Abstract
Isolating fetal erythroblasts from maternal blood offers a promising noninvasive alternative for prenatal diagnosis. The current immunoenzymatic methods of identifying fetal cells from background maternal cells postenrichment by labeling γ-globin are problematic. They are nonspecific because maternal cells may produce γ-globin, give poor hybridization efficiencies with chromosomal fluorescence in situ hybridization (FISH), and do not permit simultaneous visualization of the fetal cell identifier and the FISH signal. We describe a novel technique that allows simultaneous visualization of fetal erythroblast morphology, chromosomal FISH, and ε-globin labeled with AMCA (7-amino-4-methylcoumarin-3-acetic acid). AMCA was chosen as the fluorescent label to circumvent the problem of heme autofluorescence because the mean difference in relative fluorescence intensity between fetal erythroblasts stained positive for antiglobin antibody and autofluorescence of unstained cells was greater with AMCA (mean 43.2; 95% confidence interval [CI], 34.6-51.9; SD = 14.0) as the reporting label compared with fluorescein isothiocyanate (mean 24.2; 95% CI, 16.4-31.9; SD = 12.4) or phycoerythrin (mean 9.8; 95% CI, 4.8-14.8; SD = 8.0). Median FISH hybridization efficiency was 97%, comparable to the 98% (n = 5 paired samples) using Carnoy fixative. One ε-positive fetal erythroblast was identified among 105 maternal nucleated cells in 6 paired mixture experiments of fetal erythroblasts in maternal blood (P < .001). Male ε-positive fetal erythroblasts were clearly distinguishable from adult female ε-negative erythroblasts, with no false positives (n = 1000). The frequency of fetal erythroblasts expressing ε-globin declines linearly from 7 to 14 weeks' gestation (y = −15.8 × + 230.8;R2 = 0.8; P < .001). We describe a rapid and accurate method to detect simultaneously fetal erythroblast morphology, intracytoplasmic ε-globin, and nuclear FISH.
Introduction
Isolating fetal nucleated red blood cells (NRBCs) from maternal blood should allow first trimester noninvasive prenatal diagnosis of aneuploidy and monogenic disorders.1There are currently 3 steps: enrichment of fetal cells in maternal blood, identification of fetal cells among background maternal cells, and diagnosis using fluorescence in situ hybridization (FISH) or single-cell techniques. Antibody directed against the γ chain of fetal hemoglobin is commonly used for both the fetal cell enrichment2,3 and identification4 steps. There may be a case for using γ-globin for sorting, favoring yield over purity, but it is not nearly specific enough for accurate fetal erythroblast identification because of increased maternal fetal hemoglobin production in pregnancy5 and β thalassemia.6,7 In contrast, ε- or ζ-globin chains appear specific for fetal cells in chorionic villus supernatants7 but have not been tested in maternal blood. Whereas ζ-globin is occasionally produced in adults with α thalassemia,8 ε-globin has not been found in adult peripheral blood,9 making it the preferred fetal cell identifier.
Current rare-event enrichment protocols yield small numbers of fetal erythroblasts against a large background of maternal NRBCs.3 10 After sorting, separate fetal cell identification and genetic diagnosis procedures promote loss of rare cells and compromise specificity. Thus, an accurate slide-based identification system, which allows genetic analysis of only fetal cells among what is typically a 100- to 1000-fold higher postenrichment background of contaminating maternal erythroblasts, is desirable.
Strategies to date combining immunoenzymatic labeling of fetal antigens and chromosomal FISH4,11-13 have proved problematic. First, colored dyes such as Fast Red dissolve in the organic solvents used in FISH, while Vector Blue Substrate affects hybridization efficiency. Secondly, they necessitate either spatial orientation and relocation during subsequent analysis or cumbersome switching between light and fluorescence microscopes. To obviate this, fluorophores could be used to label fetal antigens, but their application has been limited by heme autofluorescence and overlap of colors with those used for FISH.2 Thus, no current technique allows simultaneous visualization of fetal cell marker and the nuclear hybridization signal.
We describe the development of a novel technique for simultaneous visualization of fetal NRBC morphology, intracytoplasmic ε-globin and nuclear FISH.
Materials and methods
Fetal blood samples
Fetal whole blood was obtained by ultrasound-guided transabdominal cardiocentesis before clinically indicated surgical termination of pregnancy.14 Blood collection for research was approved by the institutional ethics committee in compliance with national guidelines regarding the use of fetal tissue for research purposes. All women gave written informed consent. Gestational ages determined by crown–rump length measurement ranged from 7 to 14 weeks.
Slide preparation and controls
A total of 30 000 nucleated cells suspended in 1% bovine serum albumin in phosphate-buffered saline were cytocentrifuged onto glass slides. Chromosomal FISH hybridization efficiency was compared with standard Carnoy fixative protocols for male and female lymphocyte nuclei.15 Pure populations of K562 cells—a female erythroleukemia cell line that expresses ε-globin when cultured in 0.1 mM hemin16—and erythroblasts derived from adult bone marrow by magnetically activated cell sorting (MACS) using transferrin receptor antibody were used as positive and negative controls, respectively. Sensitivity was ascertained in mixtures of male fetal NRBCs within nucleated cells from a never-pregnant female, with ratios ranging from 1:102 to 1:105. Maternal NRBCs enriched from peripheral blood of mothers carrying a male fetus were studied for expression of ε-globin. Specificity was confirmed in mixtures of male fetal erythroblasts with female adult bone marrow erythroblasts.
Studying heme autofluorescence
To determine the limiting effect of heme autofluorescence on choosing a fluorescence label for antiglobin antibody, we studied 4 groups of 50 fetal erythroblasts from the same sample at 9 weeks' gestation. In 3 groups, the cells were stained by fluorescence immunocytochemistry using either fluorescein isothiocyanate (FITC), phycoerythrin (PE), or AMCA (7-amino-4-methylcoumarin-3-acetic acid) for either the ε- or the γ-globin chain and the fluorescence intensities of positive cells studied. The fourth group was not stained, and the autofluorescence within the cells was determined through the red (Texas Red), green (FITC), and blue (DAPI; 4′6-diamidino-2-phenylindole · 2HCl) channels. We used FITC and AMCA to label ε-globin (Europa Bioproducts, Cambridge, United Kingdom) but for PE labeled γ-globin instead because it was commercially available preconjugated (Europa Bioproducts). To compare image intensities, all images were ColourNormalised (256 gray levels; IPLab Software, Digital Scientific, Cambridge, United Kingdom) according to set criteria before analysis. Ten randomly selected clusters of 5 neighboring cells were studied for each of the 4 study groups. Clusters were labeled 1 to 10 consecutively, upon selection. Within each cell, 10 small areas within the cytoplasm were studied. A mean fluorescence intensity was calculated for each cluster of 5 cells. This number was transformed to a relative fluorescence intensity of cluster (RFIC) by making it a percentage of the 256 gray levels.17 Within each filter channel—green, red, and blue—the difference between corresponding RFICs was calculated.
Combined fluorescence immunocytochemistry and chromosomal FISH
Slides were fixed in 1:1 (vol/vol) methanol:acetone for 8 minutes at room temperature, permeabilized with 0.25% glacial acetic acid in methanol (vol/vol), and rinsed in Tris-buffered saline with Tween 20 (TBST; Dako, Carpinteria, CA). They were incubated with goat serum (Sigma Diagnostics, St Louis, MO) diluted 1:5 in TBST for 30 minutes followed by incubation with anti-ε monoclonal antibody (Europa Bioproducts) diluted 1:100 for 60 minutes and were washed twice after each incubation. Subsequent incubations were with biotinylated goat antimouse (Vector Laboratories, Burlingame, CA) and with streptavidin conjugated with AMCA (Vector Laboratories), both diluted 1:100 and incubated for 30 minutes. Reagents were diluted in TBST; incubations were in a humidifying chamber at room temperature; and washes were in TBST for 3 minutes. Slides were dehydrated through 70%, 90%, and 100% ethanol, air dried, and prepared for FISH to the sex chromosomes. The chromosome-specific centromeric repeat probes DXZ1, labeled with SpectrumOrange, and DYZ1, labeled with Spectrumgreen (Vysis, Downer's Grove, IL), were used. Five microliters of the probe, diluted 1:1 in hybridization buffer, containing 50% formamide and 10% dextran sulfate in 2 × SSC at pH 7.0, was added to each cytospin under a cover glass. Target DNA was denatured on an in situ hybridization block at 71°C for 7 minutes followed by 4 hours of hybridization at 37°C. Posthybridization washes included once in 0.4 × SSC at 72°C for 2 minutes and twice in 2 × SSC at room temperature for 2 minutes. Slides were dehydrated through an ethanol series and mounted in fluorescence antifade medium (Vector Laboratories). The slides were analyzed by epifluorescence microscopy using single band pass filters for Spectrumaqua (aqua) and Spectrumorange (orange) and a triple band pass filter set for DAPI, FITC, and Texas Red. Images were captured using a cooled charge-coupled device camera and reviewed in Quipps m-FISH software (Vysis).
Statistics
Pearson's correlation coefficient, Spearman's rho, and the Mann-Whitney were derived using SPSS Software (SPSS, Chicago, IL).
Results
Heme autofluorescence
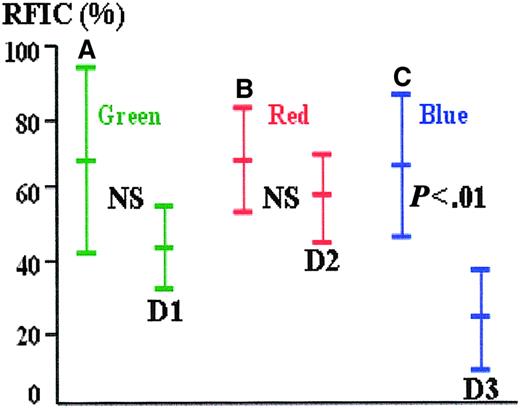
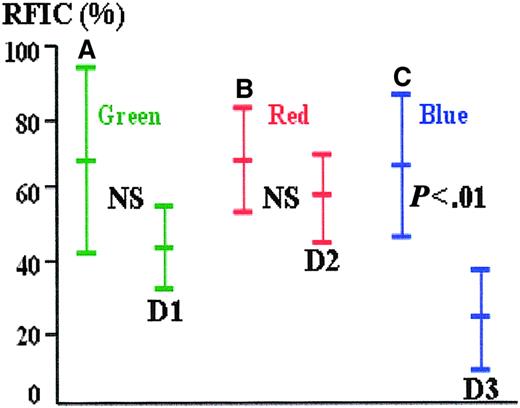
The mean difference in the RFICs between stained and unstained erythroblasts was greater with AMCA (mean 43.0; 95% confidence interval [CI], 34.2-51.8; SD = 13.9) as the reporting label compared with FITC (mean 24.1; 95% CI, 16.3-31.9; SD = 12.4) or PE (mean 9.8; 95% CI, 4.8-14.8; SD = 8.0) (Figure1). Viewed through the green channel, 55.9% of unstained cells have a greater (auto)fluorescence intensity than weakly stained positive cells; this latter group of weakly stained cells represents 23.8% of all positive cells, and 20.1% of all green cells fall within this zone of ambiguity. Similarly, through the red channel, 68.7% of unstained cells have a greater (auto)fluorescence intensity than weakly stained positive cells; this latter group of weakly stained cells represents 58.2% of all positive cells, and 46.0% of all red cells fall within this zone of ambiguity. In the remaining 79.9% of cases for green and 54% of the cases for red, stained and autofluorescent cells can be easily distinguished by optimizing the fluorescence threshold on the color histogram in the image-capture software. In contrast, there is no overlap of fluorescence between stained and unstained cells viewed through the blue channel, suggesting that confusion between positive and negative cells is unlikely. AMCA was therefore chosen to label the anti-ε globin antibody and improve specificity.
Relative fluorescence intensity of stained and unstained cells (autofluorescence) through green, red, and blue channels.
(A-C) The mean RFIC and the 95% data intervals (± 1.96 SD) of fetal erythroblasts stained with antiglobin antibodies conjugated with FITC, PE, and AMCA, respectively. (D) The autofluorescence of unstained fetal erythroblasts when viewed through the green, red, and blue filters. There is overlap between the brighter autofluoresecent and weakly stained cells in the green and red channels but not in the blue.
Relative fluorescence intensity of stained and unstained cells (autofluorescence) through green, red, and blue channels.
(A-C) The mean RFIC and the 95% data intervals (± 1.96 SD) of fetal erythroblasts stained with antiglobin antibodies conjugated with FITC, PE, and AMCA, respectively. (D) The autofluorescence of unstained fetal erythroblasts when viewed through the green, red, and blue filters. There is overlap between the brighter autofluoresecent and weakly stained cells in the green and red channels but not in the blue.
Combined fluorescence immunocytochemistry and chromosomal FISH
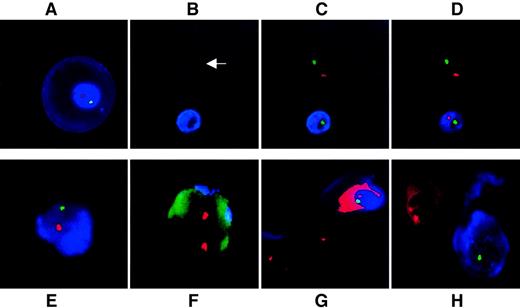
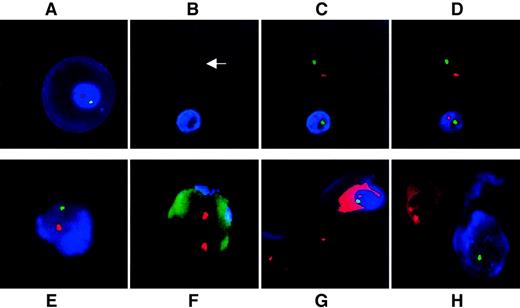
Figure 2 demonstrates simultaneous visualization of ε-globin as an intracytoplasmic fetal cell identifier and chromosomal FISH; the figure includes controls. The large ε-globin–positive erythroblast in Figure 2A is typical for 9 weeks. Use of DAPI as nuclear counterstain proved unnecessary because ε-positive erythroblasts fluoresce blue and ε-negative erythroblasts autofluoresce in the Spectrumaqua channel, clearly identifying the location of the cells and demarcating their nuclear boundaries (Figure 2B-D). The median hybridization efficiency for 2 FISH signals per AMCA-positive nucleated cell was 97%, comparable to the 98% (n = 5 sample pairs; z = 0.74, nonsignificant) obtained in control slides of male and female lymphocytes. All erythroblast-like K562 cells cultured in 0.1 mM hemin were positive for ε (n = 1,500; 3 samples of 500 cells each) whereas no adult NRBC (n = 1,000; 5 samples of 200 cells each) or white blood cell (n = 250 000; 5 samples of 50 000 cells each) expressed ε-globin protein. Specificity was thus 100%. In sample mixtures (n = 6 experiments), the technique was sensitive enough to identify consistently one ε-positive fetal NRBC among 105 adult white blood cells (P < .001) and distinguished between male fetal and adult female erythroblasts (Figure 2E-H).
Simultaneous immunophenotyping and chromosomal FISH.
(A) Primitive ε-positive fetal erythroblast at 10 weeks' gestation stained with AMCA. Cell morphology is well preserved. No DAPI has been used as counterstain because accumulation of AMCA around the nucleus acts as an excellent counterstain. (B-D) Fetal whole blood at 9 weeks' gestation. (B) Use of the Spectrumaqua channel allows the visualization of all heme-containing cells that autofluoresce. One NRBC is positive for ε and the other is negative (arrow); (C) the same group of cells viewed with the red and green filters switched on to show X and Y signals; and (D) the same group of cells viewed with the Spectrumaqua filter off. (E, F) Representative ε-positive and ε-negative cells as seen in a representative mixing experiment of male fetal erythroblasts in never-pregnant adult female nucleated cells in a ratio of 1:10−5. (E) ε-Globin–positive male fetal erythroblast. (F) Nearby ε-globin–negative female nucleated cell. The autofluorescence of the cell through the green channel was deliberately potentiated to demonstrate the outline of the female leukocyte. (G) Mixing experiment of ε-globin–positive male fetal erythroblast with K562 cells cultured in the absence of hemin. One of the X chromosome signals in both neighboring K562 cells are beyond the focal plane captured. (H) Mixing experiment of first trimester, ε-positive, male fetal erythroblasts with female adult bone marrow–derived erythroblasts. Fetal ε-positive NRBC stained with AMCA is clearly distinguished from the AMCA-negative cell. Two X signals are visible in the female erythroblast with demonstrable autofluorescence of heme through red. The Y probe is easily visualized in the male erythroblast, but the X signal is only barely visible at the nuclear periphery in this focal plane. All fluorescence images represent a single optical plane.
Simultaneous immunophenotyping and chromosomal FISH.
(A) Primitive ε-positive fetal erythroblast at 10 weeks' gestation stained with AMCA. Cell morphology is well preserved. No DAPI has been used as counterstain because accumulation of AMCA around the nucleus acts as an excellent counterstain. (B-D) Fetal whole blood at 9 weeks' gestation. (B) Use of the Spectrumaqua channel allows the visualization of all heme-containing cells that autofluoresce. One NRBC is positive for ε and the other is negative (arrow); (C) the same group of cells viewed with the red and green filters switched on to show X and Y signals; and (D) the same group of cells viewed with the Spectrumaqua filter off. (E, F) Representative ε-positive and ε-negative cells as seen in a representative mixing experiment of male fetal erythroblasts in never-pregnant adult female nucleated cells in a ratio of 1:10−5. (E) ε-Globin–positive male fetal erythroblast. (F) Nearby ε-globin–negative female nucleated cell. The autofluorescence of the cell through the green channel was deliberately potentiated to demonstrate the outline of the female leukocyte. (G) Mixing experiment of ε-globin–positive male fetal erythroblast with K562 cells cultured in the absence of hemin. One of the X chromosome signals in both neighboring K562 cells are beyond the focal plane captured. (H) Mixing experiment of first trimester, ε-positive, male fetal erythroblasts with female adult bone marrow–derived erythroblasts. Fetal ε-positive NRBC stained with AMCA is clearly distinguished from the AMCA-negative cell. Two X signals are visible in the female erythroblast with demonstrable autofluorescence of heme through red. The Y probe is easily visualized in the male erythroblast, but the X signal is only barely visible at the nuclear periphery in this focal plane. All fluorescence images represent a single optical plane.
Frequency of ε-positive fetal erythroblast in first trimester fetal blood
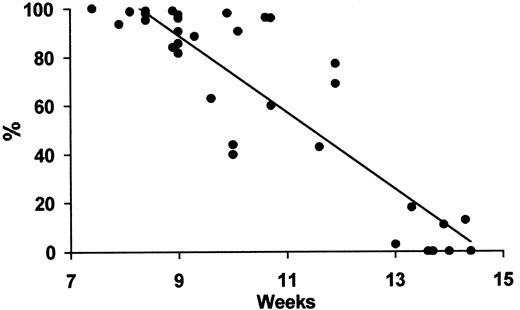
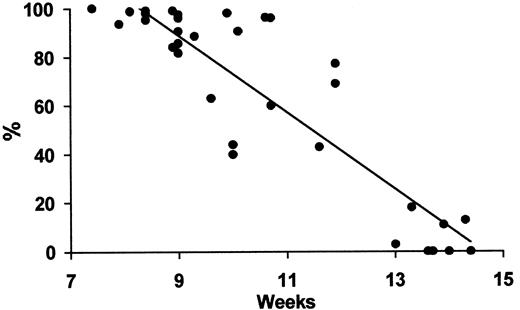
We found that the frequency of ε-positive erythroblasts in circulating fetal blood declined linearly to reach almost negligible levels by 14 weeks (Figure 3).
Fetal ε-positive erythroblasts versus gestational age.
Regression analysis of the fall in frequency of ε-containing fetal erythroblasts by gestational week during the first trimester. y = −15.8 × + 230.8; R2 = 0.8;P < .001.
Fetal ε-positive erythroblasts versus gestational age.
Regression analysis of the fall in frequency of ε-containing fetal erythroblasts by gestational week during the first trimester. y = −15.8 × + 230.8; R2 = 0.8;P < .001.
Discussion
Rare-event detection in this field poses many problems, the greatest being loss of the few precious cells during enrichment, identification, and diagnosis.18 Sorting, whether by magnetic-10 or fluorescence-based19 methods, is an essential and necessarily separate step. However, combining diagnosis with identification in situ to permit simultaneous visualization has several advantages: It limits further fetal cell loss, reduces the risk of either technique failing, and enhances specificity.
The pivotal step in developing this technique was circumventing heme autofluorescence, which had thus far been the limiting factor preventing simultaneous visualization of the fetal cell identifier and molecular genetic signal.2,4 Previous attempts at reducing or correcting autofluorescence, which involved concomitant use of dyes absorbing certain emitted wavelengths20 or mathematical manipulations of pixel intensity,17 met with little success. We chose, instead, to systematically study heme autofluorescence within first trimester fetal erythroblasts. Red and green fluorophores were found unsuitable to label intracytoplasmic globins because in a significant proportion of cases it was difficult to distinguish between stained and unstained cells; another problem is that these colors are also commonly used to label FISH probes.2 Using AMCA to label the globin eliminated this source of confusion. Blue is also the color most commonly reserved for nuclear counterstain in chromosomal FISH and, fortuitously, DNA counterstaining proved unnecessary using this technique on intact cells, because AMCA acts as surrogate counterstain. We are currently investigating why AMCA accumulates around the nucleus, sometimes appearing intranuclear in two-dimensional views such as Figure 2B,C; we postulate it may be due to leakage of hemoglobin nearer the cell membrane during permeabilization. Autofluorescence through aqua allows the recognition of heme-containing cells, a unique feature that can be exploited to differentiate between AMCA-stained and -unstained erythroblasts (Figure 2B-D).
Postenrichment frequencies of fetal erythroblasts among contaminating maternal cells range from 10−2 to 10−5, typically 10−4 for MACS-based protocols.21-23This technique was developed for application to an enriched population of cells, for which its sensitivity of 10−5 with 100% specificity should be ideal. The sensitivity is comparable to polymerase chain reaction–based methods used for the identification of fetal DNA in enriched samples24,25 without confounding false positives. The rarer an event, the greater the impact of missing a cell or inaccurate diagnosis; this level of sensitivity and specificity minimizes the possibility of such errors. Whereas heme-containing fetal erythroblasts are easily distinguishable from white blood cells that lack autofluorescence, anti–ε-globin reliably distinguishes between (ε-positive) fetal and adult NRBCs (Figure 2H). Detecting anti–ε-globin–positive cells was easy. Viewed through the blue filter, these were bright blue against a black background, rendering their identification against a contaminating maternal background potentially amenable to automation. Our high chromosomal FISH hybridization efficiency is important if diagnosis is reliant upon few cells only. Conventional immunoenzymatic staining with Vector Blue Substrate does not allow similar hybridization efficiency,4 possibly because its dense precipitate hampers penetration of FISH probes into the nucleus.
Classical data analyzing hemolysates demonstrate negligible levels of ε-globin by 12 weeks' gestation.26 By contrast, single-cell immunofluorescence cytology has confirmed the presence of ε-globin in approximately 5% of anucleate fetal erythrocytes up to 22 weeks9 and the rare fetal erythroblast at term.27 We show a linear decline in the frequency of fetal nucleated erythroid precursors through the first trimester of pregnancy, reaching negligible levels by 14 weeks. However, the data also demonstrate that nearly half of the fetal NRBCs still contained ε-globin as late as 12 weeks' gestation, defining a suitable window during which ε-globin may be useful as a fetal cell identifier.
The ability to concurrently visualize a fetal cell marker and a hybridization signal within the interphase nucleus represents a significant advance for prenatal diagnosis using fetal cells derived from maternal blood. Direct labeling with AMCA-conjugated anti-ε antibody will further abbreviate the procedure time (presently about 8 hours), bringing this technology nearer to clinical practice.
We thank M. Antoniou for providing the K562 cell line and M. Preece for statistical advice.
Supported by an Overseas Graduate Scholarship from National University of Singapore (M.C.) and by a grant from Wellbeing (C.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mahesh Choolani, Department of Maternal and Fetal Medicine, Division of Paediatrics, Obstetrics and Gynaecology, Institute of Reproductive and Developmental Biology, Imperial College School of Medicine, Hammersmith Hospital Campus, Du Cane Rd, London W12 0NN, United Kingdom; e-mail: mchoolani@cwcom.net.