Expression of tissue transglutaminase (transglutaminase II, tTG) was shown to increase drastically during monocyte differentiation into macrophages; however, its role in monocytic cells remains largely unknown. This study describes a novel function of cell surface tTG as an adhesion and migration receptor for fibronectin (Fn). Two structurally related transglutaminases, tTG and the A subunit of factor XIII (FXIIIA), are expressed on the surface of monocytic cells, whereas only surface tTG is associated with multiple integrins of the β1 and β3 subfamilies. Both surface levels of tTG and the amounts of integrin-bound tTG are sharply up-regulated during the conversion of monocytes into macrophages. In contrast, a reduction in biosynthesis and surface expression of FXIIIA accompanies monocyte differentiation. Cell surface tTG is colocalized with β1- and β3-integrins in podosomelike adhesive structures of macrophages adherent on Fn. Down-regulation of surface tTG by expression of antisense tTG construct or its inhibition by function-blocking antibodies significantly decreases adhesion and spreading of monocytic cells on Fn and, in particular, on the gelatin-binding fragment of Fn consisting of modules I6II1,2I7-9. Likewise, interfering with the adhesive function of surface tTG markedly reduces migration of myeloid cells on Fn and its gelatin-binding fragment. These data demonstrate that cell surface tTG serves as an integrin-associated adhesion receptor that might be involved in extravasation and migration of monocytic cells into tissues containing Fn matrices during inflammation.
Introduction
Monocytic cells are involved in a variety of immune and inflammatory processes. A number of proinflammatory cytokines can trigger extravasation of monocytes and stimulate their invasion into inflamed tissues where these cells play a key role in a local immune response.1-5 Several subsets of adhesion molecules on the surface of monocytes are involved in different stages of this multistep process. This involves rolling of monocytic cells along vascular endothelium, arrest and initial adhesion to endothelium and subsequent strong adhesion, and spreading and transmigration of monocytes across the endothelial monolayer as well as invasion into underlying tissues.6-8 As adhesion receptors, integrins participate in multiple aspects of monocyte adhesion and transmigration across the endothelial monolayer. The α4β1-integrin expressed on monocytic cells is involved in the arrest and initial adhesion of these cells to endothelium by binding to vascular cell adhesion molecule-1 (VCAM-1).9 In addition, binding of α4β1- and α4β7-integrins to the alternatively spliced connecting segment-1 (CS-1) domain of fibronectin (Fn) contributes to monocyte-endothelial interactions.10-15 The integrins of the β2 subfamily, αLβ2 and αMβ2, are also present on monocytes16 and because of interaction with intercellular adhesion molecule-1 (ICAM-1) mediate tight adhesion to the endothelial cells.7,17,18 Finally, the αVβ3-integrin promotes transmigration of monocytes through endothelial monolayers, likely by down-regulation of adhesive function of β2-integrins.8
Tissue transglutaminase (tTG) is a member of transglutaminase family of enzymes that covalently cross-link proteins in a Ca++-dependent manner.19 In addition, tTG has a guanosine triphosphatase (GTPase) activity20 and is involved in intracellular signaling via agonist-mediated interactions with α1B- and α1D-adrenergic receptors21 and downstream effectors such as phospholipase Cδ1.22 tTG localizes mainly in the cytoplasm, yet some amounts of the enzyme are present on the cell surface and in the extracellular matrix (ECM). tTG is able to bind and cross-link several ECM proteins.23-28 This function of extracellular tTG might help to stabilize the ECM and basement membranes.26 Among various ECM proteins, the interaction of tTG with Fn is best characterized. tTG binds to Fn in vitro with high affinity (Kd ∼8 nM) and 2:1 stoichiometry.25 This interaction is mediated by a 42-kd gelatin-binding fragment of Fn that consists of modules I6II1,2I7-9 and lacks any known integrin-binding motifs.25,29 Previous studies implicated cell surface tTG in cell adhesion and spreading although the molecular basis for this function remained unclear.30-32 We recently demonstrated that tTG interacts with integrins of the β1 and β3 subfamilies and integrin-tTG complexes are detected both inside the cell during biosynthesis and accumulate on the cell surface.33 Surface tTG mediates interaction of integrins with Fn acting as a bridge between the adhesion receptor and its ECM ligand. Thus, it serves as integrin-binding coreceptor for Fn on fibroblastic cells to promote cell adhesion and, possibly, other integrin-dependent functions.33
tTG accumulates rapidly to very high levels, up to 0.1% to 1% of total cellular protein, due to a strong increase in its biosynthesis, following induction of monocyte differentiation by adhesion to a substrate,34 various cytokines, and serum retinoids35,36 or lipopolysaccharide.34,37Similarly, an approximate 50-fold up-regulation of tTG was observed in the human monocytic leukemia cell line THP-1 after stimulation with 12-O-tetradecanoyl-phorbol-13-acetate (TPA).38 The induction of tTG protein is a specific response of monocytic cells that strictly correlates with the extent of their morphologic and functional differentiation.34,38 Transglutaminase activity in macrophages was suggested to participate in Fc receptor–mediated phagocytosis.39-41 Yet, there is a lack of information regarding the role of tTG in monocytic cell functions. Another member of transglutaminase family, the A (catalytic) subunit of coagulation protein factor XIII (FXIIIA) is also expressed by monocytes42-44; however, its functions in this cell type remain unknown. Here we demonstrate that monocyte differentiation into macrophages is accompanied by a sharp increase in the level of integrin-associated surface tTG. Cell surface tTG is colocalized with β1- and β3-integrins in podosomes, specialized adhesive structures of adherent macrophages. Down-regulation of surface tTG by expression of antisense tTG construct or its inhibition by function-blocking antibodies leads to a significant and specific decrease in adhesion and migration of monocytic cells on Fn. This suggests that interaction of integrin-bound surface tTG with the gelatin-binding domain of Fn can contribute to adhesion and migration of macrophages on Fn-containing matrices during extravasation and invasion into subendothelial tissues.
Materials and methods
Complementary DNA, antibodies, ECM proteins, and Fn fragments
Complementary DNA (cDNA) for human endothelial tTG was described earlier.45 A 1.1-kilobase (kb) fragment of tTG cDNA, cloned in antisense orientation into pcDNA 3.1neo plasmid (Invitrogen, Carlsbad, CA), was shown to efficiently inhibit expression of endogenous tTG in ECV304 endothelial cells.46 Purified human plasma Fn and its 110-kd cell-binding (III2-11) and 42-kd gelatin-binding (I6II1,2I7-9) fragments were kindly provided by Dr Kenneth Ingham (Department of Biochemistry, American Red Cross). Human laminin and human type I collagen were obtained from Gibco BRL (Rockville, MD). Anti-tTG monoclonal antibodies (mAb) CUB7402 and TG100 (Neomarkers, Freemont, CA) and rabbit polyclonal antibodies against the NH2-terminal domain of tTG(1-165) were previously shown to interfere with tTG-Fn interaction.33 In addition, we generated a novel mAb 4G3 against the Fn-binding domain of tTG, tTG(1-165), which inhibits the binding of tTG to Fn. Rabbit antibody against FXIIIA was from Calbiochem (La Jolla, CA). Several function-blocking antibodies against human integrins were used. These included anti–β1-integrin mAb JB1A, anti–β2-integrin mAb YFC118.3, and anti–β3-integrin mAb B3A (all from Chemicon, Temecula, CA). The mAb 10.1 against human CD64 (α chain of FcγRI subtype of Fc receptor) was from BD Pharmingen (San Diego, CA). The mAb 7F9 against human vinculin was described previously.33Rhodamine-phalloidin (Molecular Probes, Eugene, OR) was used to visualize F-actin.
Cell isolation, culture, and transfection
Human peripheral blood monocytes were obtained from healthy donors as described.47,48 Peripheral blood was diluted 1:2.5 in phosphate-buffered saline (PBS) and 30 mL was layered onto 15-mL Ficoll-Paque Plus (Amersham Pharmacia, Piscataway, NJ) and centrifuged at 400g for 35 minutes at 18°C. Mononuclear cells at the interface between the separating medium and the plasma were collected and washed 3 times with HEPES-buffered RPMI-1640 containing 0.5% bovine serum albumin (BSA) and 2 mM EDTA. Monocytes were purified further by counterflow centrifugal elutriation using J-6B Beckman (Palo Alto, CA) centrifuge and JE-5.0 elutriation rotor.48 The fourth cell fraction collected for further work contained a 95% pure monocyte population. Monocytes were kept in suspension culture at 37°C in serum-free RPMI containing 10 mM HEPES, 50 μM β-mercaptoethanol, 50 μg/mL kanamycin, nonessential amino acids, and 0.5% BSA. Monocytes were used for adhesion and migration assays within 24 hours after isolation. Surface expression levels of β1-, β2-, and β3-integrins and tTG on monocytes remained unchanged during that period. THP-1 cells were obtained from American Type Culture Collection (ATCC, Rockville, MD) and were grown as suspension culture in RPMI containing 10% fetal bovine serum (FBS), 10 mM HEPES, 50 μM β-mercaptoethanol, and antibiotics. For in vitro differentiation, THP-1 cells were stimulated with 150 ng/mL TPA and plated in the same medium on Fn-coated dishes. THP-1 cells (8 × 106 cells/mL) in Opti-MEM medium (Gibco BRL) were transfected by electroporation with pcDNA 3.1neo plasmid or with the same plasmid containing the 1.1-kb tTG cDNA insert in antisense orientation using 0.4-cm cuvettes (Invitrogen) and Gene Pulser II (Bio-Rad, Hercules, CA) at 250 V and 960 μF. The resulting pools of transfectants, termed THP-vector and THP-antisense, were selected for 4 to 5 weeks in 300 μg/mL G418 and tested for expression of tTG.
Metabolic labeling and cell surface biotinylation
Untreated THP-1 cells in suspension and TPA-treated THP-1 cells adherent on Fn were incubated for 12 hours in methionine, cysteine-minus Dulbecco modified Eagle medium (DMEM) with 100 μCi/mL35S-Translabel (ICN Biologicals, Irvine, CA). The35S-labeled cells were washed several times with PBS before immunoprecipitation. Untreated or EDTA-detached TPA-treated THP-1 cells were surface biotinylated in suspension by incubating for 15 minutes with 0.2 mg/mL Sulfo-NHS-Biotin (Pierce, Rockford, IL) in PBS. The reaction was stopped by addition of 20 mM TrisCl, pH 7.5, and washing of cells with PBS from excess biotin.
Analysis of integrins, tTG, and FXIIIA by immunoprecipitation and immunoblotting
Adherent or suspended 35S-labeled or surface-biotinylated THP-1 cells were washed in PBS and lysed in ice-cold RIPA buffer, containing 1% Triton X-100, 0.5% Na-deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 150 mM NaCl, 50 mM TrisCl, pH 7.5, with 0.5 mM phenylmethylsulfonyl fluoride (PMSF), 0.5 mM benzamidine, 10 μg/mL leupeptin, and 10 μg/mL aprotinin. Cell lysates were precleared by centrifugation (14 000 rpm for 30 minutes at 4°C). Then 3 × 108 cpm of protein-incorporated radioactivity (for 35S-labeled cells) or 1 mg total cell protein (for surface-biotinylated cells) was taken for each immunoprecipitation sample with antibodies (4-8 μg/sample) against integrins, tTG, or FXIIIA, followed by protein G-Sepharose.
To visualize 35S-labeled proteins, SDS-polyacrylamide gel electrophoresis (SDS-PAGE) in 10% acrylamide/0.25% bis-acrylamide gels was followed by treatment of gels with Autofluor (Amersham Pharmacia) and fluorography. To detect biotinylated proteins, proteins separated by SDS-PAGE were transferred to polyvinylidene difluoride (PVDF) membrane. After blocking the membrane with 5% BSA in TBS/0.05%Tween-20, it was developed with avidin-peroxidase (Pierce). To prove the identity of tTG and FXIIIA in the samples, the same blots were probed with mAb TG100 against tTG or polyclonal antibody to the FXIIIA, respectively.
To determine the amounts of tTG and integrin-tTG complexes in THP-vector and THP-antisense cell populations, β1-, β2-, and β3-integrins and tTG were immunoprecipitated each from 1 mg RIPA cell lysates using 4 to 8 μg mAb JB1A, mAb Y118.3, mAb B3A, and polyclonal anti-tTG antibodies, respectively, followed by protein G-Sepharose. The immune complexes were separated by SDS-PAGE, transferred to PVDF membrane, and probed with anti-tTG mAb TG100.
Quantitation of the number of tTG molecules on the surface of monocytes and THP-1 cells
To determine the number of tTG molecules on the cell surface, THP-1 cells were kept in suspension without any treatment or treated with 150 ng/mL TPA and plated for indicated periods on Fn-coated dishes. Human monocytes were used as untreated cells or cells treated with 5 ng/mL macrophage colony-stimulating factor (M-CSF) and plated on Fn-coated dishes for indicated periods. Adherent cells were detached with EDTA and washed in DMEM/2% BSA before incubation with the label. Then 2 × 105 cells were incubated in suspension for 45 minutes at 4°C with 10 μg/mL 125I-labeled mAb 4G3 against tTG (specific activity 3 × 106 cpm/μg) in DMEM containing 2% BSA. To separate from unbound label, cells were centrifuged (10 000 rpm, 5 minutes, 4°C) through 400 μL 20% sucrose in DMEM. Cell-bound radioactivity was counted in a gamma counter. Incubation with 10-fold excess unlabeled mAb 4G3 was used to determine and subtract nonspecific binding. Three independent experiments were performed with duplicate samples. The number of surface tTG molecules was determined based on 1:1 stoichiometry for mAb 4G3 binding to tTG in the presence of excess 125I-labeled mAb 4G3.
Flow cytometry
For flow cytometry, live nonpermeabilized untreated or TPA-stimulated THP-1 cells plated on Fn for indicated periods and detached by EDTA were incubated for 1 hour at 4°C with 10 μg/mL polyclonal anti-tTG antibody, polyclonal anti-FXIIIA antibody, or mAbs against human β1-, β2-, or β3-integrins. After washing with PBS, cells were fixed with 3% paraformaldehyde in PBS, washed, and then incubated with secondary fluorescein-labeled IgG. The cells were analyzed in FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Similarly, untreated and TPA-treated THP-vector and THP-antisense cell populations plated on Fn for 72 hours were analyzed for surface tTG expression by incubation for 1 hour at 4°C with 10 μg/mL polyclonal anti-tTG antibody, followed by fluorescein-labeled IgG. Three independent determinations were performed for each cell surface protein, cell type, and treatment.
Immunofluorescence
To induce differentiation, THP-1 cells were treated with 150 ng/mL TPA and plated in RPMI containing 10% FBS on Fn-coated glass coverslips for 72 hours. Live nonpermeabilzed cells were double-stained with 10 μg/mL polyclonal anti-tTG antibody and 10 μg/mL mouse mAb JB1A against human β1-integrin, mAb B3A to human β3-integrin, or mAb 10.1 against FcγRI receptor. Following incubation with primary antibodies, cells were fixed with 3% paraformaldehyde in PBS. After several washes with PBS, cells were stained with a combination of fluorescein-labeled goat anti–rabbit IgG and rhodamine-conjugated goat anti–mouse IgG (Chemicon). To simultaneously visualize cell surface tTG and cytoskeletal proteins localized at podosomes, live nonpermeabilized cells were stained with 10 μg/mL polyclonal anti-tTG antibody and fluorescein-labeled goat anti–rabbit IgG. Then cells were washed, fixed with paraformaldehyde, permeabilized with 0.1% Triton X-100 in PBS, and costained with rhodamine-phalloidin or antivinculin mAb 7F9 followed by rhodamine-labeled goat anti–mouse IgG. Stained cells were analyzed and photographed using Eclipse E800 epifluorescence microscope (Nikon, Tokyo, Japan) and Spot RT digital camera (Diagnostic Instruments, Sterling Heights, MI).
Cell adhesion and spreading assays
The 24-well tissue culture plates (Costar, Cambridge, MA) were coated with 10 μg/mL laminin, collagen, BSA, Fn, and Fn fragments and then blocked with 0.5% BSA. For adhesion studies,35S-labeled THP-1 cells or human peripheral blood monocytes in serum-free AIM-V medium (Gibco BRL) containing 0.5% BSA were used. Cells were used either without stimulation or were pretreated for 1 hour with 150 ng/mL TPA (THP-1 cells) or for 4 hours with 5 ng/mL M-CSF (human monocytes). Some cell samples were preincubated for 1 hour at 4°C with 20 μg/mL blocking polyclonal anti-tTG antibody, mAbs 4G3 or CUB7402 against tTG, polyclonal antibody to FXIIIA, function-blocking mAbs against β1-, β2-, and β3-integrins or control nonimmune mouse IgG (all antibodies were purified IgG). Then 2 × 104 cells were plated on protein-coated wells for 1 hour at 37°C in serum-free AIM-V/0.5% BSA supplemented with either 150 ng/mL TPA (THP-1 cells) or 5 ng/mL M-CSF (monocytes) and respective antibodies. Adherent cells were washed 3 times with PBS and lysed in 100 μL hot 1% SDS. Bound radioactivity was counted in a scintillation counter and converted into cell numbers by referring to the levels of35S-radioactivity incorporated per 103 cells. Three independent experiments with duplicate determinations were performed for each cell type and treatment.
For analysis of cell spreading, unlabeled THP-1 cells were treated as indicated above and following plating in serum-free AIM-V/0.5% BSA on protein-coated wells were allowed to spread for 12 hours at 37°C. Cells were washed 3 times with PBS, fixed with 3% paraformaldehyde in PBS, and then stained with Coomassie brilliant blue, destained, and photographed.
Migrationassays
Directional migration of 35S-labeled cells (5 × 104 cells/insert) in Transwells (Costar) with 5-μm pores and the membrane undersurface coated with collagen I, laminin, Fn, the 42-kd and 110-kd Fn fragments, and BSA (10 μg/mL each) was analyzed under serum-free conditions using AIM-V medium with 0.5% BSA. To stimulate chemotactic migration of cells, 125 ng/mL monocyte chemoattractant protein-1 (MCP-1) was added to lower chambers of the Transwells. THP-1 cells were used either without stimulation or were treated with 150 ng/mL TPA immediately before the assay. Human monocytes were treated with 5 ng/mL M-CSF 4 hours before the assay. Some cell samples were preincubated for 1 hour at 4°C with 20 μg/mL blocking polyclonal anti-tTG antibody, mAbs 4G3 or CUB7402 against tTG, polyclonal antibody to FXIIIA, function-blocking mAbs against β1-, β2-, and β3-integrins or control nonimmune mouse IgG. All antibodies were used as purified IgG. Differentiation agents (TPA or M-CSF) and the respective antibodies were kept in the medium during the assay. Following incubation for 4 hours at 37°C, cells transmigrated from the upper chambers to the membrane undersurface. To remove cells from the upper chambers, they were washed twice with 0.25% trypsin. Transmigrated cells were harvested from the undersurface of the inserts, the medium, and the bottom surface of the wells. The number of transmigrated cells in each sample was determined by cell lysis in 100 μL hot 1% SDS and counting 35S-radioactivity in a scintillation counter. The35S-radioactivity was converted into the number of cells by referring to the levels of 35S-radioactivity incorporated per 103 cells. Three independent experiments with duplicated measurements were performed for each cell type, protein substrate, and treatment.
Results
Monocyte differentiation is accompanied by up-regulation of integrin-associated cell surface tTG
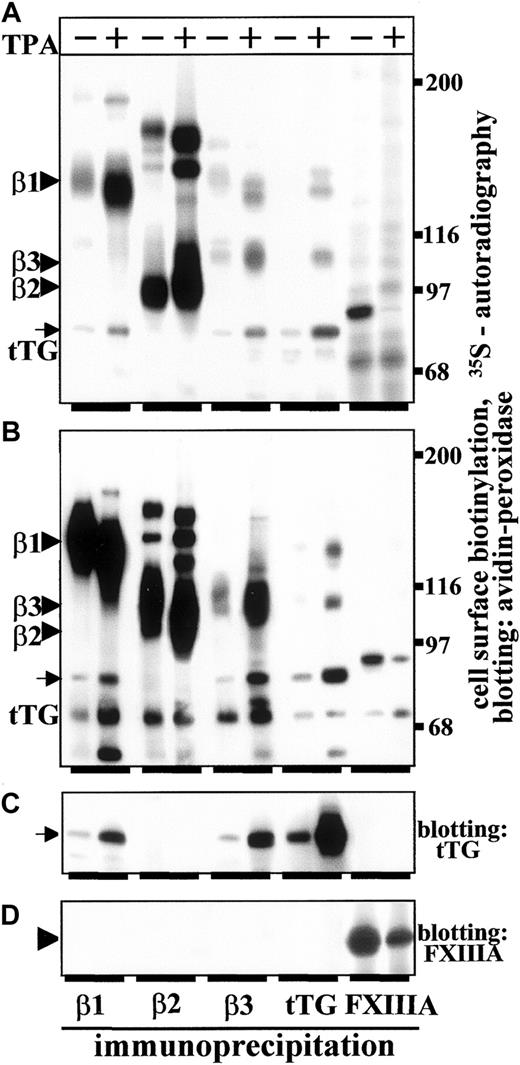
Earlier work demonstrated a drastic increase in tTG content and transglutaminase activity concomitant with monocyte conversion into macrophages.34,37-39,49 We recently showed that tTG can serve as an integrin-binding adhesion coreceptor for Fn on fibroblastic cells.33 Thus, using metabolic labeling and immunoprecipitation, we analyzed biosynthesis of tTG and association of tTG with integrins in untreated THP-1 cells in suspension and TPA-treated THP-1 cells plated on Fn for 3 days (Figure1A). Biosynthesis of tTG strongly increased, whereas the amounts of synthesized FXIIIA diminished following differentiation of THP-1 cells. We also detected an enhancement in biosynthesis of β1-, β2-, and β3-integrins in differentiated THP-1 macrophages. Notably, the amounts of tTG associated with β1- and β3-integrins were significantly elevated in TPA-treated adherent THP-1 cells (Figure 1A, arrow). In agreement with our previous data, we were unable to detect complexes of β2-integrins with tTG.33 These results were corroborated using biotinylation of cell surface proteins and immunoprecipitation of integrins, tTG, and FXIIIA from untreated and TPA-treated THP-1 cells (Figure 1B). Differentiation of THP-1 cells raised the expression levels of cell surface tTG (Figure 1B,C, arrows). In parallel, the amounts of cell surface tTG complexed with β1- and β3-integrins increased markedly in adherent TPA-treated THP-1 macrophages (Figure 1B,C, arrow). In contrast, a lack of association of FXIIIA with integrins and down-regulation of surface expression of FXIIIA were detected during differentiation of THP-1 cells (Figure 1B,D, arrowhead). Flow cytometry analysis of β1-, β2-, and β3-integrins, tTG, and FXIIIA on undifferentiated and TPA-differentiated THP-1 cells confirmed a strong approximate 8- to 10-fold increase in the amounts of surface tTG and down-regulation of surface FXIIIA (Table1).
Monocyte differentiation elevates biosynthesis of tTG and increases surface expression of tTG and the amounts of integrin-tTG complexes.
Untreated THP-1 cells (−TPA) and THP-1 cells induced to differentiate by plating on Fn for 72 hours in the presence of 150 ng/mL TPA (+TPA) were analyzed. The β1-, β2-, and β3-integrins, tTG, and FXIIIA were immunoprecipitated from RIPA lysates of 35S-labeled (A) or surface-biotinylated (B-D) untreated or TPA-treated cells. (A) Autoradiographs of 35S-labeled immune complexes analyzed by SDS-PAGE and fluorography. (B) Surface-biotinylated proteins were immunoprecipitated, separated by SDS-PAGE, and blots developed with avidin-peroxidase. To detect tTG and FXIIIA in the immunoprecipitates, the same blots as in panel B were developed with mAb TG100 against tTG (C) or polyclonal antibody to FXIIIA (D). Note an up-regulation of tTG biosynthesis and elevated amounts of tTG complexed with β1- and β3-integrins during biosynthesis in TPA-treated THP-1 cells (A, arrow). Similarly, there is an increase in surface-expressed tTG and the amounts of β1-integrin-tTG and β3-integrin-tTG complexes on the surface of THP-1 cells in response to TPA treatment (B,C, arrows). In contrast, there is a decrease in biosynthesis (A) and surface expression (B) of FXIIIA in TPA-treated THP-1 cells. Arrowhead in panel D points to FXIIIA, which does not associate with integrins. Molecular weight markers in panels A and B are shown to the right of the gels.
Monocyte differentiation elevates biosynthesis of tTG and increases surface expression of tTG and the amounts of integrin-tTG complexes.
Untreated THP-1 cells (−TPA) and THP-1 cells induced to differentiate by plating on Fn for 72 hours in the presence of 150 ng/mL TPA (+TPA) were analyzed. The β1-, β2-, and β3-integrins, tTG, and FXIIIA were immunoprecipitated from RIPA lysates of 35S-labeled (A) or surface-biotinylated (B-D) untreated or TPA-treated cells. (A) Autoradiographs of 35S-labeled immune complexes analyzed by SDS-PAGE and fluorography. (B) Surface-biotinylated proteins were immunoprecipitated, separated by SDS-PAGE, and blots developed with avidin-peroxidase. To detect tTG and FXIIIA in the immunoprecipitates, the same blots as in panel B were developed with mAb TG100 against tTG (C) or polyclonal antibody to FXIIIA (D). Note an up-regulation of tTG biosynthesis and elevated amounts of tTG complexed with β1- and β3-integrins during biosynthesis in TPA-treated THP-1 cells (A, arrow). Similarly, there is an increase in surface-expressed tTG and the amounts of β1-integrin-tTG and β3-integrin-tTG complexes on the surface of THP-1 cells in response to TPA treatment (B,C, arrows). In contrast, there is a decrease in biosynthesis (A) and surface expression (B) of FXIIIA in TPA-treated THP-1 cells. Arrowhead in panel D points to FXIIIA, which does not associate with integrins. Molecular weight markers in panels A and B are shown to the right of the gels.
Next, using binding of 125I-labeled anti-tTG mAb 4G3 to cells in suspension, we determined a time course of tTG cell surface expression in THP-1 cells during differentiation (Figure2A). The number of tTG molecules on the surface of THP-1 cells progressively increased from 6.7 ± 1.3 × 104 for unstimulated cells to 61.1 ± 12 × 104 for TPA-stimulated cells adherent to Fn. We also observed a biphasic effect of TPA on the expression of surface tTG. A first rapid phase resulted in an approximate 6-fold increase in surface tTG within 2 hours after stimulation (Figure 2A) and was not inhibited by treatment of cells with cycloheximide (data not shown). In contrast, a second phase of the increase of surface tTG entirely depended on de novo protein synthesis, started about 12 hours after cell stimulation with TPA and reached a plateau on day 3 of differentiation. We also determined the number of tTG molecules on the surface of human monocytes that were induced to differentiate with M-CSF (Figure 2B). Similarly, the number of surface tTG molecules on monocytes increased about 2-fold after 2 hours and about 5-fold after 72 hours of M-CSF treatment. Together, our data indicate that monocytic cells sharply up-regulate the amounts of integrin-associated cell surface tTG during differentiation.
Differentiation significantly increases the number of tTG molecules expressed on the surface of THP-1 monocytic cells and human peripheral blood monocytes.
THP-1-cells (A) or peripheral blood monocytes (B) were kept in suspension without treatment or plated on Fn and stimulated with 150 ng/mL TPA (A) or 5 ng/mL M-CSF (B) for indicated time periods. Cells (2 × 105) were incubated in suspension for 45 minutes at 4°C with 10 μg/mL 125I-labeled mAb 4G3 against tTG. The amounts of 125I-labeled mAb 4G3 bound to cell surfaces were determined in a gamma counter. The numbers of cell surface tTG molecules on THP-1 cells and human monocytes were determined by subtracting nonspecific binding in the presence of excess unlabeled mAb 4G3. Shown are the means of 3 separate experiments performed in duplicate.
Differentiation significantly increases the number of tTG molecules expressed on the surface of THP-1 monocytic cells and human peripheral blood monocytes.
THP-1-cells (A) or peripheral blood monocytes (B) were kept in suspension without treatment or plated on Fn and stimulated with 150 ng/mL TPA (A) or 5 ng/mL M-CSF (B) for indicated time periods. Cells (2 × 105) were incubated in suspension for 45 minutes at 4°C with 10 μg/mL 125I-labeled mAb 4G3 against tTG. The amounts of 125I-labeled mAb 4G3 bound to cell surfaces were determined in a gamma counter. The numbers of cell surface tTG molecules on THP-1 cells and human monocytes were determined by subtracting nonspecific binding in the presence of excess unlabeled mAb 4G3. Shown are the means of 3 separate experiments performed in duplicate.
tTG is codistributed with β1- and β3-integrins on the cell surface and is localized in podosomes, specialized adhesive structures of macrophages
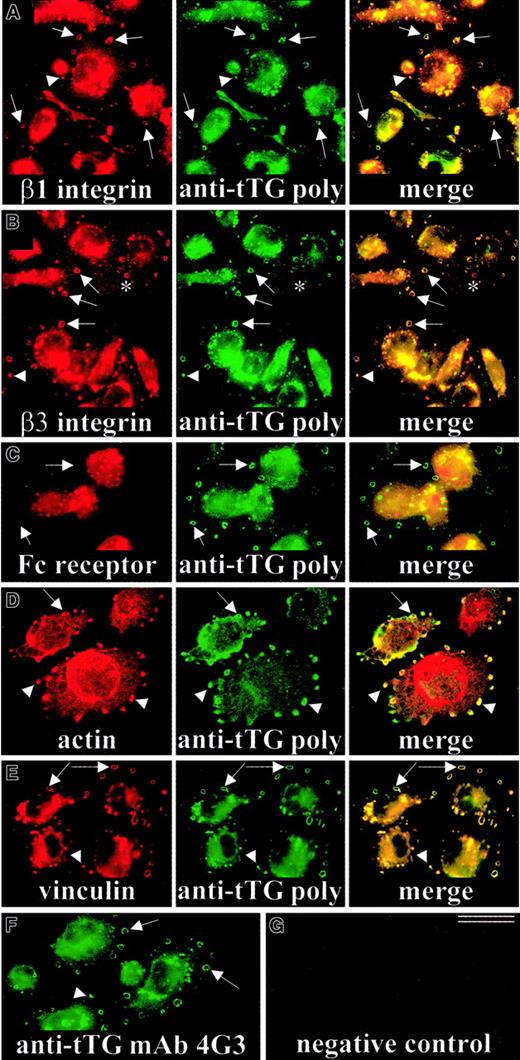
To determine localization of tTG on the cell surface, we immunostained live nonpermeabilized TPA-differentiated THP-1 cells that were plated for 3 days on Fn-coated coverslips (Figure3). tTG was abundant on the surface of THP-1 macrophages and was distinctly accumulated in ringlike structures (Figure 3A,B, arrows) and dotlike round-shaped adhesions (Figure 3A,B, arrowheads) along the cell periphery. Double-staining of differentiated THP-1 cells adherent on Fn for tTG and β1- or β3-integrins revealed their precise and extensive colocalization at podosomes. These specialized adhesive structures of macrophages are involved in adhesion, migration, and diapedesis.50-52 This localization was specific for cell surface adhesion receptors, because FcγRI receptor was not accumulated at macrophage podosomes (Figure 3C). Several cytoskeletal proteins such as actin and vinculin, which are concentrated at the cytoplasmic face of macrophage podosomes,50 also appeared codistributed with surface tTG in differentiated THP-1 cells, though to a somewhat lesser extent than integrins (Figure 3D,E, arrows, arrowheads). The mAb 4G3 also detected tTG in podosomes (Figure 3F), whereas no staining of these structures could be seen without primary antibody (Figure 3G). Thus, cell surface tTG is localized at podosomes, which serve as specialized cell-ECM adhesive contacts of macrophages.
Localization of tTG on the surface of THP-1 macrophages adherent on Fn.
THP-1 monocytic cells were induced with 150 ng/mL TPA and plated on Fn-coated coverslips for 72 hours. Live nonpermeabilized cells were double-stained for β1-integrins and tTG (A), β3-integrins and tTG (B), or FcγRI receptor and tTG using polyclonal anti-tTG antibody (C). Note an extensive colocalization of tTG with β1- and β3-integrins on the surface of THP-1 macrophages and their specific accumulation in podosomes (arrows, arrowheads). Alternatively, live nonpermeabilized cells were stained for surface tTG with polyclonal antibody, fixed, permeabilized, and then costained for F-actin (D) or vinculin (E). The mAb 4G3 also revealed localization of tTG in podosomes of THP-1 macrophages (F), whereas incubation without primary and with secondary antibody only (fluorescein isothiocyanate–labeled anti–rabbit IgG) resulted in negative background staining and no detectable staining of podosomes (G). Arrows point to ringlike and arrowheads mark round-shaped podosomes. Asterisk marks a podosome containing β3-integrins, but little or no tTG. Bar indicates 25 μm.
Localization of tTG on the surface of THP-1 macrophages adherent on Fn.
THP-1 monocytic cells were induced with 150 ng/mL TPA and plated on Fn-coated coverslips for 72 hours. Live nonpermeabilized cells were double-stained for β1-integrins and tTG (A), β3-integrins and tTG (B), or FcγRI receptor and tTG using polyclonal anti-tTG antibody (C). Note an extensive colocalization of tTG with β1- and β3-integrins on the surface of THP-1 macrophages and their specific accumulation in podosomes (arrows, arrowheads). Alternatively, live nonpermeabilized cells were stained for surface tTG with polyclonal antibody, fixed, permeabilized, and then costained for F-actin (D) or vinculin (E). The mAb 4G3 also revealed localization of tTG in podosomes of THP-1 macrophages (F), whereas incubation without primary and with secondary antibody only (fluorescein isothiocyanate–labeled anti–rabbit IgG) resulted in negative background staining and no detectable staining of podosomes (G). Arrows point to ringlike and arrowheads mark round-shaped podosomes. Asterisk marks a podosome containing β3-integrins, but little or no tTG. Bar indicates 25 μm.
Expression of antisense tTG construct in THP-1 cells reduces the level of cell surface tTG
Following transfection of antisense tTG construct into THP-1 cells and selection of stable transfectants, we analyzed the expression levels of tTG and the amounts of tTG associated with integrins in the transfectants by immunoprecipitation and immunoblotting (Figure4A). We observed a strong decrease in the total tTG content in THP-1 antisense transfectants differentiated for 72 hours in the presence of TPA, compared with vector-transfected THP-1 cells. This corresponded to significantly reduced amounts of tTG complexed with β1- and β3-integrins in the THP-antisense transfectants (Figure 4A, arrow). Analysis of surface tTG by immunostaining with polyclonal anti-tTG antibody and flow cytometry with live transfectants demonstrated an approximate 2- to 2.5-fold decrease on unstimulated and 4-fold decrease on TPA-induced THP-antisense transfectants compared with vector-transfected counterparts (Figure 4B). Thus, we generated THP-1 transfectants that express significantly reduced levels of surface tTG.
Expression of antisense tTG construct in THP-1 cells reduces tTG content, association of tTG with integrins, and tTG expression on the cell surface.
THP-1 cells were transfected with pcDNA 3.1 vector alone or with the same vector containing a 1.1-kb antisense tTG construct,46to generate stable populations of THP-vector and THP-antisense transfectants. (A) The β1-, β2-, and β3-integrins and tTG were immunoprecipitated from 1 mg RIPA cell lysates of TPA-treated transfectants. The amounts of tTG in the immunoprecipitates were determined by SDS-PAGE followed by immunoblotting with anti-tTG mAb TG100. Arrowhead points to tTG bands. Note a reduction of the tTG content and the amounts of tTG complexed with β1-integrins and β3-integrins, in the THP-antisense transfectants. (B) The levels of cell surface tTG were determined by staining of live nonpermeabilized untreated and TPA-treated transfectants with polyclonal anti-tTG antibody, followed by fluorescein-conjugated anti–rabbit IgG and flow cytometry. The amounts of surface tTG were compared to that of untreated THP-vector cells, which was taken as 100%. ░, THP-vector; ■, THP-antisense. Shown are the means of triplicate determinations.
Expression of antisense tTG construct in THP-1 cells reduces tTG content, association of tTG with integrins, and tTG expression on the cell surface.
THP-1 cells were transfected with pcDNA 3.1 vector alone or with the same vector containing a 1.1-kb antisense tTG construct,46to generate stable populations of THP-vector and THP-antisense transfectants. (A) The β1-, β2-, and β3-integrins and tTG were immunoprecipitated from 1 mg RIPA cell lysates of TPA-treated transfectants. The amounts of tTG in the immunoprecipitates were determined by SDS-PAGE followed by immunoblotting with anti-tTG mAb TG100. Arrowhead points to tTG bands. Note a reduction of the tTG content and the amounts of tTG complexed with β1-integrins and β3-integrins, in the THP-antisense transfectants. (B) The levels of cell surface tTG were determined by staining of live nonpermeabilized untreated and TPA-treated transfectants with polyclonal anti-tTG antibody, followed by fluorescein-conjugated anti–rabbit IgG and flow cytometry. The amounts of surface tTG were compared to that of untreated THP-vector cells, which was taken as 100%. ░, THP-vector; ■, THP-antisense. Shown are the means of triplicate determinations.
Down-regulation or inhibition of cell surface tTG specifically decreases adhesion of monocytic cells on Fn and its 42-kd gelatin-binding fragment
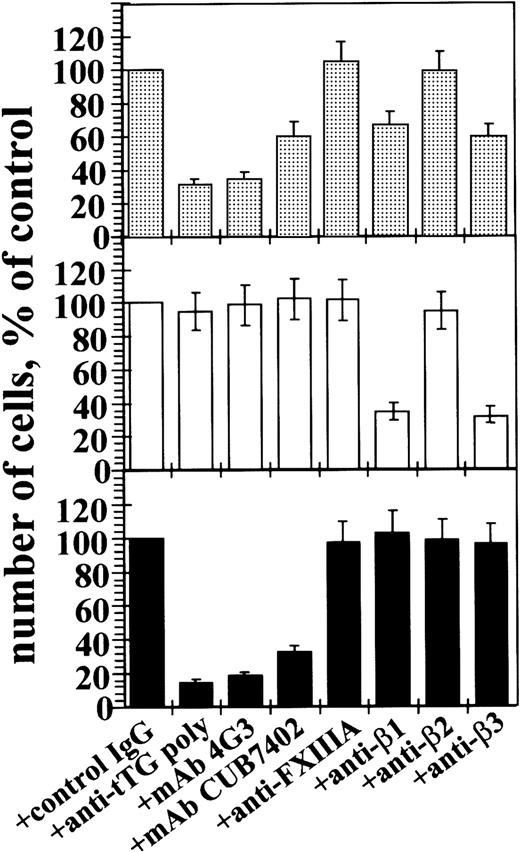
To define the role of surface tTG in adhesion of THP-1 monocytic cells, we performed quantitative adhesion assays with TPA- induced cells and several ECM proteins and Fn fragments (Figure 5, A,B).
Down-regulation or blocking cell surface tTG decreases adhesion of THP-1 cells and human peripheral blood monocytes on Fn and the 42-kd Fn fragment.
(A-C) Cells (2 × 104) were plated for 1 hour at 37°C in serum-free AIM-V medium containing 0.5% BSA on protein-coated wells. (A) THP-vector (░) and THP-antisense (▨) cells were stimulated for 1 hour with 150 ng/mL TPA and then allowed to adhere to wells coated with collagen type I (Col I), laminin (Ln), Fn, 110-kd or 42-kd Fn fragments, or BSA. Some samples of THP-vector cells were pretreated for 1 hour with function-blocking polyclonal anti-tTG antibody (■). (B) THP-vector (■) and THP-antisense cells (▧), either untreated or pretreated for 1 hour with 150 ng/mL TPA (░, vector; ▨, antisense), were allowed to adhere on Fn-coated wells. Some samples of TPA-stimulated THP-vector cells were preincubated for 1 hour with function-blocking polyclonal anti-tTG antibody, blocking mAbs against β1-, β2-, or β3-integrins, antibody against FXIIIA, or control nonimmune IgG. (C) Human peripheral blood monocytes were treated for 4 hours with 5 ng/mL M-CSF and then plated on wells coated with Fn (■) or the 42-kd fragment of Fn (▪). Monocyte samples were preincubated for 1 hour with control nonimmune IgG, function-blocking polyclonal anti-tTG antibody, mAb 4G3 against tTG, or blocking mAbs against β1-, β2-, or β3-integrins. (A-C) The antibodies were kept in the medium during the assay. Shown are the means of 3 separate experiments performed in duplicate.
Down-regulation or blocking cell surface tTG decreases adhesion of THP-1 cells and human peripheral blood monocytes on Fn and the 42-kd Fn fragment.
(A-C) Cells (2 × 104) were plated for 1 hour at 37°C in serum-free AIM-V medium containing 0.5% BSA on protein-coated wells. (A) THP-vector (░) and THP-antisense (▨) cells were stimulated for 1 hour with 150 ng/mL TPA and then allowed to adhere to wells coated with collagen type I (Col I), laminin (Ln), Fn, 110-kd or 42-kd Fn fragments, or BSA. Some samples of THP-vector cells were pretreated for 1 hour with function-blocking polyclonal anti-tTG antibody (■). (B) THP-vector (■) and THP-antisense cells (▧), either untreated or pretreated for 1 hour with 150 ng/mL TPA (░, vector; ▨, antisense), were allowed to adhere on Fn-coated wells. Some samples of TPA-stimulated THP-vector cells were preincubated for 1 hour with function-blocking polyclonal anti-tTG antibody, blocking mAbs against β1-, β2-, or β3-integrins, antibody against FXIIIA, or control nonimmune IgG. (C) Human peripheral blood monocytes were treated for 4 hours with 5 ng/mL M-CSF and then plated on wells coated with Fn (■) or the 42-kd fragment of Fn (▪). Monocyte samples were preincubated for 1 hour with control nonimmune IgG, function-blocking polyclonal anti-tTG antibody, mAb 4G3 against tTG, or blocking mAbs against β1-, β2-, or β3-integrins. (A-C) The antibodies were kept in the medium during the assay. Shown are the means of 3 separate experiments performed in duplicate.
Preincubation with function-blocking antibody against tTG did not change adhesion of THP-vector cells on collagen type I, laminin, and the 110-kd cell-binding fragment of Fn (Figure 5A). In contrast, adhesion of THP-vector cells on Fn and, in particular on the 42-kd gelatin-binding fragment of Fn,29 33 was significantly reduced by blocking anti-tTG antibody. In agreement, adhesion on collagen I, laminin, and the 110-kd Fn fragment was not altered by expression of antisense tTG construct. However, adhesion on Fn and even more so, on the 42-kd fragment of Fn, was reduced for THP-antisense transfectants compared to THP-vector control cells. Adhesion of both THP-1 vector and THP-antisense cells to Fn was induced about 6- to 7-fold by treatment with TPA, whereas it was twice lower for the antisense transfectants (Figure 5B). Treatment of uninduced THP-1 cells with antibodies against integrins or tTG had little effect on adhesion to Fn (data not shown). Function-blocking mAbs against β1- and β3-integrins, but not antibodies against β2-integrin, FXIIIA, or control nonimmune IgG, substantially reduced adhesion of TPA-stimulated THP-vector cells on Fn. However, blocking β1- or β3-integrins with specific antibodies resulted in less prominent inhibition of cell adhesion on Fn than interfering with surface tTG. Similarly, we observed a strong decrease in adhesion of M-CSF–treated human monocytes on Fn in the presence of blocking anti-tTG antibodies, whereas antibodies to β1- and β3-integrins had more limited effects (Figure 5C). Antibodies against tTG fully suppressed adhesion of human monocytes on the 42-kd Fn fragment, whereas anti-integrin antibodies had essentially no effect.
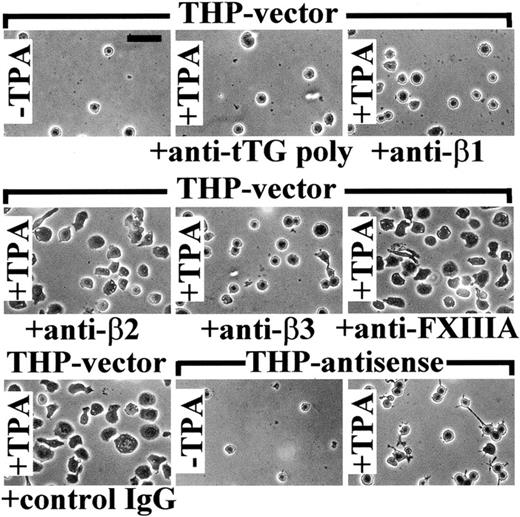
Then, we examined the role of β1- and β3-integrins and tTG in spreading of THP-1 cell on Fn. Treatment with TPA significantly increased spreading of both THP-vector and THP-antisense cells on Fn (Figure6). TPA-induced THP-antisense cells formed distinctive thin projections but overall appeared less spread on Fn than their vector-transfected counterparts. Treatment of THP-vector transfectants with blocking antibodies against β1- or β3-integrins reduced cell spreading on Fn. Yet, preincubation with blocking anti-tTG polyclonal antibody caused even more prominent inhibition of cell spreading. As with adhesion assays, antibodies to β2-integrins, FXIIIA, and control IgG had essentially no effect on spreading of THP-1 cells on Fn. Collectively, our data show that cell surface tTG contributes specifically and significantly to adhesion and spreading of monocytic cells on Fn due to high-affinity interaction with the gelatin-binding part of the Fn molecule.
Blocking antibody against tTG and expression of antisense tTG construct inhibit spreading of TPA-stimulated THP-1 cells on Fn.
The 5 × 103 THP-vector and THP-antisense cells, either untreated (−TPA) or treated for 1 hour with 150 ng/mL TPA (+TPA), were plated for 12 hours at 37°C in serum-free AIM-V medium containing 0.5% BSA on Fn-coated wells. Some samples of THP-vector cells were preincubated for 1 hour with function-blocking polyclonal anti-tTG antibody, blocking mAbs against β1-, β2-, or β3-integrins, antibody against FXIIIA, or control nonimmune IgG, before plating on Fn. The antibodies were present in the medium during the assay. Adherent cells were washed with PBS, fixed with paraformaldehyde, stained with Coomassie brilliant blue, and photographed. Bar indicates 50 μm.
Blocking antibody against tTG and expression of antisense tTG construct inhibit spreading of TPA-stimulated THP-1 cells on Fn.
The 5 × 103 THP-vector and THP-antisense cells, either untreated (−TPA) or treated for 1 hour with 150 ng/mL TPA (+TPA), were plated for 12 hours at 37°C in serum-free AIM-V medium containing 0.5% BSA on Fn-coated wells. Some samples of THP-vector cells were preincubated for 1 hour with function-blocking polyclonal anti-tTG antibody, blocking mAbs against β1-, β2-, or β3-integrins, antibody against FXIIIA, or control nonimmune IgG, before plating on Fn. The antibodies were present in the medium during the assay. Adherent cells were washed with PBS, fixed with paraformaldehyde, stained with Coomassie brilliant blue, and photographed. Bar indicates 50 μm.
Antisense inhibition or blocking cell surface tTG selectively reduces migration of monocytic cells on Fn and the 42-kd fragment of Fn
To analyze the potential involvement of surface tTG in migration of TPA-stimulated THP-1 monocytic cells, we used transmigration assays with Transwells (Costar) where the undersurface was coated with ECM proteins and Fn fragments. To ensure an efficient directional migration, MCP-1 was added to lower chambers. Only very few THP-1 cells transmigrated onto protein-coated membranes in the absence of TPA stimulation (data not shown). Treatment with blocking antibody against tTG did not change migration of TPA-induced THP-vector cells on collagen type I, laminin, and the 110-kd cell-binding fragment of Fn (Figure 7). On the contrary, blocking anti-tTG antibody significantly reduced migration of THP-vector cells on Fn and its 42-kd fragment. Likewise, migration on collagen I, laminin, and the 110-kd Fn fragment was not altered by expression of THP-antisense construct, whereas migration on Fn and the 42-kd fragment of Fn was decreased for TPA-induced THP-antisense transfectants compared to their THP-vector counterparts.
Antisense inhibition or blocking cell surface tTG reduces migration of TPA-stimulated THP-1 cells on Fn and its 42-kd fragment.
The 5 × 104 THP-vector (░) and THP-antisense (▨) cells in serum-free AIM-V medium containing 0.5% BSA were stimulated with 150 ng/mL TPA and placed for 4 hours at 37°C into upper chambers of Transwells (Costar) where undersurface of the inserts was precoated with collagen I (Col I), laminin (Ln), Fn, the 110-kd or 42-kd fragments of Fn, or BSA. Then 125 ng/mL MCP-1 was added to lower chambers. Some samples of THP-vector cells were preincubated for 1 hour with function-blocking polyclonal anti-tTG antibody that was kept in the medium during the assay (■). Shown are the means of 3 separate experiments performed in duplicate.
Antisense inhibition or blocking cell surface tTG reduces migration of TPA-stimulated THP-1 cells on Fn and its 42-kd fragment.
The 5 × 104 THP-vector (░) and THP-antisense (▨) cells in serum-free AIM-V medium containing 0.5% BSA were stimulated with 150 ng/mL TPA and placed for 4 hours at 37°C into upper chambers of Transwells (Costar) where undersurface of the inserts was precoated with collagen I (Col I), laminin (Ln), Fn, the 110-kd or 42-kd fragments of Fn, or BSA. Then 125 ng/mL MCP-1 was added to lower chambers. Some samples of THP-vector cells were preincubated for 1 hour with function-blocking polyclonal anti-tTG antibody that was kept in the medium during the assay (■). Shown are the means of 3 separate experiments performed in duplicate.
Further, we tested several antibodies against tTG, integrins, and FXIIIA in migration assays with TPA-stimulated THP-1 cells and membranes precoated with Fn and the 110-kd and 42-kd Fn fragments (Figure 8). Polyclonal antibody and mAb 4G3 against the NH2-terminal Fn-binding domain of tTG had the most striking inhibitory effects on migration of THP-1 cell on Fn. Another mAb CUB7402 against the tTG fragment (331-478), which also interferes with the interaction of surface tTG with Fn,33 decreased the migration of THP-1 cells on Fn to a lesser extent. Because the monovalent Fab fragment of anti-tTG mAb 4G3 also inhibited THP-1 cell migration on Fn (data not shown), interfering with tTG-Fn interaction rather than mere clustering of tTG on the cell surface reduced monocyte migration. Function-blocking antibodies to either β1- or β3-integrin also inhibited migration of THP-1 cells on Fn, yet they had a more modest effect than anti-tTG antibodies. Both antibodies against β1- and β3-integrins efficiently suppressed migration of THP-1 cells on the 110-kd Fn fragment, whereas anti-tTG antibodies had essentially no effect on this substrate. In contrast, all 3 antibodies against tTG had a robust inhibitory effect, whereas anti–β1- and anti–β3-integrin mAbs did not alter THP-1 cell migration on the 42-kd fragment of Fn. Antibodies against β2-integrin, FXIIIA, or control nonimmune IgG had very little if any influence on THP-1 cell migration on Fn and Fn fragments.
Blocking antibodies against tTG and β1
- or β3-integrins differentially affect migration of TPA-induced THP-1 cells on the 110-kd and the 42-kd fragments of Fn. The 5 × 104 THP-vector cells in serum-free AIM-V medium containing 0.5% BSA were stimulated with 150 ng/mL TPA and placed for 4 hours at 37°C into upper chambers of Transwells (Costar) where undersurface of the inserts was precoated with Fn (░) or its 110-kd (■) or 42-kd (▪) fragments. Then 125 ng/mL MCP-1 was added to lower chambers. Before adding cells to the Transwells, cell samples were preincubated for 1 hour with control nonimmune IgG, function-blocking polyclonal anti-tTG antibody, anti-tTG mAb 4G3 or mAb CUB7402, antibody against FXIIIA, or blocking mAbs against β1-, β2-, or β3-integrins. The antibodies were present in the medium during the assay. The numbers of transmigrated cells on each substrate in the presence of control nonimmune IgG (23.6 × 103 on Fn, 7.9 × 103 on the 110-kd fragment, and 13.7 × 103 on the 42-kd fragment) were taken as 100%. Bars depict the means of 3 separate experiments performed in duplicate.
Blocking antibodies against tTG and β1
- or β3-integrins differentially affect migration of TPA-induced THP-1 cells on the 110-kd and the 42-kd fragments of Fn. The 5 × 104 THP-vector cells in serum-free AIM-V medium containing 0.5% BSA were stimulated with 150 ng/mL TPA and placed for 4 hours at 37°C into upper chambers of Transwells (Costar) where undersurface of the inserts was precoated with Fn (░) or its 110-kd (■) or 42-kd (▪) fragments. Then 125 ng/mL MCP-1 was added to lower chambers. Before adding cells to the Transwells, cell samples were preincubated for 1 hour with control nonimmune IgG, function-blocking polyclonal anti-tTG antibody, anti-tTG mAb 4G3 or mAb CUB7402, antibody against FXIIIA, or blocking mAbs against β1-, β2-, or β3-integrins. The antibodies were present in the medium during the assay. The numbers of transmigrated cells on each substrate in the presence of control nonimmune IgG (23.6 × 103 on Fn, 7.9 × 103 on the 110-kd fragment, and 13.7 × 103 on the 42-kd fragment) were taken as 100%. Bars depict the means of 3 separate experiments performed in duplicate.
Similarly, analysis of M-CSF–treated human monocytes revealed a key role of surface tTG in MCP-1–induced transmigration on Fn and its 42-kd fragment (Figure 9). Again, a pretreatment with anti-tTG antibodies caused a strong inhibitory effect on cell migration on Fn and, even more so, on the 42-kd fragment, whereas antibodies against β1- and β3-integrins decreased the migration on Fn but did not alter the migration on the 42-kd Fn fragment. Together, these data underscore an important role of cell surface tTG as an integrin-associated coreceptor involved in migration of monocytic cells on Fn.
Blocking cell surface tTG inhibits migration of M-CSF–treated monocytes on Fn and the 42-kd Fn fragment.
Human peripheral blood monocytes (5 × 104) in serum-free AIM-V medium containing 0.5% BSA were stimulated for 4 hours with 5 ng/mL M-CSF and then placed for 4 hours at 37°C into upper chambers of Transwells (Costar) where undersurface of the inserts was precoated with Fn (■) or its 42-kd fragment (▪). Then 125 ng/mL MCP-1 was added to lower chambers. Before adding to the Transwells, monocytes were preincubated for 1 hour with control nonimmune IgG, function-blocking polyclonal anti-tTG antibody, anti-tTG mAb 4G3, or blocking mAbs against β1-, β2-, or β3-integrins. The antibodies were kept in the medium during the assay. Shown are the means of 3 separate experiments performed in duplicate.
Blocking cell surface tTG inhibits migration of M-CSF–treated monocytes on Fn and the 42-kd Fn fragment.
Human peripheral blood monocytes (5 × 104) in serum-free AIM-V medium containing 0.5% BSA were stimulated for 4 hours with 5 ng/mL M-CSF and then placed for 4 hours at 37°C into upper chambers of Transwells (Costar) where undersurface of the inserts was precoated with Fn (■) or its 42-kd fragment (▪). Then 125 ng/mL MCP-1 was added to lower chambers. Before adding to the Transwells, monocytes were preincubated for 1 hour with control nonimmune IgG, function-blocking polyclonal anti-tTG antibody, anti-tTG mAb 4G3, or blocking mAbs against β1-, β2-, or β3-integrins. The antibodies were kept in the medium during the assay. Shown are the means of 3 separate experiments performed in duplicate.
Discussion
Previous work showed that tTG content is drastically increased in monocytic cells undergoing differentiation34,37-39,49; however, the functional significance of this up-regulation of tTG remained unknown. Here we show that monocyte maturation into macrophages is accompanied by a sharp elevation of cell surface tTG. This occurs in response to inducers of monocyte differentiation (adhesion to Fn, treatment with M-CSF or other cytokines that promote macrophage phenotype) or as a result of treatment of THP-1 monocytic leukemia cells with nonspecific activators of protein kinase C (TPA). Importantly, we found that the first phase of up-regulation of surface tTG occurs rapidly within 2 hours after stimulation and does not require protein synthesis. This suggests that monocytes can elevate promptly the amounts of surface tTG in response to various activation stimuli. Mechanisms underlying this rapid up-regulation of surface tTG remain unknown. Yet, recently we reported that complexes of tTG with β1-integrins can be detected inside the cell during biosynthesis.33 Thus, translocation of integrin-tTG complexes residing in the endoplasmic reticulum and Golgi compartments might rapidly increase the amounts of surface tTG on activated monocytes. These elevated levels of tTG on the cell surface appear to be sustained throughout later stages of macrophage differentiation and should promote interaction of these cells with Fn-containing matrices. In addition, the increased amounts of surface tTG may facilitate interaction of monocytic cells with some other ECM proteins or endothelial counterreceptors capable of binding tTG.
We recently reported colocalization of surface tTG with β1-integrins at focal adhesions on the surface of fibroblasts adherent on Fn.33 In this study, we found that tTG is accumulated together with β1- and β3-integrins in podosomes, specialized adhesive structures of macrophages.50-52 This specific localization of cell surface tTG at podosomes points to a direct involvement of this protein in cell-ECM interactions.
The results presented in this work highlight a novel function of cell surface tTG as an adhesion and migration receptor on macrophages. We observed a significant decrease in adhesion and migration of monocytic cells on Fn due to down-regulation of cell surface tTG by expression of antisense construct or inhibition of tTG-Fn interaction with blocking antibodies. Notably, the magnitude of these effects exceeded those of blocking antibodies against either β1- or β3-integrins. This major role of surface tTG in the interaction of cells with Fn is based on high-affinity binding of tTG to Fn25 via interaction with its gelatin-binding domain.29 33 Thus, a significant contribution of tTG to the interaction of macrophages with Fn might be attributed to both high amounts of cell surface tTG complexed with β1- and β3-integrins in activated monocytic cells and strong association of tTG with Fn. Monocyte differentiation into macrophages is also accompanied by up-regulation of transglutaminase cross-linking activity on the cell surface primarily due to the increase in tTG expression (data not shown). However, at this moment it remains unclear whether enzymatic activity of tTG has any role in adhesion, extravasation, and invasion of activated monocytes.
Our data also point to dissimilar roles of 2 structurally related transglutaminases, tTG and FXIIIA, in monocytic cell functions. In contrast to a sharp increase in the tTG biosynthesis and content, we found a down-regulation of FXIIIA expression during monocyte differentiation. Then, as in the case of its exogenous expression in fibroblasts,33 FXIIIA failed to interact with β1- and β3-integrins, thereby suggesting distinct mechanisms of externalization and cell surface retention for these transglutaminases. Notably, we detected no contribution of surface FXIIIA in adhesion and migration of monocytic cells on Fn, likely due its inability to bind Fn.26 44
Where may the interaction of cell surface tTG on monocytic cells with the surrounding Fn-containing matrices occur in vivo? This can take place during migration of monocytic cells beneath the endothelial monolayer into underlying tissues that contain large amounts of Fn in the ECM. Yet, a rapid up-regulation of cell surface tTG on monocytic cells in response to activation stimuli suggests that tTG interaction with Fn and, possibly other tTG-binding ECM proteins or endothelial receptors, may also occur during earlier stages of monocyte extravasation. Further work is required to address a potential role of integrin-associated cell surface tTG in monocyte-endothelial interactions.
Notably, certain types of acute monocytic leukemia are characterized by 2 subpopulations of monocytes.53 54 One of these displays an increased extravasation and accumulation in various tissues due to elevated adhesive and migratory capacity. In future work it will be important to determine whether the abnormal adhesive and migratory properties of monocytic leukemia cells involve a deranged expression of cell surface tTG.
The authors thank Dr Peter Davies (University of Texas, Houston, TX) for providing tTG cDNA. We wish to thank Dr Kenneth Ingham (Department of Biochemistry, American Red Cross) for providing purified Fn fragments used in this study. We are grateful to Liubov Zaritskaya (Department of Immunology, American Red Cross) for expert technical assistance with monocyte isolation and flow cytometry analysis.
Supported by grant CA77697 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alexey M. Belkin, Department of Biochemistry, The Holland Laboratory, American Red Cross, 15601 Crabbs Branch Way, Rockville, MD 20855; e-mail: belkina@usa.redcross.org.