The adhesion and aggregation of platelets at sites of vascular injury is dependent on the initial binding of the GP Ib/V/IX receptor complex to immobilized von Willebrand factor (VWF). Under flow conditions, this interaction supports platelet translocation that is characteristically stop-start in nature. High resolution imaging of platelets during surface translocation on immobilized VWF revealed that thin membrane tethers (length: 0.91 μm-47.90 μm) were pulled from the surface of these cells. Membrane tethers were dynamic structures that extended from small, localized adhesion contacts under the influence of flow. Perfusion of platelets in the presence of blocking antibodies against integrin αIIbβ3, or over isolated A1 domains, demonstrated that the VWF–GP Ib interaction was sufficient to induce membrane tether formation. The rate and extent of tether elongation was shear-dependent (shear range: 150 s−1-10 000 s−1), with mean tether length ranging from 3.23 μm to 16.55 μm, tether frequency from 2.67% to 97.33%, and tether growth rate from 0.04 μm/sec to 8.39 μm/sec. Tether formation and retraction did not require platelet activation; however, the growth rate, lifetime, and dimensions were significantly affected by the actin polymerization inhibitor, cytochalasin D, and by chelating intracellular calcium. Single-cell analysis revealed that formation of membrane tethers regulates the stop-start phases of platelet translocation on VWF, suggesting a potentially important role for this phenomenon in regulating the dynamics of the platelet-VWF interaction under flow.
Introduction
The formation of stable adhesion contacts between circulating blood cells, such as platelets and leukocytes, and the injured vessel wall requires specialized adhesion mechanisms capable of withstanding the mechanical forces generated by flowing blood. It has recently become apparent that platelets and leukocytes utilize a similar multistep adhesion mechanism, albeit through entirely different ligand-receptor pairs,1,2 that enables efficient cell adhesion under flow. In the initial stages of platelet and leukocyte adhesion, receptor-ligand interactions with inherently rapid bond kinetics support cell tethering onto the vessel wall. These bonds are reversible, such that tethered cells subjected to hydrodynamic drag forces roll or translocate over the injured vessel wall. Rolling is indispensable for normal platelet and leukocyte function as it serves to decelerate cell movement relative to flowing blood, allowing receptors with slower bond kinetics (ie, integrins) to engage adhesive ligands and mediate firm cell adhesion.3 4
The molecular basis of cell rolling has been primarily determined from in vitro and in vivo studies on leukocytes. In rolling cells, adhesive bond formation between selectin family members and their corresponding carbohydrate ligands is balanced by continuous bond breakage. This process is facilitated by the clustering of selectins and their ligands (eg, L-selectin, PSGL-1) at the tips of microvilli.5-7 In support of this, disruption of these membrane structures with cytochalasin B, or redistribution of L-selectin from microvilli to the body of the cell, are associated with decreased leukocyte adhesion and rolling.8,9 Adhesion of leukocytes via these microvilli is thought to result in the formation of a cluster of tethered bonds that when subjected to tensile forces, results in the elongation of microvilli into thin membrane tethers. Tether formation has been proposed to account for the 2 distinct behaviors observed in rolling leukocytes, where tether stretching represents the slow, smooth rolling phase and release of membrane tethers represents the intermittent stepwise or jerky phases.10
The formation of membrane tethers has been extensively studied in erythrocytes, using micropipette techniques to extend the membrane into thin, elongated membrane tethers.11,12 During this process, the plasma membrane is thought to flow from the cell body over the underlying cytoskeletal layer, forming a hollow cylinder of lipid bilayer. In this model, the rigid membrane skeleton is unable to deform from its original state, causing it to become either fragmented or completely separated from the lipid bilayer.13 A similar response has also been described in neutrophils, whereby applying a pulling force to surface microvilli results in membrane tether extrusion.14 More recently, membrane tether formation has been directly observed in neutrophils interacting with adherent platelets as well as immobilized P-selectin.15 The extension of membrane tethers during neutrophil rolling may be important for reducing the level of stress experienced by individual receptor-ligand interactions, thereby increasing the likelihood of rolling cells forming stable adhesion contacts.
Like leukocytes, platelets have also been observed to roll in vitro4,16 and at sites of vascular injury in vivo.17 Platelet rolling not only occurs on the injured vessel wall but also on the surface of thrombi, and is mediated by the interaction between the A1 domain of immobilized von Willebrand factor (VWF) with the platelet glycoprotein (GP) Ib/V/IX receptor complex. This rolling interaction is characteristically stop-start in nature, wherein transient stationary adhesion contacts are interspersed by periods of rapid translocation. Our recent studies have demonstrated that platelets undergo changes in morphology during surface translocation, converting from disc-shaped cells into irregular spheres extending multiple filopodia16; however, to date there are no reports of membrane tether formation during platelet translocation.
In this study, we have examined platelet morphologic changes during the initial interaction of platelets with immobilized VWF under shear conditions, using high-resolution differential interference contrast microscopy. We demonstrate that platelets extend membrane tethers during surface translocation on immobilized VWF. Tether formation in rolling platelets does not require changes in either the actin or microtubular components of the cytoskeleton and occurs independently of calcium mobilization and other platelet activation events. We show that tether formation occurs as a function of shear rate and plays a potentially important role in modulating the dynamics of the platelet-VWF interaction.
Materials and methods
Materials
Cytochalasin D, vinblastine, paclitaxel, theophylline, prostaglandin E1, and anti–β-tubulin antibody were purchased from Sigma (St Louis, MO). DM-BAPTA BAPTA and the CY5-conjugated anti–mouse antibody were obtained from Molecular Probes (Eugene, OR). The anti-GP Ibα antibody, AK2, and isolated 39/34 kd dispase fragment of VWF (A1 domain) were generous donations from Professor Michael Berndt (Baker Medical Research Institute, Melbourne, Australia). The anti-integrin αIIbβ3 antibody, chimeric Fab fragment of mAb 7E3 (c7E3 Fab-abciximab) was from Eli Lilly (Centocor, Leiden, The Netherlands). Aggrastat was from Merck (Whitehouse Station, NJ). VWF was purified from plasma cryoprecipitate according to the method of Montgomery and Zimmerman.18 All other reagents were from sources described previously.19 20
Preparation of washed platelets
Washed platelets were prepared as described previously.19 Briefly, 6 volumes of whole blood was collected in 1 volume of theophylline-containing anticoagulant (90 mM sodium citrate, 7 mM citric acid, pH 4.6, 140 mM dextrose, and 70 mM theophylline) from healthy volunteers who had not received any antiplatelet medication in the preceding 2 weeks. Platelet-rich plasma was obtained through centrifugation of anticoagulated blood at 200g. Platelets were pelleted at 2000g, resuspended in platelet washing buffer (PWB) (4.3 mM Na2HPO4, 24.3 mM NaH2PO4, pH 6.5, 113 mM NaCl, 5.5 mM glucose, 0.5% [w/v] bovine serum albumin, and 10 mM theophylline), and maintained at 37°C until use.
Flow studies and analysis of tether formation
Flow assays were performed according to a modified method of Yap et al.21 Rectangular glass microcapillary tubes (Microslides; Vitro Com, Mountain Lakes, NJ) were coated with either human VWF (100 μg/mL) or an isolated 39/34 kd fragment of VWF (174 μg/mL) overnight at 4°C and subsequently blocked with 10% heat-inactivated human serum (containing 50 μg/mL phenylmethylsulfonyl fluoride) at room temperature for 60 minutes. Platelets in PWB (3 × 108/mL) were perfused through VWF-coated microcapillary tubes at 150 s−1 to enable platelet interaction with the VWF matrix. Activation inhibitors were removed by subsequent perfusion of modified Tyrode buffer (10 mM HEPES, pH 7.4, 12 mM NaHOC3, 137 mM NaCl, 2.7 M KCl, 5 mM glucose, 1 mM CaCl, and 1 mM MgCl) through the microcapillary tubes. Tether formation in translocating platelets was visualized by differential interference contrast microscopy (DMIRB Leica microscope; ×100 objective) and 10 random fields video-recorded for offline analysis. In some experiments, platelets were pretreated with the anti-integrin αIIbβ3 c7E3 Fab (20 μg/mL) or peptidomimetic Aggrastat (0.25 μg/mL) for 10 minutes to block ligand binding to integrin αIIbβ3. VWF binding to GP Ib/V/IX was blocked by pretreating platelets with the anti-GP Ibα antibody, AK2 (5 μg/mL). In experiments examining the effect of increasing shear on the rate and stability of tether formation, platelets were initially perfused through the microcapillary tubes at either 600 s−1, 1800 s−1, 5000 s−1, or 10 000 s−1. The flow was subsequently reduced to 150 s−1 for 5 seconds (to bring platelets in close proximity to the VWF surface) and the wall shear rate was subsequently increased back to its original rate. Under these conditions platelets were not observed to interact with the VWF surface at 150 s−1; however, upon increasing the wall shear rate, platelets were observed to rapidly interact and translocate across the VWF surface. In these experiments, platelet translocation velocity was determined by measuring the distance traveled (displacement) per unit time recorded. The video monitor was calibrated using a 0.01-μm stage micrometer (Olympus, Tokyo, Japan). Images were digitized using the Microcomputer Imaging Device (MCID) software (Imaging Research, Ontario, ON, Canada). To investigate the frequency of tether formation under high shear conditions (600 s−1, 1800 s−1, 5000 s−1, or 10 000 s−1), the flow assay was modified slightly. We have previously established that perfusing platelets through microcapillary tubes at high shear, in the absence of red blood cells, leads to minimal platelet-VWF interaction.16 In contrast, perfusing platelets through the capillary tubes at low flow rates, allowing the cells to come into close contact with the matrix under the influence of gravity, then suddenly exposing the cells to rapid increases in shear (ie, from 150 s−1 up to 10 000 s−1) resulted in platelets rapidly and efficiently tethering to the VWF surface. In other studies, platelets in PWB were preincubated with prostaglandin E1(0.5 μg/mL), vinblastine (10 μg/mL), or paclitaxel (2 μM) for 20 minutes prior to perfusion, or cytochalasin D (5 μM) for 10 minutes prior to perfusion. In studies examining the role of intracellular calcium in tether formation, platelets in PWB were treated with DM-BAPTA BAPTA (70 μM) for 30 minutes at 37°C. Platelets were subsequently pelleted at 2000g and resuspended in PWB in the presence of EGTA/Mg2+ (1 mM each) prior to perfusion through VWF-coated microcapillary tubes.
Immunofluorescence studies
Translocating platelets were fixed by perfusion of 4% formaldehyde in modified Tyrode buffer through microcapillary tubes for 2 minutes. Adherent cells were permeabilized with 2% Triton X-100 and stained with fluorescein isothiocyanate (FITC)–conjugated phalloidin or a monoclonal anti–β-tubulin antibody. Cells were washed with phosphate-buffered saline (PBS) prior to incubation with a CY5-conjugated anti–mouse antibody and imaged by confocal microscopy.
Scanning electron microscopy
Translocating platelets were fixed by perfusion of 2% gluteraldehyde in scanning electron microscopy buffer (100 mM Na2HPO4/NaH2PO4, pH 7.4) through microcapillary tubes for 2 minutes. The upper surface of the rectangular glass microcapillary tube was removed by scoring the glass with a diamond pen; the lower surface was retained. Adherent cells were dehydrated and critical point dried as described previously,22 then mounted on scanning electron microscopy stubs and coated with gold. Stubs were stored under desiccation until imaging on a Hitachi S570 scanning electron microscope (Tokyo, Japan) at 15 kV accelerating voltage.
Statistical analysis
Significant differences were determined using a Studentt test using the Prism software package (GraphPAD Software for Science, San Diego, CA).
Results
Tether formation during platelet translocation on VWF
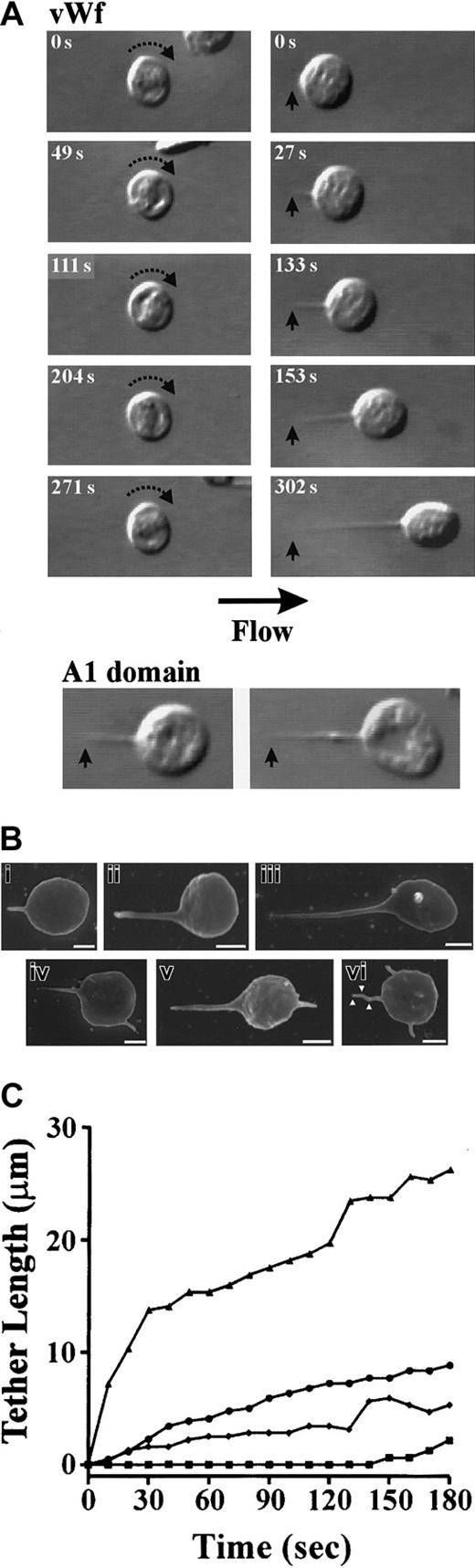
To examine the morphology of platelets during surface translocation on VWF, initial studies were performed on washed platelets under low shear conditions (150 s−1). We have previously established that these conditions allow high-resolution imaging of platelets in the absence of other blood cells.16 As demonstrated in Figure1A, platelets initially tethered to VWF as flat-disc shaped cells and translocated in a continuous stop-start manner over the VWF surface, as described previously.4 16In some cases, platelets underwent prolonged periods of stationary adhesion (> 10 seconds) that resulted in the development of single elongated membrane extensions (tethers) at the trailing edge of the cell (Figure 1A). These membrane tethers developed from small, localized adhesion contacts formed between the cell body and VWF matrix. The formation of adhesion contact points caused a slight teardrop-shaped deformation of the platelet membrane as the cell body gradually pulled away in response to the drag forces created by flow (Figure 1A). As a result, long, thin membrane tethers were seen to extend between the attachment point and the cell body (Figure 1A). In this regard, they were distinct from filopodia, as tethers were always aligned parallel to the direction of flow, with the tether attachment point facing upstream and the cell body downstream. In contrast, filopodia did not form as a result of a direct interaction between the membrane and VWF matrix, but tended to randomly extend out from the cell body in all directions as freely mobile membrane protrusions. The formation of membrane tethers was not dependent on VWF engagement of integrin αIIbβ3, as pretreating platelets with the integrin αIIbβ3 antagonists, c7E3 Fab, or Aggrastat had no inhibitory effect on tether formation. Further evidence that membrane tethers were primarily induced by the VWF–GP Ib/V/IX interaction was obtained from studies using the 39/34 proteolytic fragment of VWF, containing the GP Ibα binding domain (A1 domain). Translocation of platelets on isolated A1 domain resulted in the formation of membrane tethers that were similar in length and morphology to those observed on full-length VWF (Figure 1A). The extension of tethers on A1 domain was specific to GP Ibα as platelet adhesion was fully blocked with the anti-GP Ibα antibody ALMA12 (20 μg/mL).
Tether formation during platelet translocation on immobilized VWF.
Washed platelets (3 × 108/mL) were perfused through microcapillary tubes coated with VWF (100 μg/mL) or isolated 39/34 kd fragment of VWF (A1 domain) (174 μg/mL) at 150 s−1 as described in “Materials and methods.” (A) Digitized images of translocating platelets. VWF: During platelet translocation on immobilized VWF, 2 distinct behaviors were observed. Platelets either translocated continuously with no morphologic changes observed (left panels) or pulled fine membrane tethers (right panels). Tethers developed from specialized adhesion contacts (arrow) that did not move during tether formation. A1 domain: differential interference contrast microscopy (DIC) images of membrane tethers formed on isolated 39/34 kd fragment of VWF. (B) Scanning electron micrographs of platelet tethers. Tethers of varying lengths were pulled from the surface of translocating platelets (i-iii) and in some cases, platelets with 2 or more membrane protrusions were observed (iv-vi; see text for details). Multiple adhesion contact points (vi; white arrowheads) were also observed along the length of one tether, resulting in the generation of kinked tethers (Scale bar equals 1 μm). (C) Time-dependent change in tether length (μm) of 4 individual platelets.
Tether formation during platelet translocation on immobilized VWF.
Washed platelets (3 × 108/mL) were perfused through microcapillary tubes coated with VWF (100 μg/mL) or isolated 39/34 kd fragment of VWF (A1 domain) (174 μg/mL) at 150 s−1 as described in “Materials and methods.” (A) Digitized images of translocating platelets. VWF: During platelet translocation on immobilized VWF, 2 distinct behaviors were observed. Platelets either translocated continuously with no morphologic changes observed (left panels) or pulled fine membrane tethers (right panels). Tethers developed from specialized adhesion contacts (arrow) that did not move during tether formation. A1 domain: differential interference contrast microscopy (DIC) images of membrane tethers formed on isolated 39/34 kd fragment of VWF. (B) Scanning electron micrographs of platelet tethers. Tethers of varying lengths were pulled from the surface of translocating platelets (i-iii) and in some cases, platelets with 2 or more membrane protrusions were observed (iv-vi; see text for details). Multiple adhesion contact points (vi; white arrowheads) were also observed along the length of one tether, resulting in the generation of kinked tethers (Scale bar equals 1 μm). (C) Time-dependent change in tether length (μm) of 4 individual platelets.
Analysis of tethers by scanning electron microscopy revealed that the majority of membrane tethers formed at 150 s−1 were of similar width (mean ± SEM: 0.261 μm ± 0.01 μm; range: 0.16 μm-0.38 μm); however, the length varied considerably from 0.91 μm-10.44 μm (mean ± SEM: 3.23 μm ± 0.29 μm) (Figure1B). In some cases, platelets contained 2 or more membrane protrusions (Figure 1Biv-vi). Real-time analysis of individual platelets revealed that at any single time point, platelets only developed a single membrane tether from the point of initial contact with the VWF surface (data not shown). However, following detachment of the tether from the matrix, platelets subsequently formed a new adhesive contact point, resulting in a new membrane tether. Thus multiple membrane protrusions appear to form as a result of translation of adhesion contact points during surface translocation, with nonanchored projections representing remnants of previous tethers (see below). Furthermore, while only a single tether could form at any one time, this tether could form multiple adhesion contact points with the matrix along the entire length of the tether, resulting in the generation of kinked tethers (Figure 1Bvi).
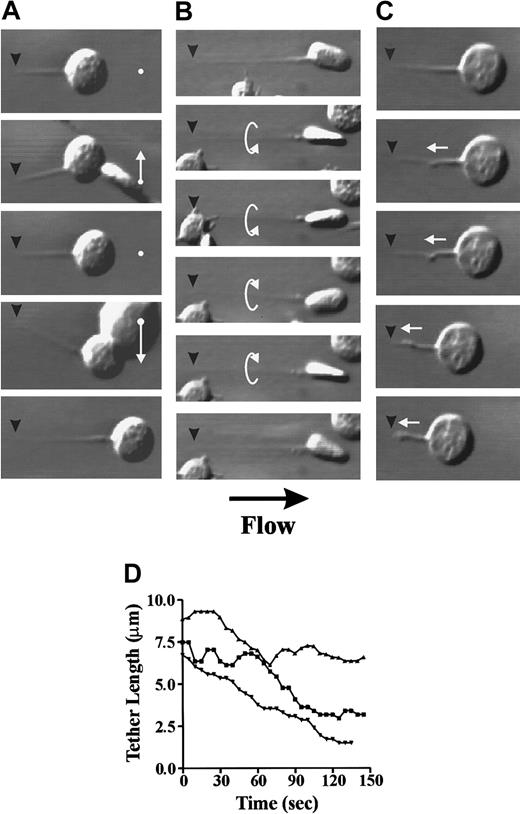
Real-time analysis of tether formation at low wall shear rates (150 s−1) revealed that in the majority of platelets, tether development was a gradual process requiring several minutes before reaching maximal tether length (Figure 1A,C). However, occasionally more rapid tether formation was observed, with platelets developing extensive membrane tethers within 10 seconds to 30 seconds (Figure 1C). In either case, maintaining stable platelet arrest during tether formation was found to be critically dependent on the attachment point, as this became the single contact point between the cell and the VWF surface. As a result, the cell body and tether were able to move freely in response to flow conditions. Tethered platelets moved in a side-to-side swinging-type motion that was induced by collision with other translocating platelets (Figure 2A) or in a rotational movement around the axis of the tether itself (Figure 2B). The strength of the tether attachment point was evident by its ability to resist the destabilizing forces generated by shear or bombardment by other translocating platelets. Furthermore, these adhesion contacts were able to sustain stable adhesion of platelets during tether contraction (Figure 2C), where the cell body was pulled against the resistive drag of flow. In this case, the tether folded back in on itself, forming membrane folds that subsequently fused together. This resulted in the formation of thicker tethers that pulled the cell body toward the tether attachment point; however, like tether formation, contraction of the membrane tethers occurred gradually over a 1-minute to 2-minute time frame under these experimental conditions (Figure 2D).
Stability and contractility of membrane tethers.
Washed platelets (3 × 108/mL) were perfused through VWF-coated (100 μg/mL) microcapillary tubes at 150 s−1 as described in “Materials and methods.” Once a tether attachment point (black arrowheads) is formed between a translocating platelet and the VWF matrix, this becomes the single point of contact during tether elongation. As a result, the cell body is able to move freely either in a side-to-side swinging motion (white arrows) induced by collision with other translocating platelets (A) or in a rotational manner around the axis of the tether (B). The tether attachment point is able to sustain stable platelet adhesion during tether contraction where the cell body is pulled against the resistive drag of flow (C). (D) Time-dependent contraction of tethers from 3 representative platelets.
Stability and contractility of membrane tethers.
Washed platelets (3 × 108/mL) were perfused through VWF-coated (100 μg/mL) microcapillary tubes at 150 s−1 as described in “Materials and methods.” Once a tether attachment point (black arrowheads) is formed between a translocating platelet and the VWF matrix, this becomes the single point of contact during tether elongation. As a result, the cell body is able to move freely either in a side-to-side swinging motion (white arrows) induced by collision with other translocating platelets (A) or in a rotational manner around the axis of the tether (B). The tether attachment point is able to sustain stable platelet adhesion during tether contraction where the cell body is pulled against the resistive drag of flow (C). (D) Time-dependent contraction of tethers from 3 representative platelets.
Effect of shear on tether formation and stability in translocating platelets
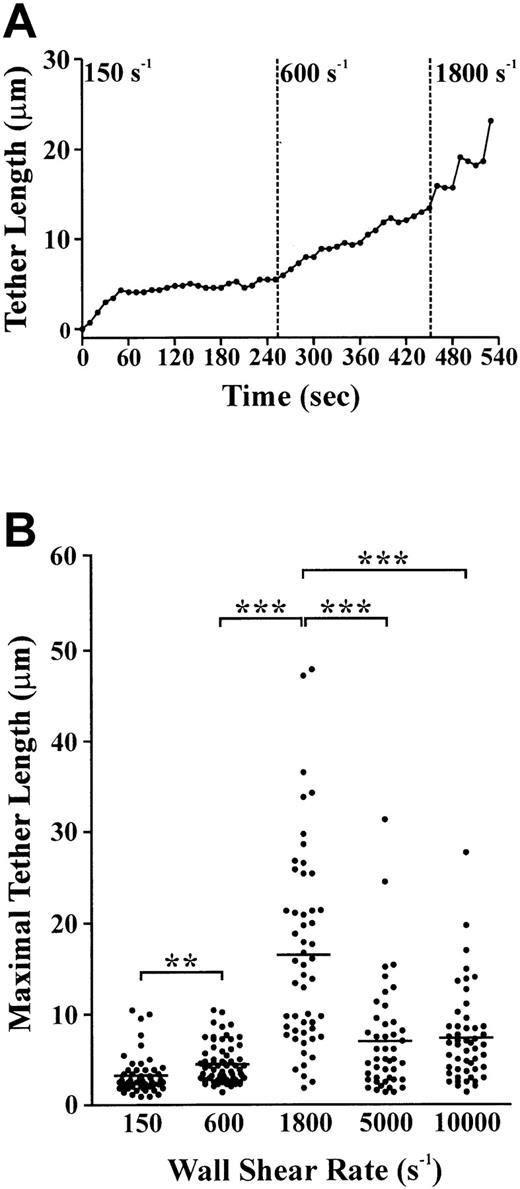
The strength of the tether attachment point was examined more closely by assessing the effect of increasing shear force on its ability to resist detachment and maintain stable platelet adhesion to immobilized VWF. As demonstrated in Figure3A, a stably adherent platelet developed a membrane tether over a 60-second time period, after which the tether length increased slowly. This plateau in tether growth rate after initial tether formation was a consistent finding in all platelets examined under low shear conditions. Incremental increases in the wall shear rate from 150 s−1 to 600 s−1 to 1800 s−1 resulted in a corresponding increase in tether length from 5.4 μm to 12.9 μm to 23.3 μm, respectively. However, at 1800 s−1 the tether contact points were unable to sustain stationary platelet adhesion. This shear-induced conversion of tethered platelets from stably adherent to rolling cells involved releasing the tether contact point from the matrix. At no stage was the membrane tether observed to detach from the cell body (data not shown). Instead, the tether participated in the translocation process along with the cell body (Figure 4A), or alternatively, retracted back into the cell (Figure 4B). The retraction process involved contraction of the tether into small membrane blebs along the length of the tether, gradually receding back into the cell (Figure 4B,C).
Effect of shear on tether length and stability.
(A) Single-cell analysis of tether length as a function of shear. A membrane tether is pulled from a single platelet over a 50-second time period after which the tether length is maintained. Increasing the wall shear rate from 150 s−1 to 600 s−1 to 1800 s−1 results in a corresponding increase in tether length. After 80 seconds at 1800 s−1, the tether detaches, allowing the cell to reinitiate translocation. (B) Washed platelets were perfused through VWF-coated microcapillary tubes at 150 s−1 for 5 seconds, after which the wall shear rate was either maintained at 150 s−1 or increased to 600 s−1, 1800 s−1, 5000 s−1, or 10 000 s−1 for analysis of tether length. The maximal tether length was determined immediately prior to release of the tether attachment point. These results are from 4 independent experiments (P < .05; P < .001).
Effect of shear on tether length and stability.
(A) Single-cell analysis of tether length as a function of shear. A membrane tether is pulled from a single platelet over a 50-second time period after which the tether length is maintained. Increasing the wall shear rate from 150 s−1 to 600 s−1 to 1800 s−1 results in a corresponding increase in tether length. After 80 seconds at 1800 s−1, the tether detaches, allowing the cell to reinitiate translocation. (B) Washed platelets were perfused through VWF-coated microcapillary tubes at 150 s−1 for 5 seconds, after which the wall shear rate was either maintained at 150 s−1 or increased to 600 s−1, 1800 s−1, 5000 s−1, or 10 000 s−1 for analysis of tether length. The maximal tether length was determined immediately prior to release of the tether attachment point. These results are from 4 independent experiments (P < .05; P < .001).
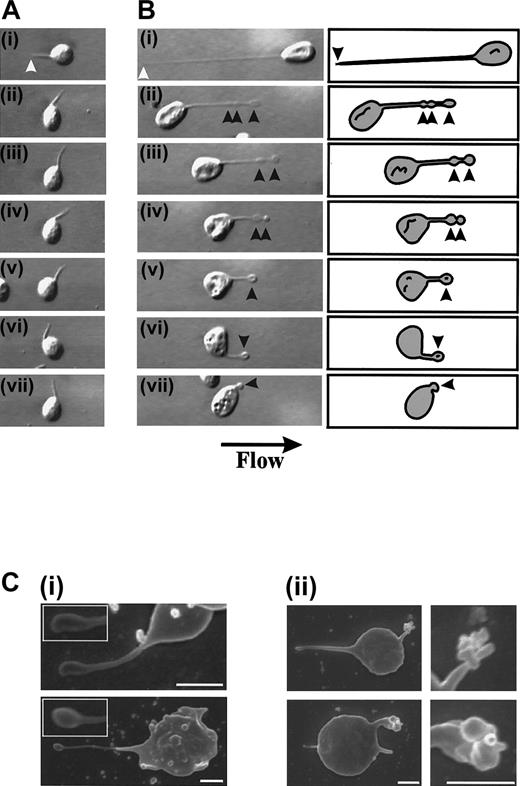
Retraction of membrane tethers.
Washed platelets (3 × 108/mL) were perfused through VWF-coated (100 μg/mL) microcapillary tubes at 150 s−1and the wall shear rate increased to 1800 s−1 to induce tether detachment. (A,B) (i) Membrane tethers formed on immobilized VWF. The white arrowheads refer to the tether attachment point. (ii-vii) The tether attachment point releases its contact with the VWF matrix, causing the platelet to translocate in the direction of flow. Tethers either remained extended, actively participating in the translocation process (A), or alternatively, retracted back into the body of the cell (B). Tether retraction occurred through the formation of flat, ball-like structures along the length of the tether (arrowheads) while receding into the cell body. (C) Scanning electron micrographs demonstrating the early (i) and late phases (ii) of tether retraction (scale bar equals 1 μm).
Retraction of membrane tethers.
Washed platelets (3 × 108/mL) were perfused through VWF-coated (100 μg/mL) microcapillary tubes at 150 s−1and the wall shear rate increased to 1800 s−1 to induce tether detachment. (A,B) (i) Membrane tethers formed on immobilized VWF. The white arrowheads refer to the tether attachment point. (ii-vii) The tether attachment point releases its contact with the VWF matrix, causing the platelet to translocate in the direction of flow. Tethers either remained extended, actively participating in the translocation process (A), or alternatively, retracted back into the body of the cell (B). Tether retraction occurred through the formation of flat, ball-like structures along the length of the tether (arrowheads) while receding into the cell body. (C) Scanning electron micrographs demonstrating the early (i) and late phases (ii) of tether retraction (scale bar equals 1 μm).
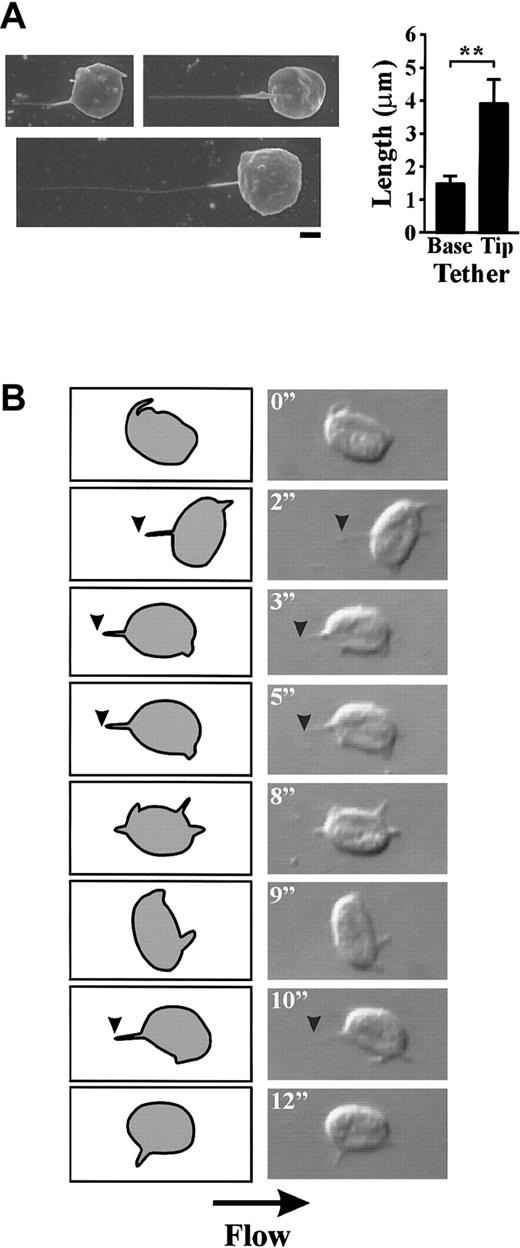
In further studies, we examined whether translocating platelets were able to form membrane tethers at shear rates of more than 150 s−1. As demonstrated in Figure 3B, translocating platelets formed membrane attachment points and tethers at all wall shear rates examined. Quantitation of the length of membrane tethers revealed a shear-dependent increase in the mean ± SEM tether length from 3.23 μM ± 0.29 μM at 150 s−1 up to 16.55 μM ± 1.5 μM at 1800 s−1. At wall shear rates greater than 1800 s−1 there was a significant decrease in the length of tethers formed (mean ± SEM: 7.00 μm ± 0.90 μm [5000 s−1]; 7.36 μm ± 0.72 μm [10 000 s−1]) (Figure 3B). Interestingly, this decrease in tether length correlated with a change in the morphology of the tethers, with the width appearing to be much thinner than those forming at 150 s−1 to 600 s−1. Visualization of tethers formed at 5000 s−1 by scanning electron microscopy revealed that these tethers were distinct from those formed at lower shear conditions (refer to Figure 1B) in that they were thicker at the base of the cell body (mean ± SEM: 0.22 μm ± 0.022 μm) than at the distal end of the tether (mean ± SEM: 0.04 μm ± 0.01 μm). Furthermore, the proximal thicker end of the tether was significantly shorter (mean length ± SEM: 1.48 μm ± 0.24 μm) than the distal thinner end (mean length ± SEM: 3.91 μm ± 0.74 μm) (Figure5A).
Dynamics of tether formation under high shear conditions.
Washed platelets (3 × 108/mL) were perfused through VWF-coated (100 μg/mL) microcapillary tubes at 5000 s−1(A) Scanning electron micrographs of membrane tethers (scale bar equals 1 μm). These tethers consisted of a thicker proximal end that was significantly shorter than the thinner distal end (graph) (P < .05). (B) Digitized images (right panels) and corresponding schematics (left panels) of a platelet forming multiple membrane tethers during surface translocation at 5000 s−1. During translocation, a specialized adhesion contact forms (arrowheads) at 2 inches resulting in the formation of a thin membrane tether. This tether is rapidly released and a second membrane tether forms at 3 inches. This tether remains attached to the matrix for a slightly longer period of time (2 seconds), then detaches and a third tether forms at 10 inches.
Dynamics of tether formation under high shear conditions.
Washed platelets (3 × 108/mL) were perfused through VWF-coated (100 μg/mL) microcapillary tubes at 5000 s−1(A) Scanning electron micrographs of membrane tethers (scale bar equals 1 μm). These tethers consisted of a thicker proximal end that was significantly shorter than the thinner distal end (graph) (P < .05). (B) Digitized images (right panels) and corresponding schematics (left panels) of a platelet forming multiple membrane tethers during surface translocation at 5000 s−1. During translocation, a specialized adhesion contact forms (arrowheads) at 2 inches resulting in the formation of a thin membrane tether. This tether is rapidly released and a second membrane tether forms at 3 inches. This tether remains attached to the matrix for a slightly longer period of time (2 seconds), then detaches and a third tether forms at 10 inches.
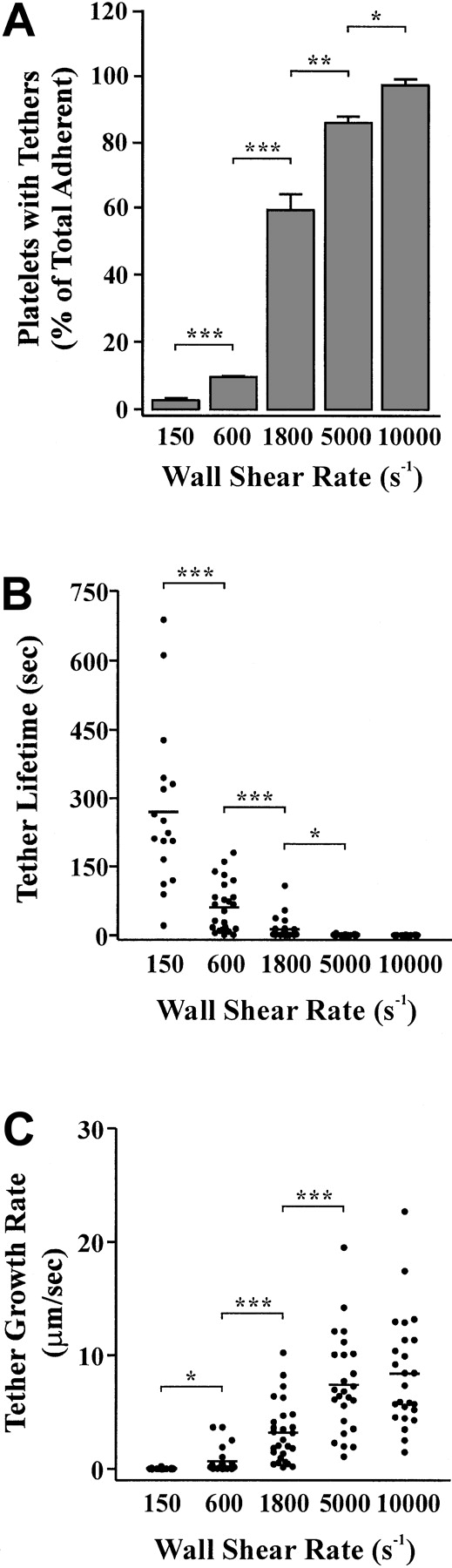
Analysis of individual platelets during surface translocation at high wall shear rates (5000 s−1) revealed the dynamic and rapid nature of tether formation. As demonstrated in Figure 5B, a translocating platelet formed 3 separate tether attachment points (at 2 seconds, 3 seconds, and 10 seconds) over a 12-second time period. These adhesive contacts resulted in the rapid formation of thin membrane tethers that subsequently detached from the matrix and retracted back into the cell body (Figure 5B). The retraction process appeared to occur in a similar manner to that described at lower shear conditions (refer to Figure 4B) with the membrane contracting into ball-like structures that then fully retracted back into the cell body. In contrast to our findings at low shear, these tethers retracted rapidly (1-2 seconds) in a “springlike” manner. Analysis of the frequency of tether formation demonstrated the shear-dependency of this phenomenon. For example, at a low shear rate (150 s−1), less than 5% of translocating cells developed membrane tethers, whereas at 10 000 s−1 more than 95% of cells extended these processes (Figure 6A). Although the formation of membrane adhesive contacts and tethers occurred far more readily under high shear conditions (5000 s−1-10 000 s−1), they were unable to sustain stable platelet adhesion for periods of more than 2 seconds (Figure 2B). As demonstrated in Figure 6B, the average time of tether duration (lifetime) decreased significantly with increasing shear (Figure 6B). This decrease in tether lifetime was associated with an increase in the average tether growth rate from 0.04 μm/sec at 150 s−1 up to 8.39 μm/sec at 10 000 s−1 (Figure 6C).
Effect of wall shear rate on tether frequency, lifetime, and growth rate.
Washed platelets were perfused through VWF-coated (100 μg/mL) microcapillary tubes at either 150 s−1, 600 s−1, 1800 s−1, 5000 s−1, or 10 000 s−1. Translocating platelets from 10 random fields were video-recorded for analysis of tether frequency (A), lifetime (B), or growth rate (C) as described in “Materials and methods.” These results are from 3 independent experiments ( P < .1;P < .05; P < .001).
Effect of wall shear rate on tether frequency, lifetime, and growth rate.
Washed platelets were perfused through VWF-coated (100 μg/mL) microcapillary tubes at either 150 s−1, 600 s−1, 1800 s−1, 5000 s−1, or 10 000 s−1. Translocating platelets from 10 random fields were video-recorded for analysis of tether frequency (A), lifetime (B), or growth rate (C) as described in “Materials and methods.” These results are from 3 independent experiments ( P < .1;P < .05; P < .001).
Effect of tether formation on platelet translocation
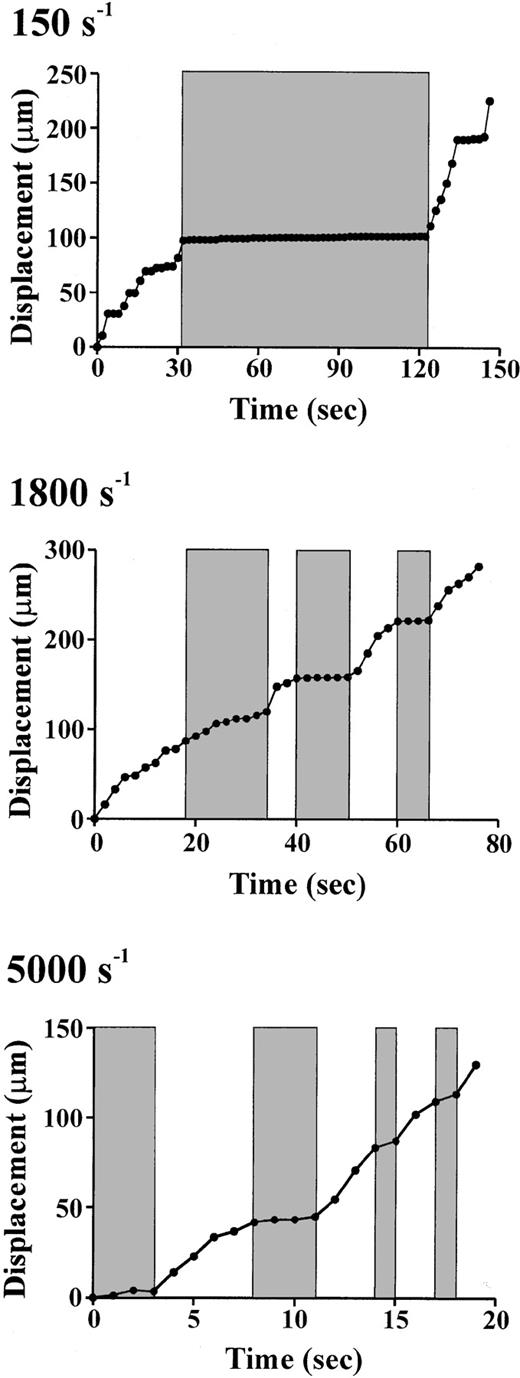
To gain insight into the potential role of tether formation in regulating the dynamics of the platelet-VWF interaction, we examined the velocity of platelet translocation before, during, and after tether formation. As demonstrated in Figure 7, platelets translocated in a stop-start manner over a broad range of shear conditions (150 s−1-5000 s−1). At low shear rates (150 s−1), the formation of a membrane tether (shaded region) resulted in a sudden cessation in platelet movement followed by a slight increase in the displacement of the cell centroid over time as the tether gradually lengthens. At the point in which the tether attachment point is unable to maintain stable adhesion with the matrix (124"), the platelet reinitiates surface translocation. It should be noted that not all stationary phases were associated with membrane tether formation (eg, see Figure 7; 18-28 seconds and 134-146 seconds), raising the possibility that additional mechanisms regulate the dynamics of the platelet-VWF interaction. In contrast to low shear, at 1800 s−1 and 5000 s−1 tether development was far more rapid (occurring over a few seconds) and continuous, with multiple tethers forming during translocation (shaded regions in Figure7). In most cases, the development of tethers at these higher wall shear rates correlated with the short periods of stationary adhesion or the “stop” phase of the translocation process, whereas the “start” phase of translocation was a result of the tether releasing its contact point from the matrix. However, when extremely long membrane tethers developed rapidly (eg, Figure 7; first tether at 1800 s−1 [18 seconds]), a significant difference in translocation velocity prior to and during tether formation was not observed.
Role of tether formation in regulating the dynamics of platelet translocation.
Membrane tether dynamics regulate the stop-start pattern of platelet translocation at low (150 s−1), intermediate (1800 s−1), and high (5000 s−1) shear rates. Real-time analysis of the displacement (μm) of the cell centroid demonstrates that the formation of membrane tethers (shaded regions) results in a distinct pause in platelet translocation (stop phase), whereas release of tethers is associated with platelet translocation in the direction of flow (start phase) (unshaded regions). Note that each shaded bar at 1800 s−1 and 5000 s−1represents the formation of a new membrane tether.
Role of tether formation in regulating the dynamics of platelet translocation.
Membrane tether dynamics regulate the stop-start pattern of platelet translocation at low (150 s−1), intermediate (1800 s−1), and high (5000 s−1) shear rates. Real-time analysis of the displacement (μm) of the cell centroid demonstrates that the formation of membrane tethers (shaded regions) results in a distinct pause in platelet translocation (stop phase), whereas release of tethers is associated with platelet translocation in the direction of flow (start phase) (unshaded regions). Note that each shaded bar at 1800 s−1 and 5000 s−1represents the formation of a new membrane tether.
Role of the cytoskeleton in regulating platelet tether formation
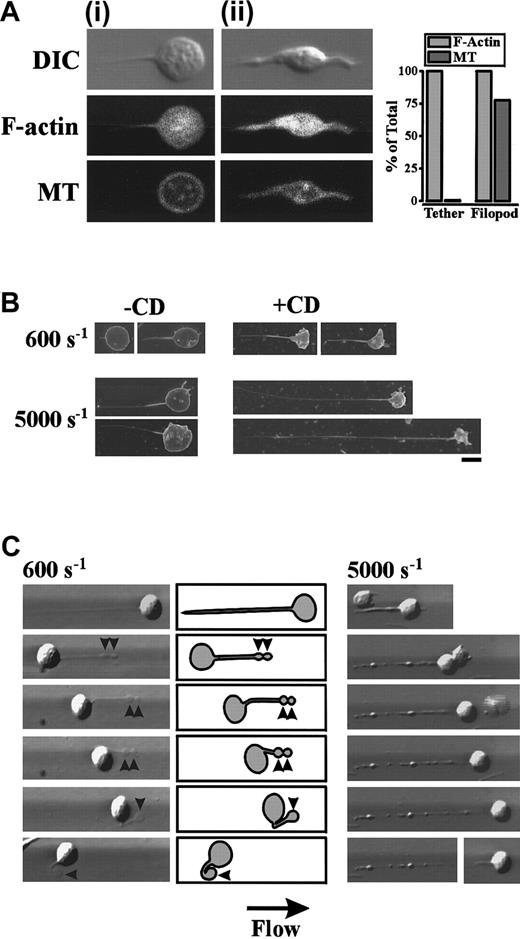
In further studies, we examined the role of the platelet cytoskeleton in promoting membrane tether formation. Initially we examined for the presence of actin filaments and/or microtubular structures within membrane tethers. As demonstrated in Figure8A, staining of the actin cytoskeleton with FITC-conjugated phalloidin revealed that all cells exhibited a diffuse pattern of staining within the cell body and membrane tether. In contrast, immunofluorescent analysis of the same cells using a β-tubulin antibody revealed a distinct microtubule ring around the circumference, with no staining of microtubules within the membrane tether (Figure 8A). In contrast, filopodial projections in translocating platelets stained strongly for both actin filaments and microtubules (Figure 8Aii), further highlighting the structural differences between filopodia and membrane tethers.
Role of the platelet cytoskeleton in regulating tether formation.
(A) Washed platelets were perfused through VWF-coated microcapillary tubes at 150 s−1 for 5 seconds, after which the wall shear rate was increased to 600 s−1. Translocating platelets containing either membrane tethers (Ai) or filopodial projections (Aii) were fixed and stained with FITC-conjugated phalloidin (F-actin) and an anti–β-tubulin (MT) antibody. Platelet morphology was visualized by DIC. The percentage of platelets with F-actin or microtubular staining within membrane tethers and filopodia was quantified from 105 platelets (bar graph). (B,C) Washed platelets were pretreated with vehicle alone or cytochalasin D (5 μM) prior to perfusion through VWF-coated microcapillary tubes at 150 s−1 for 5 seconds. The wall shear rate was either maintained at 150 s−1 or increased to 600 s−1, 1800 s−1, 5000 s−1, or 10 000 s−1. (B) Scanning electron micrographs of representative cells forming tethers at 600 s−1 and 5000 s−1 in the absence (-CD) or presence (+CD) of cytochalasin D (scale bar equals 1 μm). (C) 600 s−1: Digitized images (left panels) and corresponding schematics (right panels) demonstrating that cytochalasin D has no effect on tether release or retraction under low shear conditions. Once released, the tether contracts into flat ball-like structures (arrowheads) that gradually recede into the cell body. 5000 s−1: Cytochalasin D treatment of platelets results in the formation of unstable tethers at higher wall shear rates. Initially a fine, long membrane tether is pulled from the platelet surface that becomes progressively thicker as membrane pulls from the cell body into the tether. Further extension of this thicker membrane tether results in the formation of rounded ball-like structures along the length of the tether resulting in a beadlike appearance that becomes increasingly unstable. Ultimately the cell body detaches from the tether and reinitiates its surface translocation.
Role of the platelet cytoskeleton in regulating tether formation.
(A) Washed platelets were perfused through VWF-coated microcapillary tubes at 150 s−1 for 5 seconds, after which the wall shear rate was increased to 600 s−1. Translocating platelets containing either membrane tethers (Ai) or filopodial projections (Aii) were fixed and stained with FITC-conjugated phalloidin (F-actin) and an anti–β-tubulin (MT) antibody. Platelet morphology was visualized by DIC. The percentage of platelets with F-actin or microtubular staining within membrane tethers and filopodia was quantified from 105 platelets (bar graph). (B,C) Washed platelets were pretreated with vehicle alone or cytochalasin D (5 μM) prior to perfusion through VWF-coated microcapillary tubes at 150 s−1 for 5 seconds. The wall shear rate was either maintained at 150 s−1 or increased to 600 s−1, 1800 s−1, 5000 s−1, or 10 000 s−1. (B) Scanning electron micrographs of representative cells forming tethers at 600 s−1 and 5000 s−1 in the absence (-CD) or presence (+CD) of cytochalasin D (scale bar equals 1 μm). (C) 600 s−1: Digitized images (left panels) and corresponding schematics (right panels) demonstrating that cytochalasin D has no effect on tether release or retraction under low shear conditions. Once released, the tether contracts into flat ball-like structures (arrowheads) that gradually recede into the cell body. 5000 s−1: Cytochalasin D treatment of platelets results in the formation of unstable tethers at higher wall shear rates. Initially a fine, long membrane tether is pulled from the platelet surface that becomes progressively thicker as membrane pulls from the cell body into the tether. Further extension of this thicker membrane tether results in the formation of rounded ball-like structures along the length of the tether resulting in a beadlike appearance that becomes increasingly unstable. Ultimately the cell body detaches from the tether and reinitiates its surface translocation.
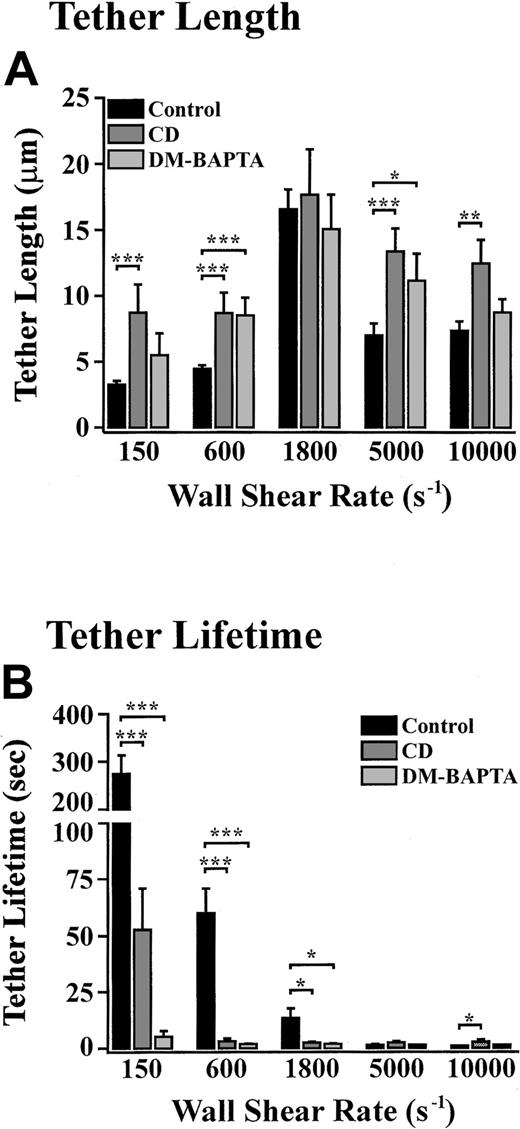
To investigate the potential importance of the actin cytoskeleton in regulating tether formation and retraction, platelets were pretreated with the actin polymerisation inhibitor, cytochalasin D. Under all shear conditions (150 s−1-10 000 s−1), cytochalasin D did not prevent the formation of membrane tethers (Figure 8B and data not shown). However, at low wall shear rates, (150 s−1-600 s−1) tethers forming in the presence of cytochalasin D were finer and significantly longer than those observed in untreated platelets (Figure 8B and Figure 9A). Cytochalasin D treatment of platelets also had a significant effect on tether duration, with the average tether lifetime reduced by 80.6% and 95.0% at 150 s−1 and 600 s−1, respectively (Figure9B). Analysis of tether formation in real time revealed that cytochalasin D did not affect tether retraction following release of the tether attachment point at these low wall shear rates. This retraction occurred in a similar manner to that described for untreated platelets, where the extended membrane tether contracted into flat, ball-like structures that gradually receded into the cell body (Figure 8C; 600 s−1). In contrast, at higher wall shear rates (5000 s−1-10 000 s−1) translocating platelets initially pulled fine membrane tethers that were also significantly longer than those formed from untreated platelets (Figure 8B and Figure 9A). The tether lifetime of cytochalasin D–treated platelets under these rapid flow conditions was also prolonged compared with that observed with untreated platelets (Figure 9B). However, once formed, these extended membrane tethers became progressively thicker as the membrane from the cell body was pulled into the tether itself. Further extension of these thicker membrane tethers resulted in the formation of rounded ball-like structures along the length of the tether resulting in a beadlike appearance (Figure 8C). These irregular membrane tethers were unable to remain associated with the cell body at such high wall shear rates, and as such, the cell body could be seen to detach from the tether and reinitiate its surface translocation (Figure 8C, 5000 s−1, lowest panel). Tethers that had detached from the surface of cytochalasin D–treated platelets also translocated in the direction of blood flow, often depositing small membrane fragments along the VWF matrix. In contrast to the effects of cytochalasin D, pretreating platelets with the microtubule disrupting agent, vinblastine, or the microtubule stabilizer, paclitaxel, had no effect on tether growth rate, dimensions, lifetime, retraction, or stability at all shear rates examined (150 s−1-10 000 s−1) (data not shown).
Role of cytoskeletal reorganization and intracellular calcium in regulating tether dynamics.
Platelets were pretreated with vehicle alone (control), cytochalasin D (CD) (5 μM), or DM-BAPTA (70 μM) prior to perfusion through VWF-coated microcapillary tubes at 150 s−1 for 5 seconds. The wall shear rate was either maintained at 150 s−1 or increased to 600 s−1, 1800 s−1, 5000 s−1, or 10 000 s−1. Translocating platelets from 10 random fields were video-recorded for analysis of tether length (A) and lifetime (B). These results are from 3 independent experiments (P < .1; P < .05;P < .001).
Role of cytoskeletal reorganization and intracellular calcium in regulating tether dynamics.
Platelets were pretreated with vehicle alone (control), cytochalasin D (CD) (5 μM), or DM-BAPTA (70 μM) prior to perfusion through VWF-coated microcapillary tubes at 150 s−1 for 5 seconds. The wall shear rate was either maintained at 150 s−1 or increased to 600 s−1, 1800 s−1, 5000 s−1, or 10 000 s−1. Translocating platelets from 10 random fields were video-recorded for analysis of tether length (A) and lifetime (B). These results are from 3 independent experiments (P < .1; P < .05;P < .001).
Role of platelet activation and calcium in regulating tether dynamics
We have recently demonstrated that VWF engagement of GP Ib induces increases in cytosolic calcium and reorganization of actin filaments, via signaling processes sensitive to the inhibitory effects of cAMP.16 To investigate the ability of elevated levels of cAMP to inhibit the formation of membrane tethers, platelet translocation studies were performed in the presence of the cAMP phosphodiesterase inhibitor, theophylline. Interestingly, perfusion of platelets under these conditions had no effect on the rate, extent, or morphology of tethers pulled from the surface of translocating platelets (data not shown). Furthermore, combining theophylline with a stimulator of adenylyl cyclase, prostaglandin E1, also had no effect on tether formation. Chelating cytosolic calcium, by pretreating platelets with DM-BAPTA, did not inhibit tether formation; however, these tethers were longer (Figure 9A) and finer (data not shown), similar to those observed with cytochalasin D. However, unlike cytochalasin D, DM-BAPTA did not induce the formation of unstable tethers. Reducing cytosolic calcium with DM-BAPTA did not affect the retraction of membrane tethers but did have a significant effect on the shear-dependent decrease in tether lifetime observed in control platelets (Figure 9B). As a result, the average lifetime of tethers was significantly shorter at 150 s−1, 600 s−1, and 1800 s−1 (4.86 sec, 1.52 sec, and 1.51 sec, respectively) than in untreated controls (270.0 sec-13.07 sec).
Discussion
The studies presented here demonstrate a new platelet phenomenon, the shear-dependent elongation of membrane tethers during surface translocation on immobilized VWF. Membrane tethers are dynamic structures that extend from small, localized adhesion contacts under the influence of hydrodynamic shear stress. Interestingly, all aspects of tether dynamics, including tether growth rate and dimensions, lifetime of adhesive contact with the matrix, and retractile dynamics are influenced by the shear conditions experienced by the cell. Tether formation appears to regulate, at least in part, the stop-start nature of platelet translocation on VWF, raising the interesting possibility that tether formation represents a previously hitherto unrecognized mechanism regulating platelet adhesion under flow.
Recent studies have demonstrated that neutrophils also form membrane tethers during surface translocation,15 although in these cells, tether formation is dependent on the interaction between PSGL-1 and immobilized P-selectin. In contrast to the VWF–GP Ib interaction, PSGL-1–P-selectin bonds can only support neutrophil tethering and rolling under low shear conditions (50 s−1-300 s−1), thereby restricting analysis of the effects of hemodynamic forces on neutrophil tethers to a relatively narrow shear range. Despite this, we have noted important similarities between neutrophils and platelets with respect to tether dynamics. In both cells, the rate of tether formation and adhesive contact lifetime is dependent on the level of shear. Furthermore, in both of these cells, tethers were observed to retract back into the cell following release of the tether attachment point. Although direct comparison of shear forces operating on these cells is difficult, due to marked differences in cell shape and size (discoid platelets, diameter 1 μm-2 μm; round neutrophils, diameter ∼14 μm), the neutrophil is likely to experience shear forces at least 50-fold greater than platelets, based on the formula F = 32r2τ (where τ is the shear stress).23 These differences may partially explain why platelets develop membrane tethers slowly (mean: 0.042 μm/sec) and display a prolonged lifetime (275 sec) at a low shear (150 s−1) compared with neutrophil tether growth rates of ∼6 μm/sec and tether lifetimes of less than 1 sec at 100 s−1. Furthermore, under these low shear conditions, the frequency of tether formation previously reported for neutrophils was far greater (frequency: 32%) than in platelets (mean: 2.67%). Interestingly, subjecting platelets to shear forces approximating those experienced by neutrophils, by increasing the wall shear rate by ∼50-fold (5000 s−1-10 000 s−1), resulted in a dramatic transformation in tether dynamics, with platelets displaying a more rapid tether growth rate (mean: 7.89 μm/sec) and shorter lifetime (mean: 0.87 sec), similar to those reported in neutrophils.
It is of interest that less than 5% of platelets at low shear developed membrane tethers during surface translocation. This low frequency of tether formation is not due to the presence of a distinct subpopulation of “tether-forming” platelets, as increasing the wall shear rate up to 10 000 s−1 resulted in nearly all cells developing tethers. Furthermore, our analysis of tether formation during platelet rolling revealed that not every GP Ib/V/IX–VWF interaction results in tether formation. Instead, platelets often translocated continuously for several seconds without forming observable membrane tethers. The explanation for this apparent heterogeneity is not known, although one possibility is that multiple GP Ib/V/IX receptors are required to bind simultaneously in order to induce and maintain stable platelet adhesion to immobilized VWF. This has previously been proposed for neutrophils, where clusters of L-selectin and PSGL-1 localized at the tips of surface microvilli mediate adhesion and tether formation. However, this mechanism may not be relevant to platelets as they do not exhibit any microvilluslike membrane projections, nor has it been reported that GP Ib/V/IX exists in clusters on the platelet surface. An alternative possibility is that differences in the association of GP Ib/V/IX receptors with the underlying membrane cytoskeleton influence their propensity to promote tether formation. Up to 30% of GP Ib/V/IX complexes have been demonstrated not to be associated with the membrane skeleton.24 It is possible that exerting a pulling force on receptors with little or no cytoskeletal association may result in deformation of the plasma membrane and tether formation. Such a concept has previously been suggested to play an important role in determining whether neutrophil microvillus extension results in tether formation.14 15
The results presented here demonstrate for the first time that the VWF–GP Ib/V/IX interaction can support prolonged stationary cell adhesion (> 10 seconds) under physiologic flow conditions, independent of integrin αIIbβ3, through the generation of membrane tethers. Two lines of evidence suggest that this prolonged interaction was not due to incomplete blockade of integrin αIIbβ3. First, exposing tethered platelets to high wall shear rates (5000 s−1) always induced rapid disruption (< 5 seconds) of the adhesive contacts. In contrast, this was never observed with platelets forming stationary adhesion contacts in the absence of a blocking integrin αIIbβ3 antibody (S.M.D. and S.P.J., unpublished data, 2001). Second, prolonged adhesion contacts were also observed with platelets interacting with isolated A1 domains. The ability of platelets to form prolonged adhesion contacts at physiologic shear rates is of particular interest in light of the intrinsic rapid kinetics of the VWF–GP Ib interaction. Under all shear conditions, the rapid association rate of the VWF–GP Ib bonds leads to efficient platelet capture on the adhesive surface, whereas the rapid rate of bond dissociation favors continual translation of adhesion contacts, resulting in platelet translocation. It is unlikely that the adhesive contact points responsible for tether formation have distinct kinetic properties from other VWF–GP Ib bonds, as this would be expected to result in prolonged adhesion contact over all shear rates tested. It is more likely that clustering or other cooperative effects between adhesive bonds promotes sustained cell adhesion. Future studies will be required to address this issue.
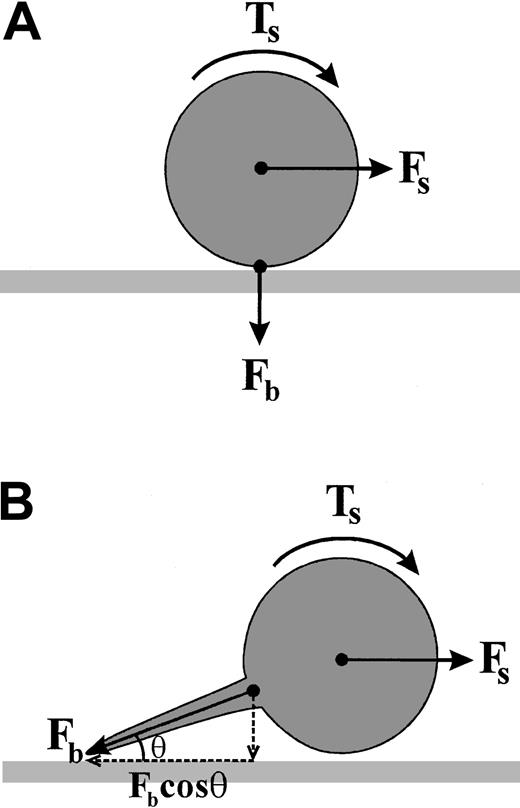
The low percentage of platelets forming tethers under low shear conditions suggests that this phenomena is unlikely to play a major role in regulating platelet translocation velocity in the venous circulation. However, at high shear the vast majority of platelets develop membrane tethers, raising the interesting possibility that tether formation is a key step in the platelet adhesion process under high shear. These observations may not be surprising given that tether formation has been proposed to reduce the level of force exerted on adhesive bonds.14 As demonstrated in Figure10, in the case of reversible adhesive interactions, such as those formed between VWF and GP Ib/V/IX, hydrodymanic forces generated by flowing blood rapidly undermine the formation of stationary adhesion contacts. By forming tethers, adhesive bonds more effectively oppose shear forces by increasing the moment arm (see Figure 10 legend for detailed explanation). Thus platelets, as well as leukocytes, appear to have evolved tethering mechanisms that serve to retard the velocity of cell translocation, albeit transiently, thereby increasing the probability of receptors with intrinsically slow kinetics (ie, integrins) forming irreversible adhesion contacts.
Model depicting the mechanism by which tether formation reduces the force on adhesive bonds.
(A) A cell adherent within a flow field experiences a hydrodynamic shear force (Fs) that generates a torque (Ts) about the centroid of the cell. In order for the cell to remain stably adherent, the force of the adhesive bond (Fb) must oppose the shear force (Fs) in an equal and opposite manner. The ability of Fb to oppose Fs is in part dependent on the off rate of this adhesive interaction. Bonds that have an intrinsically rapid dissociation rate, such as the GP Ib/V/IX–VWF interaction, are unable to oppose FS, leading to cell translocation in the direction of flow. (B) Tether formation between the cell body and the adhesive surface gives mechanical advantage to the adhesive bond. The bond force Fb is composed of both horizontal and vertical components, dependent on the angle θ, to which it is applied at the adhesive surface. Tether formation increases the horizontal component of Fb (Fbcosθ) opposing Fs. In the case of a stationary cell within a flow field, the horizontal component of Fb must be equal and opposite to Fs. As the tether length increases, the proportion of Fb directly opposing Fs increases, thereby increasing the likelihood that the adhesive contact will remain stable. It should be noted that this model deals with the static situation in which no work is done. However, the dynamic process of tether formation, that is elongation of the plasma membrane, potentially involves considerable work, with the elastic energy stored in the tether also opposing the applied shear force.
Model depicting the mechanism by which tether formation reduces the force on adhesive bonds.
(A) A cell adherent within a flow field experiences a hydrodynamic shear force (Fs) that generates a torque (Ts) about the centroid of the cell. In order for the cell to remain stably adherent, the force of the adhesive bond (Fb) must oppose the shear force (Fs) in an equal and opposite manner. The ability of Fb to oppose Fs is in part dependent on the off rate of this adhesive interaction. Bonds that have an intrinsically rapid dissociation rate, such as the GP Ib/V/IX–VWF interaction, are unable to oppose FS, leading to cell translocation in the direction of flow. (B) Tether formation between the cell body and the adhesive surface gives mechanical advantage to the adhesive bond. The bond force Fb is composed of both horizontal and vertical components, dependent on the angle θ, to which it is applied at the adhesive surface. Tether formation increases the horizontal component of Fb (Fbcosθ) opposing Fs. In the case of a stationary cell within a flow field, the horizontal component of Fb must be equal and opposite to Fs. As the tether length increases, the proportion of Fb directly opposing Fs increases, thereby increasing the likelihood that the adhesive contact will remain stable. It should be noted that this model deals with the static situation in which no work is done. However, the dynamic process of tether formation, that is elongation of the plasma membrane, potentially involves considerable work, with the elastic energy stored in the tether also opposing the applied shear force.
We thank Prof Michael Berndt and Dr Francois Lanza for helpful suggestions and the generous donation of monoclonal antibodies.
Supported by grants from the National Health and Medical Research Council of Australia, National Heart Foundation of Australia, and the Welcome Trust (London, UK).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shaun P. Jackson, Australian Centre for Blood Diseases, Monash Medical School, Box Hill Hospital, Box Hill, Victoria 3127, Australia; e-mail: shaun.jackson@med.monash.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal