Platelet adhesion and aggregation at sites of vascular injury are critically dependent on the interaction between von Willebrand factor (VWF) and 2 major platelet adhesion receptors, glycoprotein (GP) Ib/V/IX and integrin αIIbβ3. GP Ib/V/IX binding to VWF mediates platelet tethering and translocation, whereas activation of integrin αIIbβ3 promotes cell arrest. To date, the signaling pathways used by the VWF-GP Ib/V/IX interaction to promote activation of integrin αIIbβ3, particularly under shear, have remained poorly defined. In this study, the potential involvement of type 1 phosphoinositide (PI) 3–kinases in this process was investigated. Results show that platelet adhesion and spreading on immobilized VWF results in a specific increase in the PI 3–kinase lipid product, PtdIns(3,4)P2. Under static conditions, inhibiting PI 3–kinase with LY294002 or wortmannin did not prevent platelet adhesion, integrin αIIbβ3activation, or platelet spreading although it significantly delayed the onset of these events. In contrast, PI 3–kinase inhibition under shear dramatically reduced both platelet adhesion and spreading. Real-time analysis of intracellular calcium demonstrated that under static conditions inhibiting PI 3–kinase delayed the onset of intracellular fluxes in adherent platelets, but did not affect the final magnitude of the calcium response. However, under shear, inhibiting PI 3–kinase dramatically reduced intracellular calcium mobilization and integrin αIIbβ3 activation, resulting in impaired thrombus growth. The studies demonstrate a shear-dependent role for PI 3–kinase in promoting platelet adhesion on immobilized VWF. Under static conditions, platelets appear to mobilize intracellular calcium through both PI 3–kinase–dependent and –independent mechanisms, whereas under shear PI 3–kinase is indispensable for VWF-induced calcium release.
Introduction
Platelet adhesion and aggregation at sites of blood vessel injury are essential for the arrest of bleeding and for the maintenance of vascular integrity. Under conditions of rapid blood flow, the formation of the primary hemostatic plug requires the synergistic contribution of multiple platelet receptor-ligand interactions,1,2 foremost of which involves the sequential binding of von Willebrand factor (VWF) to platelet adhesion receptors, glycoprotein (GP) Ib/V/IX and integrin αIIbβ3. The VWF-GP Ib/V/IX interaction is the first step in the hemostatic process that tethers platelets to the site of vascular injury. A characteristic feature of this adhesive interaction is its rapid reversibility such that platelets translocate (roll) on immobilized VWF. During surface translocation, GP Ib/V/IX transduces signals (outside-in signaling) to induce platelet activation, converting integrin αIIbβ3 from a low- to a high-affinity state (inside-out signaling) capable of engaging the C1 domain of VWF.3-5 Recent in vivo studies in mice lacking VWF or fibrinogen or both have demonstrated the central role for VWF in promoting platelet-vessel wall and platelet-platelet adhesive interactions during thrombus growth.6 However, despite its fundamental importance, the mechanisms linking the VWF-GP Ib/V/IX interaction to activation of integrin αIIbβ3, particularly under shear conditions, remain poorly defined.
An important signaling pathway operating in all mammalian cells involves the activation of one or more members of the phosphoinositide (PI) 3–kinase family. These enzymes phosphorylate membrane inositol phospholipids at the 3-OH position and have been classified into 3 distinct classes based on their primary structure and in vitro lipid substrate specificity.7 8 It is likely that all mammalian cells express representatives of each of the 3 types of PI 3–kinases; however, only the type 1 PI 3–kinases are capable of phosphorylating all 3 conventional lipids—PtdIns, PtdIns(4)P, PtdIns(4,5)P2—in vitro, generating PtdIns(3)P, PtdIns(3,4)P2 and PtdIns(3,4,5)P3, respectively. PtdIns(3,4)P2 and PtdIns(3,4,5)P3are considered the primary output signals of type 1 PI 3–kinases in vivo, and have been implicated in regulating a diverse range of cellular processes, including cell growth, prevention of apoptosis, glucose transport, and cytoskeletal reorganization.
A great deal of information on the function of type 1 PI 3–kinases in cells has been based on the use of the structurally distinct pharmacologic inhibitors, LY294002 and wortmannin. These inhibitors have been used to study the role of type 1 PI 3–kinases in platelets and have demonstrated a potentially important role for these enzymes in regulating the adhesive and signaling function of integrin αIIbβ3.9 For example, evidence from the study of Glanzmann thrombasthenic platelets (congenitally deficient in integrin αIIbβ3) indicates that the generation of the PI 3–kinase lipid product, PtdIns(3,4)P2, is primarily dependent on signaling downstream of integrin αIIbβ3.10 Furthermore, several integrin αIIbβ3–dependent functional responses, including platelet spreading11 and irreversible platelet aggregation,12 are dependent on PI 3–kinases. Although these studies have defined an important role for type 1 PI 3–kinases in integrin αIIbβ3 signaling (inside-out signaling) there is less convincing evidence for an absolute requirement for PI 3–kinases in inducing activation of integrin αIIbβ3 activation (outside-in signaling) by the majority of physiologic platelet stimuli studied to date.13
A potential role for type 1 PI 3–kinases in GP Ib/V/IX signaling has been suggested by the observation that VWF binding to GP Ib/V/IX can induce the cytoskeletal association and activation of p85/p110 form of type 1 PI 3–kinase.14 More recently, VWF stimulation of platelets was shown to initiate complex formation between the p85 subunit of PI 3–kinase and GP Ib/V/IX.15 In this report we have investigated the potential involvement of type 1 PI 3–kinases in shear-dependent signaling between GP Ib/V/IX and integrin αIIbβ3.
Materials and methods
Materials
LY294002, EGTA-am, and adenosine 5′-O-(1-Thio-triphosphate) (ATPαS) were from Calbiochem (La Jolla, CA). Adenosine 3′-phosphate 5′-phosphosulfate (A3P5PS) was purchased from Sigma (St Louis, MO). Wortmannin was purchased from Sapphire Bioscience (Crow's Nest, New South Wales, Australia). Oregon Green 488 BAPTA-1, am and Fura Red, am were from Molecular Probes (Eugene, OR). DiOC6 was obtained from Sigma. Apyrase was purified from potatoes according to the method of Molnar and Lorand.16 Human VWF was purified to homogeneity from plasma cryoprecipitate according to the method of Montgomery and Zimmerman.17 AR-C69931MX was generously supplied by Astrazeneca R & D Charnwood (Leicestershire, England). All other reagents were obtained from sources described previously.14,18 19
Antibodies
PAC-1 monoclonal antibody was from Becton Dickinson (Victoria, Australia) and fluorescein isothiocyanate (FITC)–conjugated antimouse IgM (α-IgM) antibody was from Southern Biotechnology Associates (Birmingham, AL).
In vitro static and flow studies
For washed platelet and platelet reconstitution studies, blood was collected from healthy volunteers who had not received any antiplatelet medication in the preceding 2 weeks. Platelets were isolated and washed as described previously18 and resuspended in modified Tyrode buffer (10 mM Hepes, 12 mM NaHCO3, pH 7.4, 137 mM NaCl, 2.7 mM KCl, 5 mM glucose), containing 1 mM CaCl2 or 1 mM MgCl2 or both where indicated. Autologous red blood cells were obtained by an initial centrifugation of anticoagulated whole blood at 200g for 30 minutes. The platelet-rich plasma was removed and red blood cells were washed 3 times with washing buffer (10 mM Hepes, pH 7.4, 140 mM NaCl, 5 mM glucose). Plasma was obtained from centrifugation of anticoagulated blood (15 mM trisodium citrate, pH 7.4) at 2000g for 10 minutes. Washed platelets were pretreated with vehicle alone (dimethyl sulfoxide [DMSO]), LY294002 (0-25 μM), or wortmannin (0-100 nM) for 15 minutes, or alternatively pretreated with apyrase (up to 16.5 U/mL) or the adenosine diphosphate (ADP) receptor antagonists, AR-C69931MX (100 nM) and A3P5PS (200 μM), and ATPαS (50-100 μM) for 30 minutes before static and flow-based assays were performed (150 s-1 or 1800 s-1) according to a modified method of Yuan et al20 and Cooke et al,21respectively. In control studies, we demonstrated that the concentrations of apyrase, ATPαS, AR-C69931MX, and A3P5PS used in our experimental assays abolished platelet aggregation or platelet shape change in washed platelets induced by 10 μM exogenous ADP. In some studies, washed platelets were reconstituted with either red blood cells alone (50% [vol/vol] autologous packed red blood cells), in the presence of 0.4 U/mL apyrase (ADPase activity), or red blood cells and plasma prior to perfusion through VWF-coated microcapillary tubes. Analysis of platelet adhesion and surface area was performed as described by Yap et al22 and expressed as a percent of control. For whole blood studies, anticoagulated blood (15 mM trisodium citrate, pH 7.4) was pretreated with vehicle alone (DMSO), LY294002 (0-200 μM), apyrase (8.25 U/mL), ATPαS (100 μM), or AR-C69931MX (100 nM) and A3P5PS (200 μM) for 10 minutes before perfusion through VWF-coated microcapillary tubes at 1800 s-1. In control studies, we confirmed that the concentrations of apyrase, ATPαS, AR-C69931MX, and A3P5PS used in our experimental assays prevented platelet aggregation in whole blood induced by 10 μM exogenous ADP using a sensitive single-platelet detection assay.
Platelet imaging studies
In static adhesion assays, washed platelets were allowed to adhere to VWF-coated coverslips in the presence of PAC-1 antibody (1 μg/mL). In flow adhesion assays, washed platelets reconstituted with red blood cells were perfused through microcapillary tubes for 5 minutes prior to the perfusion of PAC-1 (1 μg/mL) over adherent platelets for 15 minutes. Adherent platelets were fixed, incubated with a FITC-conjugated anti-IgM antibody, and visualized using confocal microscopy (× 63W; Leica TCS SP; Leica, Heidelberg, Germany). For the imaging of platelet thrombi, adherent platelets were fixed and stained with DiOC6 (1 μM). Thrombi were imaged using fluorescent confocal microscopy and reconstructed in Voxblast (Vaytek, Fairfield, IA).
Quantitation of platelet thrombi
Following formation of thrombi, red cells were lysed by perfusing 1% ammonium oxalate through the microcapillary tubes. Adherent platelets were then lysed with 0.1% Triton lysis buffer and lactate dehydrogenase (LDH) levels determined using the COBAS MIRA S Chemistry System (Roche, Somerville, NJ). This quantitative method was validated independently by examining thrombus dimensions by confocal imaging, according to the method of Kulkarni et al.23
Analysis of 32P-labeled phospholipids
Platelets were labeled with inorganic 32P (11.1 MBq [0.3 mCi/mL]) for 2 hours at 37°C and the level of phospholipids determined as described by Carter et al.24Platelets in suspension (resting) or adherent to VWF were lysed with radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris HCl, pH 7.2, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 158 mM NaCl), and phospholipids extracted with a chloroform/methanol-based mixture (100 μL chloroform/methanol, 1:2, 75 μL 3.1 M HCl, 100 μL chloroform). Following deacylation with methylamine/butanol/methanol (42:9:47) phospholipids were identified by SAX high-performance liquid chromatography (HPLC) analysis using3H-labeled commercial standards.
Preparation of scanning electron microscopy samples
Adherent platelets were fixed with 2% glutaraldehyde in 100 mM Na2HPO4/NaH2PO4, pH 7.4, for 60 minutes then incubated with 1% OsO4 in 100 mM Na2HPO4/NaH2PO4, pH 7.4, for 30 minutes. The fixed platelets were dehydrated by successive immersions in increasing concentrations of ethanol followed by critical point drying. The coverslips or microcapillary tubes, which had been dissected laterally, were mounted on scanning electron microscopy stubs and coated with gold prior to imaging using an Hitachi S570 scanning electron microscope.
Ratiometric calcium measurements
Analysis of intracellular calcium fluxes was performed according to the method of Yap et al.22 Briefly, platelets were loaded with calcium indicator dyes, Oregon Green 488 BAPTA-1,am (1 μM), and Fura Red, am (1.25 μM) for 30 minutes at 37°C, then washed and resuspended in Tyrode buffer containing either extracellular calcium (1 mM) or EGTA/Mg++(1 mM each), prior to incubation with PI 3–kinase inhibitors. Changes in the concentration of cytosolic calcium were derived from the ratio of signal intensity in the Oregon Green and Fura Red channels using confocal microscopy. The calcium dynamics in individual platelets were monitored every 0.586 seconds over a 73.25-second or 37.5-second time interval for static or flow experiments, respectively, and recorded for off-line single cell analysis as described previously.22The number of platelets demonstrating oscillatory calcium transients over the time period examined was determined. Platelet translocation was determined based on the displacement of a tethered platelet from its original point over a 37.5-second period.
Statistical analysis
Significant differences were detected using Student ttest and one-way ANOVA, using the Prism software package (Graphpad Software for Science, San Diego, CA).
Results
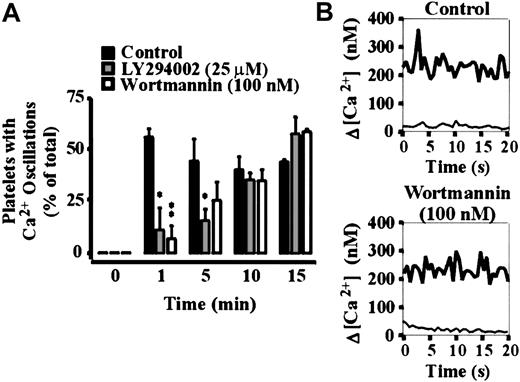
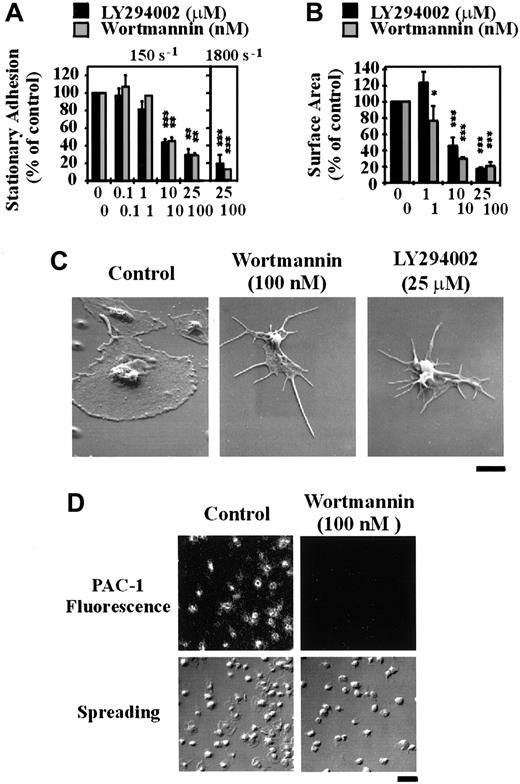
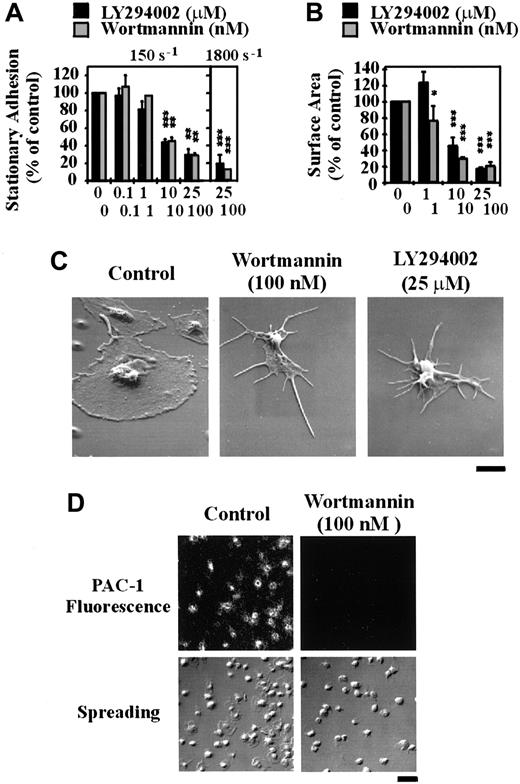
To investigate the potential involvement of type 1 PI 3–kinases in regulating platelet adhesion, integrin αIIbβ3 activation and spreading on immobilized VWF, washed platelets were pretreated with the well-characterized pharmacologic PI 3–kinase inhibitors, LY294002 or wortmannin. As demonstrated in Figure 1A, wortmannin or LY294002 exhibited a modest dose-dependent inhibitory effect on platelet adhesion under static conditions, with a maximal inhibition of 22% ± 3.45% (P < .01) using LY294002 (25 μM) and 30% ± 4.81% (P < .01) with wortmannin (100 nM). These inhibitors did not, however, inhibit integrin αIIbβ3 activation, as assessed by binding of the activation-specific monoclonal antibody, PAC-1 (Figure 1B). In control studies, we demonstrated that integrin αIIbβ3 activation under these experimental conditions was not dependent on released ADP, because pretreating platelets with apyrase (16.5 U/mL; Figure 1B) or with the ADP receptor antagonists, AR-C69931MX (α-P2Y12) and A3P5PS (α-P2Y1) or ATPαS (α-P2Y12 and α-P2Y1) (data not shown) did not prevent PAC-1 binding. Examination of platelet morphology demonstrated that pretreatment of platelets with wortmannin (0-100 nM) or LY294002 (0-25 μM) resulted in a minor, yet significant (P < .05), effect on the ability of cells to spread (∼11% reduction in platelet surface area in LY294002- and wortmannin-treated platelets [Figure2A]). High-resolution imaging of spread platelets demonstrated that a significant proportion (> 50%) of LY294002- and wortmannin-treated cells exhibited irregular cell margins and had not fully spread (Figure 2B). Real-time analysis of platelet spreading demonstrated an important role for PI 3–kinase in regulating the rate of platelet spreading. For example, control platelets adhering to VWF extended dynamic filopodial projections that extended and retracted from the cell surface. The formation of these projections was closely followed by the extension of lamellipodial sheets, resulting in full platelet spreading over 10 to 15 minutes (Figure 2C). Pretreating platelets with LY294002 (25 μM) or wortmannin (100 nM) slightly delayed the onset of filopodial formation (typically by < 3 minutes; Figure 2C). In contrast to cells pretreated with vehicle, there was a consistent delay in the subsequent formation of lamellipodial sheets (10-15 minutes), such that platelet spreading typically occurred over 20 to 30 minutes.
The role of PI 3–kinase in regulating platelet adhesion and integrin αIIbβ3 activation under static conditions and the effect of apyrase on VWF-induced integrin αIIbβ3 activation.
Washed platelets (1.5 × 108/mL) were incubated with vehicle alone (control) or the indicated concentrations of LY294002 (0-25 μM) or wortmannin (0-100 nM) for 15 minutes or with apyrase (16 U/mL) for 30 minutes. Platelets were allowed to adhere and spread on immobilized human VWF (10 μg/mL) for 60 minutes under static conditions. In panel B, pretreated platelets were incubated with PAC-1 antibody, prior to adhesion and spreading. These results demonstrate (A) the effect of LY294002 or wortmannin on the level of platelet adhesion, and (B) the effect of LY294002, wortmannin, or apyrase on PAC-1 binding as visualized by confocal microscopy (× 63 W objective; bar = 10 μm). Statistical analysis of the results was performed using a t test and the P values are indicated where appropriate (P < .01**). Results are the mean ± SEM of 3 experiments, and images are from a single experiment representative of 3.
The role of PI 3–kinase in regulating platelet adhesion and integrin αIIbβ3 activation under static conditions and the effect of apyrase on VWF-induced integrin αIIbβ3 activation.
Washed platelets (1.5 × 108/mL) were incubated with vehicle alone (control) or the indicated concentrations of LY294002 (0-25 μM) or wortmannin (0-100 nM) for 15 minutes or with apyrase (16 U/mL) for 30 minutes. Platelets were allowed to adhere and spread on immobilized human VWF (10 μg/mL) for 60 minutes under static conditions. In panel B, pretreated platelets were incubated with PAC-1 antibody, prior to adhesion and spreading. These results demonstrate (A) the effect of LY294002 or wortmannin on the level of platelet adhesion, and (B) the effect of LY294002, wortmannin, or apyrase on PAC-1 binding as visualized by confocal microscopy (× 63 W objective; bar = 10 μm). Statistical analysis of the results was performed using a t test and the P values are indicated where appropriate (P < .01**). Results are the mean ± SEM of 3 experiments, and images are from a single experiment representative of 3.
The role of PI 3–kinase in regulating platelet spreading under static conditions.
Washed platelets (1.5 × 108/mL for panels A and B or 1.5 × 107/mL for panel C) were incubated with vehicle alone (control) or the indicated concentrations of LY294002 (0-25 μM) or wortmannin (0-100 nM) for 15 minutes. These results demonstrate (A) the mean surface area of adherent platelets, and (B) the morphology of spread platelets as determined by scanning electron microscopy (bar = 2 μm). Statistical analysis of the results was performed using a t test and the P values are indicated where appropriate (P < .05*). Results are the mean ± SEM of 3 experiments, and images are from a single experiment representative of 3. In panel C, platelet spreading was visualized in real time by differential interference contrast microscopy and recorded on video for off-line analysis (× 100 oil objective; bar = 5 μm). The images presented are typical of cells from a single experiment and are representative of 3 independent experiments.
The role of PI 3–kinase in regulating platelet spreading under static conditions.
Washed platelets (1.5 × 108/mL for panels A and B or 1.5 × 107/mL for panel C) were incubated with vehicle alone (control) or the indicated concentrations of LY294002 (0-25 μM) or wortmannin (0-100 nM) for 15 minutes. These results demonstrate (A) the mean surface area of adherent platelets, and (B) the morphology of spread platelets as determined by scanning electron microscopy (bar = 2 μm). Statistical analysis of the results was performed using a t test and the P values are indicated where appropriate (P < .05*). Results are the mean ± SEM of 3 experiments, and images are from a single experiment representative of 3. In panel C, platelet spreading was visualized in real time by differential interference contrast microscopy and recorded on video for off-line analysis (× 100 oil objective; bar = 5 μm). The images presented are typical of cells from a single experiment and are representative of 3 independent experiments.
To investigate whether the inability of PI 3–kinase inhibitors to prevent integrin αIIbβ3 activation and platelet spreading was due to incomplete inhibition of PI 3–kinase, we examined the effects of these inhibitors on the generation of PI 3–kinase lipid products. As demonstrated in Figure3A, PtdIns(3,4)P2 was undetectable in resting platelets. However, this lipid increased to at least 0.15% of total PI levels following platelet spreading on immobilized VWF (Figure 3A). This increase in PtdIns(3,4)P2is similar to that previously reported for thrombin-stimulated platelets and platelets spread on fibrinogen.11 25 In contrast, the level of PtdIns(3)P was unaltered in these cells and PtdIns(3,4,5)P3 was not detected (data not shown). Pretreatment of platelets with either LY294002 or wortmannin led to a dose-dependent inhibition of PtdIns(3,4)P2 production in spreading platelets, with a maximal inhibition of about 95% and 100% using LY294002 (25 μM) and wortmannin (100 nM), respectively (Figure3A,B). The effects of these inhibitors were specific in that they did not inhibit the levels of PtdIns, PtdIns(4)P, and PtdIns(4,5)P2 (Figure 3C). Further evidence that LY294002 (25 μM) and wortmannin (100 nM) effectively inhibited PI 3–kinase was obtained from studies demonstrating that both inhibitors prevented integrin αIIbβ3 activation and spreading of platelets on a fibrinogen matrix (Figure 3D). In addition, both inhibitors completely eliminated other PI 3–kinase–dependent events such as phosphorylation of the downstream PI 3–kinase target, PKB/Akt, and platelet aggregation induced by heat-aggregated IgG and CD-9 (data not shown).
The effect of LY294002 and wortmannin on VWF-induced generation of PtdIns(3,4)P2 and on integrin αIIbβ3 activation and spreading on immobilized fibrinogen.
Washed platelets (1.5 × 108/mL) were incubated with vehicle alone (control) or the indicated concentrations of LY294002 (0-25 μM) or wortmannin (0-100 nM) for 15 minutes. (A-C) Platelets were suspended in Tyrode buffer (A, resting) and allowed to spread on immobilized VWF (10 μg/mL) for 60 minutes under static conditions (A, spreading: control and LY294002). Platelets in suspension or adherent on the matrix were subsequently lysed with RIPA buffer, lipids extracted and analyzed by SAX HPLC. These results demonstrate: (A) the generation of the PI 3–kinase lipid product, PtdIns(3,4)P2 (PI[3,4]P2), in resting platelets and in spread platelets pretreated with vehicle (control) or LY294002 (25 μM); (B) the level of inhibition of PtdIns(3,4)P2 production in spreading platelets pretreated with LY294002 (0-25 μM; solid line) or wortmannin (0-100 nM; dotted line); and (C) the effect of LY294002 and wortmannin on the levels of PtdInsP (PI), PtdIns(4)P (PI[4]P), and PtdIns(4,5)P2(PI[4,5]P2) in spreading platelets. (D) Pretreated platelets were incubated with PAC-1 antibody prior to adhesion and spreading on immobilized fibrinogen (100 μg/mL) for 60 minutes. Adherent platelets were fixed and stained with a FITC-conjugated secondary antibody prior to visualization by confocal microscopy (× 63W objective). (A-D) Images and graphs are from a single experiment representative of 3.
The effect of LY294002 and wortmannin on VWF-induced generation of PtdIns(3,4)P2 and on integrin αIIbβ3 activation and spreading on immobilized fibrinogen.
Washed platelets (1.5 × 108/mL) were incubated with vehicle alone (control) or the indicated concentrations of LY294002 (0-25 μM) or wortmannin (0-100 nM) for 15 minutes. (A-C) Platelets were suspended in Tyrode buffer (A, resting) and allowed to spread on immobilized VWF (10 μg/mL) for 60 minutes under static conditions (A, spreading: control and LY294002). Platelets in suspension or adherent on the matrix were subsequently lysed with RIPA buffer, lipids extracted and analyzed by SAX HPLC. These results demonstrate: (A) the generation of the PI 3–kinase lipid product, PtdIns(3,4)P2 (PI[3,4]P2), in resting platelets and in spread platelets pretreated with vehicle (control) or LY294002 (25 μM); (B) the level of inhibition of PtdIns(3,4)P2 production in spreading platelets pretreated with LY294002 (0-25 μM; solid line) or wortmannin (0-100 nM; dotted line); and (C) the effect of LY294002 and wortmannin on the levels of PtdInsP (PI), PtdIns(4)P (PI[4]P), and PtdIns(4,5)P2(PI[4,5]P2) in spreading platelets. (D) Pretreated platelets were incubated with PAC-1 antibody prior to adhesion and spreading on immobilized fibrinogen (100 μg/mL) for 60 minutes. Adherent platelets were fixed and stained with a FITC-conjugated secondary antibody prior to visualization by confocal microscopy (× 63W objective). (A-D) Images and graphs are from a single experiment representative of 3.
Our recent studies have demonstrated that VWF-induced integrin αIIbβ3 activation is critically dependent on intracellular calcium mobilization.22 To examine the relationship between inhibition of PI 3–kinase and calcium release, cytosolic calcium levels were monitored during platelet spreading on VWF. As demonstrated in Figure 4, panels A and B, resting platelets exhibited a cytosolic calcium level below 50 nM, which did not undergo oscillations. Platelets firmly adherent to VWF underwent dynamic calcium oscillations in about 60% of cells (Figure 4A). The range of calcium concentrations varied widely between individual cells, with calcium oscillations typically fluctuating between 150 and 800 nM.22 Pretreatment of platelets with wortmannin (100 nM) delayed the onset of the oscillatory calcium transients by 5 to 10 minutes (Figure 4A); however, they did not affect the final magnitude of the response (Figure 4B). Similar results were obtained with LY294002 (25 μM; Figure 4A). The inhibitory effect of LY294002 and wortmannin on the initiation of the oscillatory calcium transients was consistent with the delayed spreading response in these cells. Because PI 3–kinase has been implicated in regulating calcium influx as well as the release of intracellular stores, we investigated the effect of LY294002 and wortmannin on intracellular calcium mobilization. Consistent with our recent findings,22chelating extracellular calcium with the calcium chelator, EGTA, did not prevent oscillatory calcium transients, but had a major inhibitory effect (∼75%) on the magnitude of the calcium response. Pretreatment of EGTA-treated platelets with LY294002 or wortmannin delayed the onset of oscillatory calcium transients by 5 to 10 minutes; however, the final magnitude of these oscillatory calcium responses was unaffected by these inhibitors (data not shown), suggesting that the primary function of PI 3–kinase is to regulate intracellular calcium mobilization.
The role of PI 3–kinase in regulating platelet calcium responses under static conditions.
Calcium indicator dye-loaded platelets were incubated with vehicle alone (control), LY294002 (25 μM), or wortmannin (100 nM) for 15 minutes prior to adhesion on immobilized VWF (10 μg/mL). Adherent platelets were allowed to spread in the presence of extracellular Ca++ (1 mM) for up to 60 minutes under static conditions. Changes in the cytosolic calcium concentration of adherent cells were monitored at the indicated time points by confocal microscopy (× 63W objective) and fluorescence ratios quantified. The results presented in panel A demonstrate the percentage of platelets undergoing oscillatory calcium transients at the indicated time points. Results represent mean ± SEM from 3 to 5 independent experiments. The results presented in panel B show a representative calcium oscillation profile of individual vehicle-treated (control) and wortmannin-treated platelets at 0 minute (thin line) and after 15 minutes of spreading (bold line). Statistical analysis was performed using a ttest comparing control versus LY294002- or wortmannin-treated platelets (P < .05*; P < .01**).
The role of PI 3–kinase in regulating platelet calcium responses under static conditions.
Calcium indicator dye-loaded platelets were incubated with vehicle alone (control), LY294002 (25 μM), or wortmannin (100 nM) for 15 minutes prior to adhesion on immobilized VWF (10 μg/mL). Adherent platelets were allowed to spread in the presence of extracellular Ca++ (1 mM) for up to 60 minutes under static conditions. Changes in the cytosolic calcium concentration of adherent cells were monitored at the indicated time points by confocal microscopy (× 63W objective) and fluorescence ratios quantified. The results presented in panel A demonstrate the percentage of platelets undergoing oscillatory calcium transients at the indicated time points. Results represent mean ± SEM from 3 to 5 independent experiments. The results presented in panel B show a representative calcium oscillation profile of individual vehicle-treated (control) and wortmannin-treated platelets at 0 minute (thin line) and after 15 minutes of spreading (bold line). Statistical analysis was performed using a ttest comparing control versus LY294002- or wortmannin-treated platelets (P < .05*; P < .01**).
To investigate the functional importance of PI 3–kinase under shear conditions, we examined the effect of LY294002 and wortmannin on platelet adhesion and spreading on immobilized VWF using an in vitro flow-based adhesion assay. In contrast to adhesion assays performed under static conditions, inhibition of PI 3–kinase with either wortmannin or LY294002 profoundly affected the ability of platelets to adhere to a VWF matrix under flow. As demonstrated in Figure5A, under low shear conditions (150 s-1) pretreating platelets with LY294002 or wortmannin inhibited platelet adhesion in a dose-dependent manner, with maximal inhibition of about 70% (P < .05). Under high shear conditions (1800 s-1), inhibiting PI 3–kinase reduced platelet adhesion by up to 90% (P < .001). Real-time analysis of platelet adhesion under flow revealed that LY294002 or wortmannin did not affect the ability of cells to tether or translocate on the VWF matrix but dramatically reduced the ability of these cells to form stationary adhesion contacts (data not shown). This reduction in stationary adhesion correlated with more than 95% reduction in PAC-1 binding (Figure 5D). Continuous observation of translocating platelets for prolonged periods (20 minutes) demonstrated that these cells were unable to form stable adhesion contacts at all times points examined (data not shown). Inhibition of PI 3–kinase had no effect on the ability of platelets to change shape and extend filopodia on immobilized VWF; however, lamellipodial formation was completely inhibited (Figure 5B,C). In further control studies, we confirmed that ADP was not essential for stationary platelet adhesion under high shear conditions, because platelets pretreated with the ADP receptor antagonists, ATPαS (100 μM) or AR-C69931MX/A3P5PS (100 nM and 200 μM, respectively), or with apyrase (8.25 U/mL) adhered normally to the VWF matrix (data not shown).
The role of PI 3–kinase in regulating platelet adhesion, spreading, and integrin αIIbβ3activation under flow conditions.
Washed platelets (1.5 × 108/mL) were incubated with vehicle alone (control) or the indicated concentrations of LY294002 (0-25 μM) or wortmannin (0-100 nM) for 15 minutes. Pretreated platelets were either perfused immediately over immobilized VWF (100 μg/mL) at 150 s-1 or reconstituted with red blood cells prior to perfusion at 1800 s-1. In some experiments, tethered platelets were incubated with PAC-1 antibody prior to fixation and staining with a FITC-conjugated secondary antibody. These results demonstrate the effect of the PI 3–kinase inhibitors on (A) the level of stationary platelet adhesion and (B) the mean surface area of adherent platelets following perfusion at 150 s-1 or 1800 s-1; (C) the morphology of adherent platelets as visualized by scanning electron microscopy (bar = 2 μm); and (D) platelet spreading and PAC-1 binding (bar = 10 μm). (A,B) Results represent mean ± SEM from 4 independent experiments. Statistical analysis was performed using a t test comparing control versus LY294002- or wortmannin-treated platelets (P < .05*;P < .01**; P < .001***). (C,D) Images are from a single experiment representative of 3.
The role of PI 3–kinase in regulating platelet adhesion, spreading, and integrin αIIbβ3activation under flow conditions.
Washed platelets (1.5 × 108/mL) were incubated with vehicle alone (control) or the indicated concentrations of LY294002 (0-25 μM) or wortmannin (0-100 nM) for 15 minutes. Pretreated platelets were either perfused immediately over immobilized VWF (100 μg/mL) at 150 s-1 or reconstituted with red blood cells prior to perfusion at 1800 s-1. In some experiments, tethered platelets were incubated with PAC-1 antibody prior to fixation and staining with a FITC-conjugated secondary antibody. These results demonstrate the effect of the PI 3–kinase inhibitors on (A) the level of stationary platelet adhesion and (B) the mean surface area of adherent platelets following perfusion at 150 s-1 or 1800 s-1; (C) the morphology of adherent platelets as visualized by scanning electron microscopy (bar = 2 μm); and (D) platelet spreading and PAC-1 binding (bar = 10 μm). (A,B) Results represent mean ± SEM from 4 independent experiments. Statistical analysis was performed using a t test comparing control versus LY294002- or wortmannin-treated platelets (P < .05*;P < .01**; P < .001***). (C,D) Images are from a single experiment representative of 3.
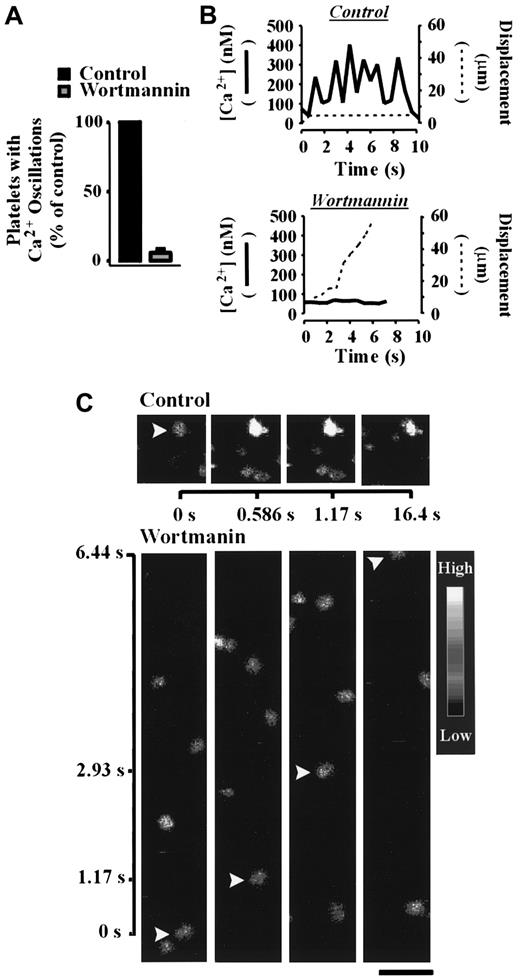
Analysis of cytosolic calcium during shear-dependent platelet adhesion demonstrated a critical role for PI 3–kinase in this process. As demonstrated in Figure 6A, pretreatment of platelets with wortmannin (100 nM) in the presence of EGTA abolished sustained calcium oscillations in more than 90% of platelets relative to control platelets. Whereas control platelets underwent strong oscillatory calcium transients and formed stationary adhesion contacts, wortmannin-treated platelets (100 nM) translocated continuously and exhibited minor calcium oscillations (Figure 6B,C). Similar results were obtained with LY294002 (data not shown). These studies demonstrate an important requirement for PI 3–kinase in promoting calcium mobilization under flow.
The role of PI 3–kinase in regulating calcium mobilization under flow conditions.
Calcium indicator dye-loaded platelets were incubated with vehicle alone (control) or wortmannin (100 nM) for 15 minutes prior to reconstitution with red blood cells and perfusion over immobilized VWF (100 μg/mL) at 1800 s-1. Changes in the cytosolic calcium concentration of adherent cells were monitored by confocal microscopy (× 63W objective) and fluorescence ratios quantified. The results presented in panel A demonstrate the effect of wortmannin on the percentage of cells undergoing oscillatory calcium transients relative to untreated (control) platelets. The images and results presented in panels B and C show a representative calcium oscillation response (solid line) and the displacement (dotted line) of individual vehicle- (control) and wortmannin-treated platelets under shear conditions. Results and images are from a single experiment representative of 3. Bar = 10 μm.
The role of PI 3–kinase in regulating calcium mobilization under flow conditions.
Calcium indicator dye-loaded platelets were incubated with vehicle alone (control) or wortmannin (100 nM) for 15 minutes prior to reconstitution with red blood cells and perfusion over immobilized VWF (100 μg/mL) at 1800 s-1. Changes in the cytosolic calcium concentration of adherent cells were monitored by confocal microscopy (× 63W objective) and fluorescence ratios quantified. The results presented in panel A demonstrate the effect of wortmannin on the percentage of cells undergoing oscillatory calcium transients relative to untreated (control) platelets. The images and results presented in panels B and C show a representative calcium oscillation response (solid line) and the displacement (dotted line) of individual vehicle- (control) and wortmannin-treated platelets under shear conditions. Results and images are from a single experiment representative of 3. Bar = 10 μm.
To investigate the potential importance of PI 3–kinase in regulating thrombus formation under experimental conditions more closely simulating those experienced by platelets in vivo, flow studies were performed on anticoagulated whole blood. As demonstrated in Figure7A, perfusing anticoagulated whole blood through VWF-coated microcapillary tubes (1800 s-1) for 5 minutes resulted in the formation of platelet-rich thrombi. Analysis of these thrombi by confocal imaging demonstrated that they covered approximately 30% to 40% of the VWF-coated surface and had a height of 7 to 10 μm (Figure 7A). In contrast, pretreating whole blood with increasing concentrations of LY294002 resulted in a dose-dependent inhibition of thrombus growth (Figure 7A,B). Real-time analysis of LY294002-treated platelets (50-200 μM) revealed that the majority of tethered cells rolled along the VWF matrix and did not form stationary adhesion contacts, similar to that observed with washed platelets (data not shown). It should be noted that the requirement for higher LY294002 concentrations to inhibit platelet adhesion in whole blood is presumably due to plasma protein binding to LY294002, because a 5- to 10-fold increase in concentration of this reagent was required to inhibit CD-9–induced platelet aggregation in platelet-rich plasma relative to washed platelets (data not shown). Further evidence that PI 3–kinase was essential for platelet thrombus formation on VWF was derived from studies using wortmannin. In these studies, washed platelets were initially pretreated with wortmannin (100 nM) to irreversibly inhibit PI 3–kinase. The cells were subsequently reconstituted with red blood cells and plasma and examined for their ability to form stationary adhesion contacts on immobilized VWF. Similar to that observed with LY294002, wortmannin completely inhibited the ability of platelets to form stationary adhesion contacts and thrombi on VWF (data not shown).
The role of PI 3–kinase in regulating platelet thrombus formation under flow conditions.
Anticoagulated whole blood was incubated with vehicle alone (control) or the indicated concentrations of LY294002 (0-200 μM) for 15 minutes prior to perfusion over immobilized VWF (100 μg/mL) at 1800 s-1. In panel A, thrombi were fixed and incubated with DiOC6. Platelet thrombi were reconstructed using a computer-assisted image analysis program. The upper panels represent an oblique view to demonstrate surface coverage; the lower panels represent a side-on view to demonstrate differences in thrombus height. The results in panel B demonstrate the effect of PI 3–kinase inhibition on thrombus formation.
The role of PI 3–kinase in regulating platelet thrombus formation under flow conditions.
Anticoagulated whole blood was incubated with vehicle alone (control) or the indicated concentrations of LY294002 (0-200 μM) for 15 minutes prior to perfusion over immobilized VWF (100 μg/mL) at 1800 s-1. In panel A, thrombi were fixed and incubated with DiOC6. Platelet thrombi were reconstructed using a computer-assisted image analysis program. The upper panels represent an oblique view to demonstrate surface coverage; the lower panels represent a side-on view to demonstrate differences in thrombus height. The results in panel B demonstrate the effect of PI 3–kinase inhibition on thrombus formation.
Discussion
The results presented here demonstrate for the first time a key signaling role for type 1 PI 3–kinases in linking the VWF-GP Ib/V/IX interaction to integrin αIIbβ3activation under physiologic flow conditions. This conclusion is primarily based on studies using the pharmacologic inhibitors, LY294002 and wortmannin. Wortmannin, a fungal metabolite that irreversibly inhibits the catalytic function of PI 3–kinase, has been well characterized to specifically inhibit PI 3–kinase at concentrations up to 100 nM.26 At higher concentrations, wortmannin also inhibits PI 4-kinase, myosin light-chain kinase, and phospholipase A2. LY294002, a quercetin analogue that reversibly inhibits PI 3–kinase, has no inhibitory effects on these enzymes and has generally been regarded as a selective PI 3–kinase inhibitor at concentrations up to 25 μM.27 However, a recent study has demonstrated that casein kinase 2 (CK2), a serine-threonine kinase involved in promoting cell growth, is also inhibited by LY294002 at low micromolar concentrations.28 CK2 is not, however, inhibited by wortmannin at concentrations as high as 1 μM, suggesting that the inhibitory effects of LY294002 in our studies are unlikely to be primarily due to effects on CK2. To further strengthen our hypothesis that PI 3–kinase is the responsible enzyme for shear-dependent platelet adhesion, we have performed dose-response studies correlating the inhibitory effects on platelet adhesion with the cellular levels of PtdIns(3,4)P2. In all studies we observed an excellent correlation between these events, thereby providing strong evidence for a key role for type 1 PI 3–kinases in this process.
Our observations that PI 3–kinase inhibitors did not block platelet spreading on VWF under static conditions was somewhat surprising given previous reports demonstrating an absolute requirement for this enzyme in promoting platelet spreading on a fibrinogen matrix.11 Several lines of evidence suggest that this difference was not due to incomplete inhibition of PI 3–kinase, but, in fact, reflects a specific difference between platelet spreading on fibrinogen versus VWF. First, we have demonstrated that both LY294002 and wortmannin effectively inhibited generation of the PI 3–kinase lipid product, PtdIns(3,4)P2. Furthermore, inhibition of PI 3–kinase activity with both inhibitors completely blocked PKB/Akt phosphorylation (unpublished observations, February 2001). Second, we demonstrated that both LY294002 and wortmannin completely inhibited platelet spreading on immobilized fibrinogen under identical conditions to those used in our spreading studies on VWF. Finally, both inhibitors abolished other PI 3–kinase–dependent processes, including platelet aggregation induced by heat-aggregated IgG or anti-CD9 antibodies.
Our findings demonstrating an important role for PI 3–kinase in regulating calcium mobilization in adherent platelets supports a growing body of evidence for an important link between PI 3–kinase signaling and calcium fluxes. PI 3–kinase has been demonstrated to regulate both calcium influx and mobilization, although the extent of its involvement in these processes appears to be specific for both cell type and stimulus. A role for PI 3–kinase, in particular its PtdIns(3,4,5)P3 lipid product, in calcium influx has been demonstrated through studies in platelets and T cells. For example, platelets deficient in src homology 2 (SH2)–containing inositol 5′ phosphatase (SHIP) exhibited a specific increase in the levels of PtdIns(3,4,5)P3, resulting in enhanced transmembrane calcium flux.29 Similarly, addition of exogenous PtdIns(3,4,5)P3 to T cells promotes calcium influx, without affecting intracellular calcium mobilization.30 In other cell types, including platelet-derived growth factor (PDGF)–stimulated NIH 3T3 cells31 and COS-1 cells,32 and antigen-stimulated B cells,33 inhibiting PI 3–kinase primarily leads to a decrease in IP3 generation and calcium release from intracellular stores. PtdIns(3,4,5)P3 has been implicated in this process because microinjection of pleckstrin homology (PH) domains that specifically bind this lipid decrease IP3 formation and calcium mobilization in COS-1 cells32 and megakaryocytes,34 respectively. Although we were unable to detect a rise in PtdIns(3,4,5)P3levels following platelet spreading on VWF, the level of increase in PtdIns(3,4)P2 reported here is in agreement with that demonstrated for platelets adherent and spread on immobilized fibrinogen.11 The most likely explanation for only observing a rise in PtdIns(3,4)P2 in our studies is that in vitro generation of PtdIns(3,4,5)P3 occurs more rapidly and precedes the appearance of PtdIns(3,4)P2,35suggesting that PtdIns(3,4,5)P3 is likely to be detected at an earlier time point.
In all studies to date, PI 3–kinase has been demonstrated to primarily serve as a modulator of phospholipase Cγ (PLCγ) activation and IP3 formation and was not found to be essential for calcium release. In contrast, in our studies PI 3–kinase appears to have an essential role for sustained calcium oscillations under shear conditions. Moreover, the effects of PI 3–kinase inhibitors on the time course and magnitude of the calcium responses in our static assays appears distinct from that previously described. For example, we have demonstrated that inhibiting PI 3–kinase prolongs the onset of calcium mobilization, but does not have any effect on the final magnitude of the calcium response. Thus, PI 3–kinase appears to be essential for inducing the “early” release of calcium from internal stores and does not appear to be required for subsequent calcium influx. These observations raise the possibility that PI 3–kinase inhibitors prevent the formation of irreversible platelet adhesion under shear conditions, because this “early” calcium response may be essential for activation of integrin αIIbβ3. In this context, it is of interest that prolonged interaction of translocating platelets with the VWF matrix (∼20 minutes) did not result in stationary platelet adhesion. It is therefore possible that PI 3–kinase participates in a shear-specific signaling pathway. This latter possibility would be in keeping with previous studies in endothelial cells in which shear-induced nitric oxide production was dependent on the activation of PI 3–kinase.36 37
A key outstanding issue is the mechanism by which the VWF-GP Ib interaction induces PI 3–kinase activation. Previous studies examining shear-induced platelet activation have suggested that platelet activation induced by the VWF–GP Ib interaction occurs indirectly through the release of ADP.38-40 Dense granule ADP has a well-defined role in promoting platelet activation by a number of platelet agonists and has been demonstrated to induce PI 3–kinase activation.41 However, we do not believe that this is the major mechanism for PI 3–kinase activation during platelet adhesion to VWF, because ADP receptor antagonists do not prevent activation of integrin αIIbβ3 under these experimental conditions. A recent study by Canobbio et al42 has suggested that VWF-induced platelet activation is critically dependent on FcγRIIA and thromboxane2 (TXA2) generation. FcγRIIA has previously been postulated to be physically and functionally linked to GP Ib/V/IX and activates platelets through a PI 3–kinase–dependent mechanism.43-45 However, this signaling pathway appears distinct from those operating during platelet adhesion to VWF under flow22 and during shear-induced platelet aggregation,39 because VWF-dependent platelet activation under these conditions is insensitive to the inhibitory effects of aspirin.
A number of recent studies from several independent laboratories have suggested that GP Ib/V/IX can signal directly to regulate the affinity status of integrin αIIbβ3 independent of ADP, TXA2, and Fc receptors.22,46,47 The precise mechanism by which GP Ib signals has not been elucidated, although one potential mechanism for PI 3–kinase activation involves direct binding to the 14.3.3ζ adapter protein. The 14.3.3ζ protein is physically linked to the cytoplasmic tail of GP Ibα and has recently been suggested to be important for GP Ib/V/IX-induced integrin αIIbβ3 activation, based on studies of Chinese hamster ovary (CHO) cell lines expressing GP Ib/IX and integrin αIIbβ3.47 Although there is evidence for complex formation between 14.3.3ζ and the p85/p110 form of PI 3–kinase in T cells,48 there is as yet no evidence that this association promotes kinase activation. A recent study by Munday et al15 has suggested a direct association between GP Ib/V/IX and PI 3–kinase; however, the significance of this association, given its low stoichiometry, remains unclear. It is possible that PI 3–kinase activation in VWF-stimulated platelets is in part regulated through integrin αIIbβ3outside-in signaling. Previous studies have highlighted the synergistic contribution of GP Ib/V/IX and integrin αIIbβ3 to cytoskeletal signaling complex formation18,49 and have also demonstrated that direct ligand binding to integrin αIIbβ3 is sufficient to induce PI 3–kinase activation and a selective increase in the cellular levels of PtdIns(3,4)P2.12These observations, combined with the demonstration that ligand binding to integrin αIIbβ3 induces intracellular calcium mobilization, raises the interesting possibility that integrin αIIbβ3-dependent PI 3–kinase activation may play an important role in promoting calcium flux in VWF-stimulated platelets. Future studies are currently under way to examine the relative contribution of GP Ib/V/IX and integrin αIIbβ3 to PI 3–kinase activation and calcium mobilization in VWF-stimulated platelets.
In conclusion, our studies demonstrate for the first time an essential role for type 1 PI 3–kinases in regulating the earliest steps of the hemostatic process, namely, the shear-induced activation of integrin αIIbβ3 following platelet adhesion to VWF. In this context, it is of interest that wortmannin was originally described as a hemorrhagic factor,50,51 a finding that has not been easily reconciled with in vitro studies demonstrating a modest effect of wortmannin on platelet activation induced by the majority of physiologic agonists studied to date.9 Although it remains to be established whether the bleeding diathesis induced by wortmannin is primarily due to effects on platelets, and in particular GP Ib/V/IX signaling, our findings nonetheless provide important new insight into the functional role of PI 3–kinase in regulating the hemostatic function of platelets.
We would like to thank Suhasini Kulkarni for technical assistance.
Supported by grants from the National Health and Medical Research Council of Australia, the National Heart Foundation of Australia, and the Welcome Trust. C.Y. is a recipient of the Australian Post-Graduate Research Award. K.A. is a National Health and Medical Research Council C. J. Martin/R.G. Menzies Fellow.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shaun P. Jackson, Australian Centre for Blood Diseases, Dept of Medicine, Monash Medical School, Box Hill Hospital, Arnold St, Box Hill, Victoria 3128, Australia; e-mail:shaun.jackson@med.monash.edu.au.



![Fig. 3. The effect of LY294002 and wortmannin on VWF-induced generation of PtdIns(3,4)P2 and on integrin αIIbβ3 activation and spreading on immobilized fibrinogen. / Washed platelets (1.5 × 108/mL) were incubated with vehicle alone (control) or the indicated concentrations of LY294002 (0-25 μM) or wortmannin (0-100 nM) for 15 minutes. (A-C) Platelets were suspended in Tyrode buffer (A, resting) and allowed to spread on immobilized VWF (10 μg/mL) for 60 minutes under static conditions (A, spreading: control and LY294002). Platelets in suspension or adherent on the matrix were subsequently lysed with RIPA buffer, lipids extracted and analyzed by SAX HPLC. These results demonstrate: (A) the generation of the PI 3–kinase lipid product, PtdIns(3,4)P2 (PI[3,4]P2), in resting platelets and in spread platelets pretreated with vehicle (control) or LY294002 (25 μM); (B) the level of inhibition of PtdIns(3,4)P2 production in spreading platelets pretreated with LY294002 (0-25 μM; solid line) or wortmannin (0-100 nM; dotted line); and (C) the effect of LY294002 and wortmannin on the levels of PtdInsP (PI), PtdIns(4)P (PI[4]P), and PtdIns(4,5)P2(PI[4,5]P2) in spreading platelets. (D) Pretreated platelets were incubated with PAC-1 antibody prior to adhesion and spreading on immobilized fibrinogen (100 μg/mL) for 60 minutes. Adherent platelets were fixed and stained with a FITC-conjugated secondary antibody prior to visualization by confocal microscopy (× 63W objective). (A-D) Images and graphs are from a single experiment representative of 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/1/10.1182_blood.v99.1.151/6/m_h80121959003.jpeg?Expires=1765927666&Signature=ySuT0X5VvfNnNLbyGdU6sAoy4KHQrykuqT02AgINwkaNgtZy7fC1lMfj0e8sJEFVzBIZAItNEa3UpgGXC0x32LJPVIx9zeDdL~R5XOYOM-Dy9a0bYx8nUMykXX7GApO7lBRFU7aHcB3oMRfygS3tOKDPQJd7y~ORR0tZdX6-07dNN3lxUxfDkbkPtpxUvUmGyhtJwjx9z-m6jIuwMPEm464SEUrShUCDlWdYqQ1IQRBLgDwU82657rmyMHfiIi7KIuelsPcS-qWXyfhVOjT7xPRvWPLIuqHtQhjHAUK3xl6foj9vsoFXp65W8CA3jhs8B1IEN~o41As7WXN0ghtngg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)






![Fig. 3. The effect of LY294002 and wortmannin on VWF-induced generation of PtdIns(3,4)P2 and on integrin αIIbβ3 activation and spreading on immobilized fibrinogen. / Washed platelets (1.5 × 108/mL) were incubated with vehicle alone (control) or the indicated concentrations of LY294002 (0-25 μM) or wortmannin (0-100 nM) for 15 minutes. (A-C) Platelets were suspended in Tyrode buffer (A, resting) and allowed to spread on immobilized VWF (10 μg/mL) for 60 minutes under static conditions (A, spreading: control and LY294002). Platelets in suspension or adherent on the matrix were subsequently lysed with RIPA buffer, lipids extracted and analyzed by SAX HPLC. These results demonstrate: (A) the generation of the PI 3–kinase lipid product, PtdIns(3,4)P2 (PI[3,4]P2), in resting platelets and in spread platelets pretreated with vehicle (control) or LY294002 (25 μM); (B) the level of inhibition of PtdIns(3,4)P2 production in spreading platelets pretreated with LY294002 (0-25 μM; solid line) or wortmannin (0-100 nM; dotted line); and (C) the effect of LY294002 and wortmannin on the levels of PtdInsP (PI), PtdIns(4)P (PI[4]P), and PtdIns(4,5)P2(PI[4,5]P2) in spreading platelets. (D) Pretreated platelets were incubated with PAC-1 antibody prior to adhesion and spreading on immobilized fibrinogen (100 μg/mL) for 60 minutes. Adherent platelets were fixed and stained with a FITC-conjugated secondary antibody prior to visualization by confocal microscopy (× 63W objective). (A-D) Images and graphs are from a single experiment representative of 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/1/10.1182_blood.v99.1.151/6/m_h80121959003.jpeg?Expires=1766150881&Signature=OvnPRIAl1jSWIifVF4dNsSJW7wm4mRR8IbApJ3DpuLZA31YdzO6E7UEd8LoWRnL3oBSo~JyeAno6yb7rqE-NmI82G5CahpcviiWoXCoCxfiSO-8bNrd820D82RmrjM-yAuCoRANw6YXPPFA4cxr6x9ddvXLjfAZgQFwOiZ3rVvy-lGwV01aivtOPQPHeccZVIAxu0MopizV6ucNmVrfyD1W~9mY82XTYgIt-0w2cKZ92MdrBL05maObX1OiKPs0eg2X7PKCcmmFyWIh7LZxwLqw-4EqnPkpPDjEEJwuq~fI5DR6QGVzHqIdIqF58UmvUhJuQaoTmTESOjcHZ0lAy7A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)