If CD38 at a particular cut-off has a Youden index of 0, there is no predictive ability; if it has a score of 100, death is perfectly predicted.
Results
We studied 145 patients with B-cell CLL. There were 88 men and 57 women. Age at diagnosis ranged from 35 to 93 years. One hundred twenty-two patients had stage A, 12 had stage B, and 11 had stage C disease; 91 had stable disease and 54 had progressive disease. At the time of analysis 46 patients had died, 24 of causes related to B-CLL and 22 of unrelated causes.
Tumor cells with mutated IgVH genes were found in 95 (66%) patients, whereas 50 (34%) patients had cells with unmutated IgVH genes. The cells were CD38− in 84 (58%) patients and CD38+ in 61 (42%) patients. Numbers of patients in each category in each Binet stage at diagnosis is shown in Table 1.
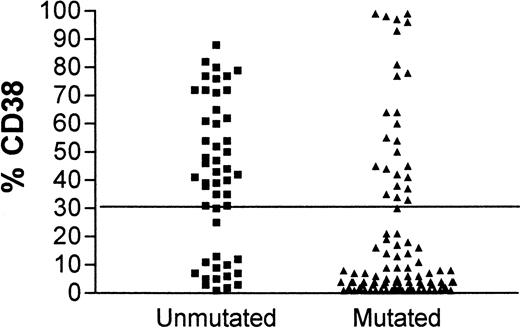
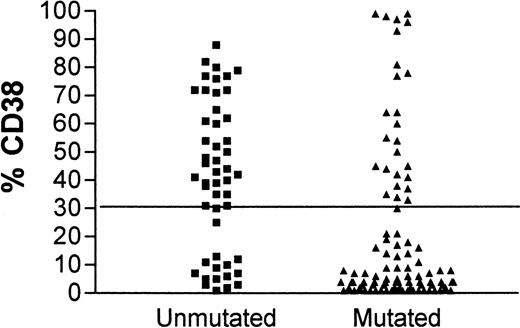
Although contingency tables show a highly significant association between CD38 expression and unmutated IgVH genes (Fisher exact test, P < .0001) and between CD38 expression and IgVH mutations and clinical stage (P = .025 and .0002, respectively), for individual patients there are important discordances. Figure1 shows the concordance for CD38 positivity and unmutated IgVH genes. In 15 patients with unmutated IgVH genes, less than 30% of cells expressed CD38, and in 26 patients with mutatedIgVH genes, more than 30% of cells expressed CD38. Forty-one of 145 (28.3%) patients had discordant results for the 2 assays. Discordant markers occurred as frequently in patients with stable disease as in those with progressive disease.
CD38 expression in leukemia.
Comparison of CD38 expression in leukemic cells of patients with B-CLL whose cells have mutated (▴) or unmutated (▪)IgVH genes.
CD38 expression in leukemia.
Comparison of CD38 expression in leukemic cells of patients with B-CLL whose cells have mutated (▴) or unmutated (▪)IgVH genes.
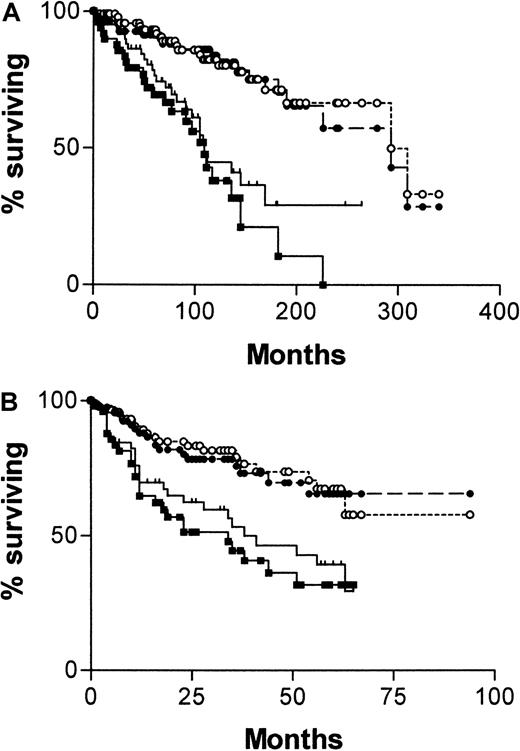
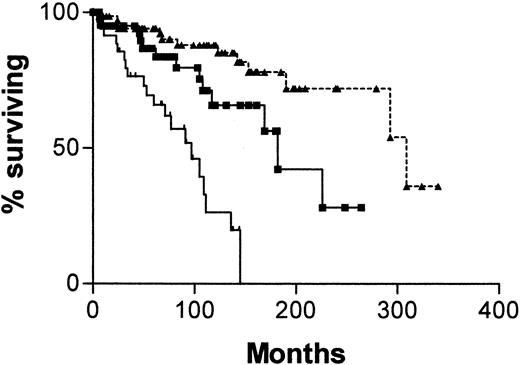
Figure 2A shows a Kaplan-Meier survival curve from diagnosis comparing patients with mutated and unmutatedIgVH genes and those with CD38+ and CD38− tumor cells. Both systems have strong predictive power for survival. For IgVH, median survival times were 109 months (95% confidence interval [CI], 90-128) for unmutated cases and 293 months (95% CI, 184-402) for mutated cases. The difference between the curves is significant (χ2 = 28.9; P < .0001). For CD38, median survival times are also 109 months (95% CI, 73-145) for positive cases and 293 months (95% CI, 158-428) for negative cases. Again the curves are significantly different (χ2 = 12.3; P = .0004). Figure 2B shows a Kaplan-Meier survival curve for the same groups, taking the date of the sample used to determine CD38 expression as point zero. Again, both systems have strong predictive power for survival, but because the analyses began well into the natural history of the disease, the length of survival is necessarily shorter. For IgVH, median survival times are 34 months for unmutated cases but are not reached for mutated cases. The difference between the curves is significant (χ2 = 15.64; P < .0001). For CD38, median survival times are 38 months for positive cases and are not reached for negative cases. Again, the curves are significantly different (χ2 = 8.31; P = .004).
Survival curves of 145 patients with B-CLL.
Comparisons were made of patients whose cells are CD38+(, n = 60) or CD− (●, n = 85) and have mutated (○, n = 95) or unmutated (▪, n = 50) IgVHgenes from date of diagnosis of CLL (A) or from date of CD38 estimation (B).
Survival curves of 145 patients with B-CLL.
Comparisons were made of patients whose cells are CD38+(, n = 60) or CD− (●, n = 85) and have mutated (○, n = 95) or unmutated (▪, n = 50) IgVHgenes from date of diagnosis of CLL (A) or from date of CD38 estimation (B).
Using Cox proportional hazards regression analysis, we compared IgVH status and CD38 positivity with other prognostic factors available to us—namely, Binet stage, typical or atypical morphology, progressive or stable disease (progressive disease included lymphocyte doubling time of less than 12 months as one of the criteria), and the 2 most common karyotypic abnormalities, trisomy 12 and abnormalities at 13q14. Only stage IgVH status and CD38 positivity had independent prognostic power (Table2). Probability values for the interactions between CD38 and IgVH gene status are P = .56 for all deaths and P = .63 for CLL related deaths.
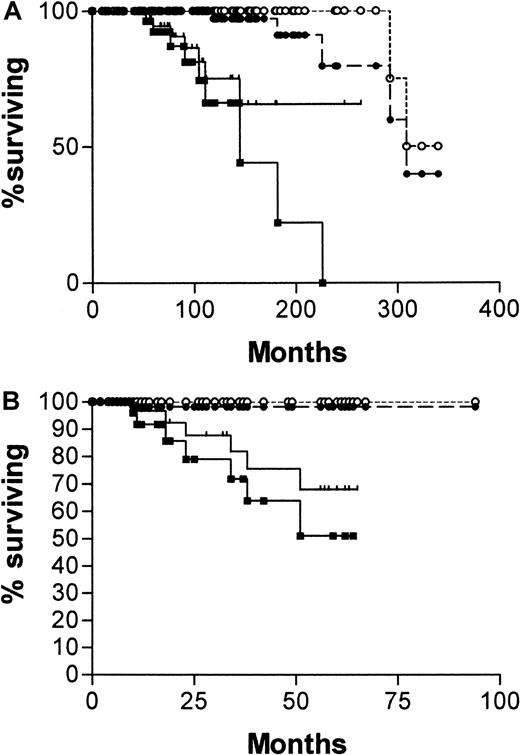
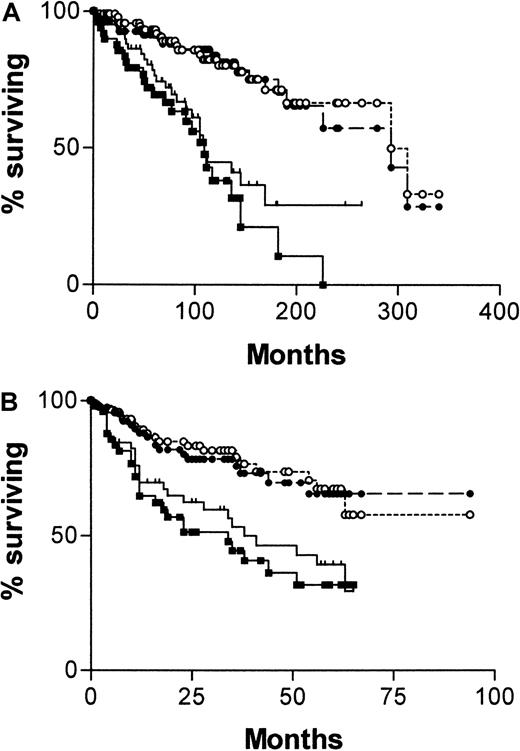
Diagnosis was made in the overwhelming proportion of patients (84%) in this series at stage A disease. What is required of a prognostic factor in stage A disease is the ability to predict those patients who will die early of CLL. Although CD38 expression and the absence of IgVH mutations were effective in this task, the IgVH assay was more reliable. Comparison of survival curves plotted from the date of diagnosis for patients with stage A disease, censoring for patients dying of causes unrelated to B-CLL (Figure3A) are as follows: median survival for CD38−, 309 months; CD38+, not yet reached; χ2 = 16.08, P < .0001. Median survival for mutated IgVH, 324 months; unmutated, 145 months; χ2 = 44.8, P < .0001. When the curves are plotted from the date of the CD38 estimation (Figure 3B), the discrimination is similar, though median survivals are not reached for any of the curves. Patients with unmutatedIgVH genes have significantly worse chances for survival than those with somatic mutations (χ2 = 21.4,P < .0001), and those whose cells express CD38 have significantly worse chance for survival than those whose cells do not (χ2 = 7.39, P = .0066).
Survival curves of stage A patients with B-CLL.
One hundred twenty-two patients from date of diagnosis (A) and 113 from date of CD38 estimation (B), censored for deaths unrelated to B-CLL. (A) CD38+(, n = 45); CD38− (●, n = 77); mutatedIgVH genes (○, n = 88); unmutatedIgVH genes (▪, n = 34). (B) CD38+(, n = 41); CD38− (●, n = 72); mutatedIgVH genes (○, n = 84); unmutatedIgVH genes (▪, n = 29). Numbers of patients analyzed by the 2 curves are different because 9 patients progressed beyond stage A between diagnosis and estimation of CD38.
Survival curves of stage A patients with B-CLL.
One hundred twenty-two patients from date of diagnosis (A) and 113 from date of CD38 estimation (B), censored for deaths unrelated to B-CLL. (A) CD38+(, n = 45); CD38− (●, n = 77); mutatedIgVH genes (○, n = 88); unmutatedIgVH genes (▪, n = 34). (B) CD38+(, n = 41); CD38− (●, n = 72); mutatedIgVH genes (○, n = 84); unmutatedIgVH genes (▪, n = 29). Numbers of patients analyzed by the 2 curves are different because 9 patients progressed beyond stage A between diagnosis and estimation of CD38.
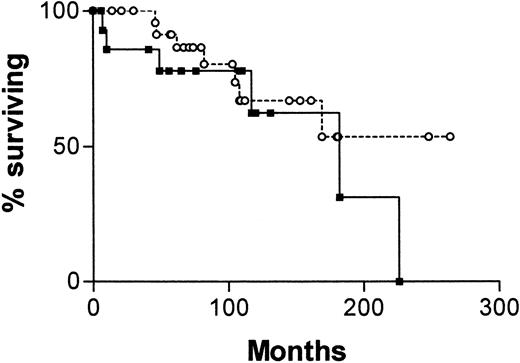
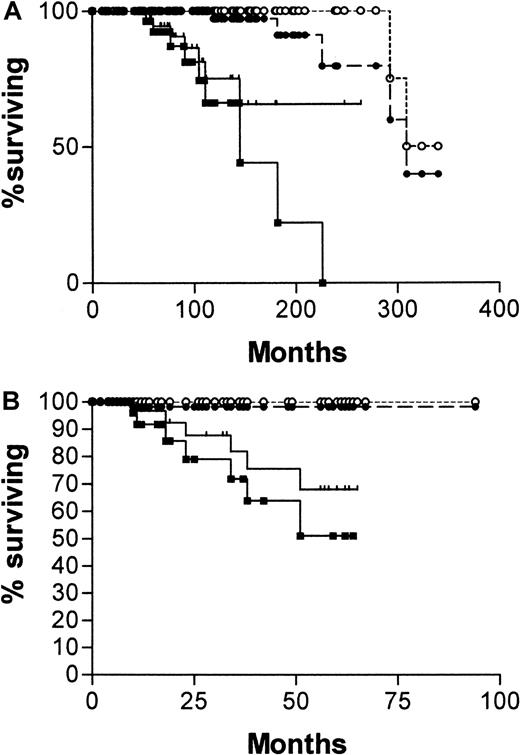
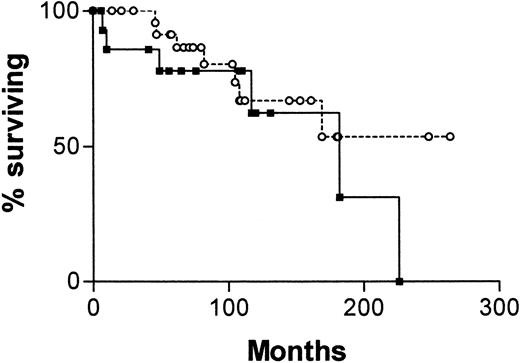
Figure 4 is a survival curve for all patients in whom IgVH mutations and CD38 status are discordant. There is no significant difference between the curve for CD38+/IgVH mutated patients and the curve for CD38−/IgVH unmutated patients. However, there were only 13 deaths among the 41 patients with discordant results. The median survival for the first group was not reached, and for the second group it was 182 months (P = .16).
Survival curves for 41 patients with B-CLL.
From date of diagnosis, comparing patients whose cells are CD38+ and have mutated IgVH genes (○, n = 25) with those whose cells are CD38− and have unmutated IgVH genes (▪, n = 16).
Survival curves for 41 patients with B-CLL.
From date of diagnosis, comparing patients whose cells are CD38+ and have mutated IgVH genes (○, n = 25) with those whose cells are CD38− and have unmutated IgVH genes (▪, n = 16).
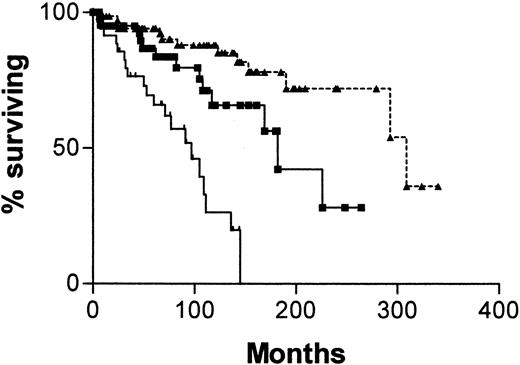
Given that the survival curves for patients with both types of discordant results are not significantly different, we decided to analyze the whole group in comparison with patients who were concordant for both assays. Figure 5 is a survival curve comparing patients whose cells were CD38+ with unmutated IgVH genes with those whose cells were CD38− with mutated V genes and with those who were discordant for the 2 assays. The median survival for patients whose cells were CD38+ with unmutatedIgVH genes was 97 months (95% CI, 65-129 months), for those whose cells were CD38− with mutatedIgVH genes it was 309 months (95% CI, 189-429 months), and for those with discordant results it was 182 months. Differences between these survival curves are significant (χ2 = 32.47; P < .0001).
Survival curves for 145 patients with B-CLL.
From date of diagnosis, comparing patients whose cells are CD38+ with unmutated IgVH genes (, n = 34) with those whose cells are CD38− with mutatedIgVH genes (▴, n = 70) and those whose cells gave discordant results for the 2 assays (▪, n = 41).
Survival curves for 145 patients with B-CLL.
From date of diagnosis, comparing patients whose cells are CD38+ with unmutated IgVH genes (, n = 34) with those whose cells are CD38− with mutatedIgVH genes (▴, n = 70) and those whose cells gave discordant results for the 2 assays (▪, n = 41).
Reproducibility of CD38 expression after cell storage
For 4 patients, cells were harvested from 9 separate tubes cryopreserved at the same time and containing separate aliquots taken from the same blood sample. They were labeled with antibodies against CD5, CD19, and CD38, as described earlier, and 6 replicates were counted from each tube. Reproducibility was very high. Mean percentages of CD38+ cells for the 4 patients were, respectively, 60.1 (SD, 1.007), 84.6 (SD, 1.475), 40.4 (SD, 0.979), and 10.7 (SD, 1.065).
To assess the effect of cryopreservation, 10 samples were tested for CD38 expression fresh and after freezing–thawing. Spearman correlation showed close agreement between the 2 measurements (r = 0.9515; P = .0001). Mean and SD for the fresh samples were 27.98 and 39.22, and they were 28.44 and 39.07 for the frozen samples.
CD38 expression during disease
In 6 patients, CD38 expression on samples taken on the same day from blood and bone marrow did not differ. For one, a lymph node sample taken on the same day gave the same result.
Sequential samples cryopreserved over periods ranging from 6 months to 9 years were harvested from 41 patients. Percentages of CD38+ cells were assayed and compared. Following Damle et al,2 differences smaller than 10% were ignored. For 8 patients there were increases in CD38 positivity over time; 2 showed decreases, and 31 were stable. Details of the 10 patients with changes in CD38 expression are given in Table 3. In 2 patients (patients 20 and 143), increasing CD38 expression was associated with increasing white blood cell count (37→145 and 8.3→17.8, respectively) independent of systemic symptoms, change in stage, change in cellular morphology, or treatment. In one patient (patient 12), the disease was clearly progressing and necessitated treatment. Individual measurements were taken at presentation, after treatment with chlorambucil and epirubicin, at first relapse after treatment, at second partial response after retreatment with chlorambucil, at second relapse, and at third partial response after treatment with fludarabine After each treatment, the residual cells expressed greater quantities of CD38; at the time of the final measurement, cell morphology became atypical. For 5 patients (patients 25, 29, 37, 144, and 185), CD38 expression also increased after courses of treatment with either chlorambucil or fludarabine, and it was consistent with the treatment selectively eliminating CD38− cells. For one of these patients (patient 25), a 9-month period off all treatment was associated with recovery of the lymphocyte cell count, with most of the recovering cells CD38−.
Of the 2 remaining patients, patient 157 had stable CLL but became increasingly hypogammaglobulinemic. The increased expression of CD38 was associated with a series of chest infections. Patient 125 had concurrent chronic myelomonocytic leukemia. As the monocyte count rose, CD38 expression by lymphocytes decreased.
Discussion
Although there are a number of valuable prognostic factors for B-CLL, clinical stage, according to the Binet13 or the Rai17 scoring system, is the one most commonly used. The common belief, supported by a meta-analysis,11 is that advanced-stage B-CLL requires treatment but that early-stage disease is best left until progression occurs. However, this analysis applies to treatment with alkylating agents and may not hold good for newer agents such as fludarabine. Moreover, approximately half the early-stage cases eventually progress and require treatment.11
French18 and Spanish19 CLL study groups identified good risk factors—normal hemoglobin count, low lymphocyte count, nondiffuse bone marrow infiltration, and lymphocyte doubling time of more than 1 year—which identified a subgroup of patients with smoldering CLL within Binet stage A or Rai stage 0 or 1 disease. However, in 10% of these patients, the disease still progresses and requires treatment.
IgVH gene analysis distinguishes between subtypes with different prognoses that arise from cells at different stages of maturation. We have previously shown that the presence of unmutated IgVH genes correlates with progressive disease, advanced stage, atypical morphology, and trisomy 12.1 Expression of high levels of CD38 is also an adverse prognostic factor. It has been shown to correlate with advanced stage, atypical morphology, higher lymphocyte count, and diffuse bone marrow infiltration.20 In this study we have confirmed the observation of Damle et al2 that there is a statistically significant correlation between CD38 expression and the presence of unmutated IgVH genes. However, though there is some overlap, the 2 tests do not identify the same patients. In this study CD38 expression and IgVH mutations give discordant results in 28.3% of patients; by multivariate analysis, they were independent prognostic indicators.
Our results differ from those of Damle et al,2 but the reason for this is not obvious. Our earlier study5 used anti-CD5–anti-CD20 mAbs, which was criticized because some B-CLL cells express low levels of CD20. In this study we have eliminated this source of discrepancy by using the same anti-CD5–anti-CD19–anti-CD38 antibodies and the same fluorochromes as Damle et al.2 It is possible that the larger number of patients in this study has better revealed the discordance between CD38 expression and IgVH mutational status.
Perhaps of more importance, CD38 expression did not remain constant throughout the course of the disease. Several patterns of CD38 positivity may be seen. Although in some patients all the cells are CD38+ or CD38p−, in many patients a mixture of CD38+ and CD− cells is seen. The composition of this mixture changed in 24.4% of patients examined. In most patients, changes in CD38 expression were associated with treatment of CLL, with a suggestion that chemotherapy selectively kills CD38− cells. In one patient, the recovering cells after several rounds of chemotherapy were strongly CD38+, and the morphology of the cells became atypical. In one patient, stopping all therapy was associated with a recovery of CD38− cells. However, we also saw increases in the proportion of CD38+cells in patients with progressive disease without treatment.
A decrease in CD38 expression in a single patient responding to treatment with chlorambucil has been reported,21 though the same group found no change in CD38 expression in another 16 patients.2 Recently, an additional study has shown an increase in the proportion of CD38+ cells in 1 of 10 patients investigated.22 This patient had clearly progressive disease but received no treatment.
In 2 of our patients, changes in the proportion of cells expressing CD38 were not associated with progression. One had stable disease but contracted recurrent chest infections; the other had concurrent chronic myelomonocytic leukemia. As the monocyte count increased, the expression of CD38 by the lymphocytes decreased.
CD38 is involved in interactions between cells of the immune system.7 Expression of CD38 on B-CLL cells is up-regulated by exposure to α-, β-, or γ-interferon in vitro.9 It will be important to investigate whether exposure to other cytokines also alters expression. It might be expected that the expression of CD38 on B-CLL cells could vary, not only with disease progression but also with the presence of intercurrent illness. Several groups have disputed whether CD38 expression is or is not a useful prognostic marker.23-29 Its variability might explain why different groups have gotten different results.
The variability of CD38 expression calls into question its value as a prognostic factor at diagnosis. Given this, we have plotted survival curves from the date of diagnosis and from the date of the CD38 sample. Although the latter gave shorter median survival times in some patients because they had already survived for a long time before the test was performed, both sets of curves demonstrate thatIgVH mutation and CD38 expression are excellent prognostic indicators in CLL.
In this series, the prognostic information given by CD38 expression and the presence of IgVH mutations is complementary. If both adverse factors are present, a short survival may be predicted even in stage A patients. Such patients then become candidates for controlled trials examining the value of early intensive treatment. If neither adverse factor is present, a median survival of 25 years may be predicted. For most patients with B-CLL such a prediction implies that no treatment will ever be required. Patients who have discordant results with these assays have a median survival of 15 years.
There is still a need to evaluate other prognostic indicators in the context of CD38 expression and IgVH mutations. In particular serum levels of soluble CD23,30,31 soluble CD44,32 serum thymidine kinase,33,34 and β2-microglobulin33,35 should be studied. Unfortunately, we do not have stored sera to carry out this investigation. A recent study suggested that the prognostic effect of CD38 expression masked that of high levels of β2-microglobulin.22
The prognostic effect of the 2 most common abnormalities, trisomy 12 and abnormalities at chromosome 13q14, are encompassed within the effect of IgVH mutations.36However, more information on the effect of 11q23 and p53abnormalities in relation to CD38 and IgVH is required.4 35 Clearly, further studies are also required to confirm the lability of CD38 expression in B-CLL.
Prognostic factors are important in CLL. A sizable proportion of patients are managed by watchful waiting because meta-analysis has shown that stage A patients do not benefit from early treatment with chlorambucil.11 Nevertheless, roughly half of such patients will eventually require treatment and might be better served by early treatment with one of the newer agents, such as fludarabine. Some prognostic factors, such as stage or marrow histology, depend on progression already having taken place for their value. At present onlyIgVH mutations have been shown to be intrinsic to the nature of the disease and not to change during its course. Unfortunately, their detection is technically difficult and available in only a few laboratories. Other prognostic variables, including CD38 expression, must be evaluated prospectively.
We thank Dr Bob Chapman for help with the statistical data and Stuart Lanham and Gavin Babbage for technical help.
Supported by grants from Tenovus, the Leukaemia Research Fund, and the Bournemouth Leukaemia Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Terry Hamblin, Department of Haematology, Royal Bournemouth Hospital, Castle Lane East, Bournemouth, BH7 7DW, United Kingdom; e-mail: terjoha@aol.com.