Abstract
Chronic immune thrombocytopenic purpura (ITP) is an autoimmune disease caused by platelet destruction resulting from autoantibodies against platelet surface proteins, particularly platelet glycoprotein IIb/IIIa (αIIbβ3). To localize the auto-epitopes on platelet αIIbβ3, the binding of autoantibodies to Chinese hamster ovary (CHO) cells expressing either αIIbβ3 or αvβ3was studied. Thirteen of 14 ITP autoantibodies bound only to CHO cells expressing αIIbβ3. Because these 2 integrins have the same beta chain (β3), these results show that most epitopes in chronic ITP are dependent on the presence of glycoprotein αIIb.
Introduction
Chronic immune thrombocytopenic purpura (ITP) is an autoimmune disorder characterized by the production of antiplatelet antibodies that bind to platelet surface membrane proteins, resulting in platelet destruction. Approximately 75% of these autoantibodies bind to platelet antigens that lie on either platelet glycoprotein (GP) IIb/IIIa (αIIbβ3) or GPIb/IX.1 2 In the current study, we evaluated the binding of platelet-associated anti-αIIbβ3 antibodies from ITP patients to Chinese hamster ovary (CHO) cells expressing either αIIbβ3 or the vitronectin receptor, αvβ3. Because these 2 integrins have the same beta chain (β3), it allowed us to evaluate the relative importance of αIIb and β3 as sites of auto-epitopes in chronic ITP.
Study design
Samples were obtained from 14 patients with chronic ITP who had high-titer autoantibodies to platelet αIIbβ3, 2 patients with high-titer anti-GPIb/IX antibodies (one with chronic ITP and one with a drug [procainamide]-dependent antibody), and one patient with posttransfusion purpura who had anti-β3 alloantibodies. Platelets from 3 normal subjects were obtained as controls.
Platelet-associated and plasma antibody eluates were prepared by acid elution as previously described.3 Before use in the binding assay, the eluates were ultracentrifuged for 30 minutes at 20 000 rpm and pre-adsorbed with untransfected CHO cells (2 × 107 cells/mL eluate) to prevent nonspecific binding.
The αIIbβ3 complex-specific monoclonal antibody (mAb) AP2 was provided by Dr Thomas Kunicki (The Scripps Research Institute, La Jolla, CA), and the αv-specific mAb LM142 was obtained from Chemicon (Temecula, CA).
Stably transfected CHO cell lines expressing wild-type αIIbβ3 or αvβ3have been described previously.4 Briefly, full-length cDNAs for αIIb, αv, and β3were subcloned into the vector CDM8, and CHO cells were cotransfected with the appropriate α subunit (αIIb or αv) and β3. The expressed receptors were detected, and clonal cell lines were established with the use of fluorescence-activated cell sorting (FACS) with appropriate monoclonal antibodies. Expression of both the α and β chains was confirmed using appropriate mAbs and FACS.
The CHO cells were harvested from tissue culture flasks with 0.05% trypsin–0.53 mM EDTA in Hank's balanced salt solution. After centrifugation for 5 minutes at 200g, the cells were resuspended in 10 mL Tyrode FACS buffer (0.137 M NaCl, 12 mM NaHCO3, 2.6 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 0.1% bovine serum albumin, 0.1% dextrose, and 5 mM HEPES) containing 0.2 mg/mL soybean trypsin inhibitor and 0.2% fetal calf serum. After 2 washes in Tyrode FACS buffer, the cells were resuspended to a concentration of 107/mL. Each eluate was incubated separately with A5 cells (expressing αIIbβ3), B10 cells (expressing αvβ3), or nontransfected CHO cells. Fifty-microliter aliquots of CHO cell suspension (5 × 105 cells) were transferred to the required number of v-bottom microtiter wells. Autoantibody eluate (175 μL) or monoclonal antibody (100 μL of either 15 μg/mL, if purified, or 1:1000 dilution, if ascites) was added, and the mixture was incubated overnight at 4°C. After 3 washes in Tyrode FACS buffer, the pellets were resuspended in either 100 μL fluorescein isothiocyanate–conjugated goat antihuman IgG or 100 μL FITC-conjugated goat antimouse IgG (Vector Laboratories, Burlingame, CA) at a concentration of 15 μg/mL. After a 30-minute incubation on ice and 3 washes, the cells were resuspended in Tyrode FACS buffer and analyzed on a Facscalibur (Becton Dickinson, Mountain View, CA).
Results and discussion
We studied platelet eluates from 15 patients with chronic ITP. Clinical details and antiplatelet antibody results are summarized in Table 1. There were 9 female and 6 male patients whose ages ranged from 20 to 70 years. Five patients had other autoimmune disorders, such as antiphospholipid syndrome (patients ITP-4 and -5), autoimmune hemolytic anemia (patients ITP-5, -8, -9) and Crohn disease of the colon (patient ITP-6). Thirteen of the 15 ITP patients had undergone splenectomy, and all had relapses after surgery. Fourteen patients had high-titer anti-αIIbβ3 antibodies, and one of these also had anti-GPIb/IX antibodies (patient ITP-9). The remaining ITP patient (patient ITP-15) had only anti-GPIb/IX antibodies. In addition, we studied platelet eluates from one patient with an anti-GPIb/IX drug antibody (procainamide), one patient with posttransfusion purpura caused by anti-PlA1 alloantibodies, and 3 control subjects.
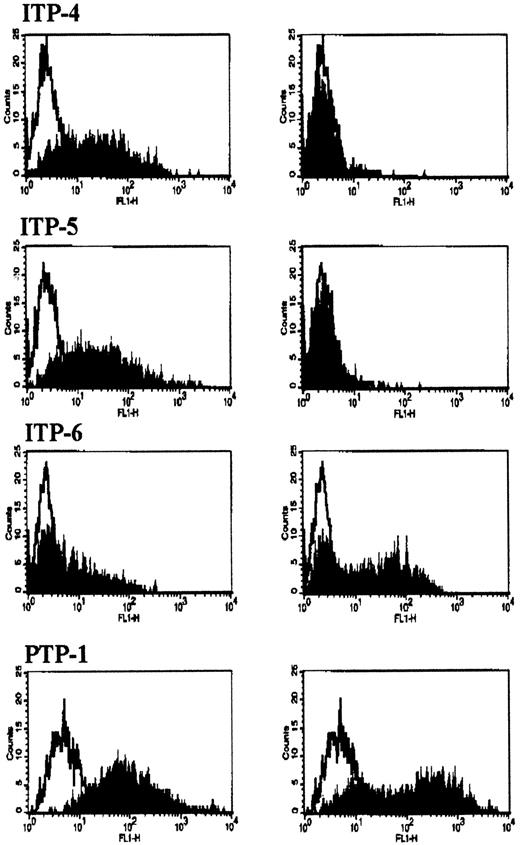
Platelet eluates that had been preadsorbed with untransfected CHO cells were incubated with CHO cells expressing αIIbβ3, CHO cells expressing αvβ3, or untransfected CHO cells. Antibody binding was determined by flow cytometry. Representative studies are shown in Figure 1. Of the 14 platelet eluates from patients with chronic ITP with high-titer autoantibodies to αIIbβ3, 13 bound only to CHO cells expressing αIIbβ3. The remaining platelet eluate (patient ITP-6) bound to CHO cells expressing either αIIbβ3 or αvβ3. Eluates from 3 normal subjects and from the 2 patients with anti-GPIb/IX antibodies (ITP-15 and the patient with the drug-dependent antibody) showed no binding to either of the CHO cell lines. The eluted alloantibody from the patient with posttransfusion purpura and anti-β3 alloantibodies bound to both αIIbβ3 and αvβ3transfected CHO cells as expected because both expressed β3.
Binding of antibodies to CHO cells expressing αIIbβ3 or αvβ3.
CHO cells, expressing either αIIbβ3 (left column) or αvβ3 (right column), were incubated with platelet-associated autoantibody from ITP patients (ITP-4, -5, -6) or allo-antibody from a patient with posttransfusion purpura (PTP-1), and bound antibody was detected by FACS analysis using FITC-antihuman IgG. In each panel, binding to CHO cells, expressing the glycoprotein complex (shaded profile), is compared to binding to native CHO cells (open profile).
Binding of antibodies to CHO cells expressing αIIbβ3 or αvβ3.
CHO cells, expressing either αIIbβ3 (left column) or αvβ3 (right column), were incubated with platelet-associated autoantibody from ITP patients (ITP-4, -5, -6) or allo-antibody from a patient with posttransfusion purpura (PTP-1), and bound antibody was detected by FACS analysis using FITC-antihuman IgG. In each panel, binding to CHO cells, expressing the glycoprotein complex (shaded profile), is compared to binding to native CHO cells (open profile).
These results indicate that anti-αIIbβ3 autoantibodies from most patients with chronic ITP (13 of 14) bind to epitopes localized on αIIb. Although it is likely that association with β3 is required for the proper folding of αIIb and epitope formation, it is clear from these studies that binding of these 13 autoantibodies to antigen does not occur in the absence of αIIb or in the presence of β3 coupled to αv. One autoantibody, from patient ITP-6, bound to both αIIbβ3- and αvβ3-expressing cells, indicating that either (1) the autoepitope is dependent on the presence of β3, (2) the autoepitope is present on both αIIb and αv because these molecules are partially homologous, or (3) the patient has multiple autoantibodies with some binding to αIIb and others to αv. Additional studies will be needed to clarify this.
These findings are consistent with previous results from this laboratory. In earlier studies, we produced a series of large peptides spanning the human β3 molecule and evaluated the ability of auto-antibodies from patients with chronic ITP to bind to these peptides. Platelet-associated autoantibodies from only one of 33 patients with ITP showed convincing binding to any of these β3 peptides.3 Earlier studies have also shown that many ITP auto-epitopes are cation dependent.5 6The formation of configurational epitopes, caused by cation-dependent folding, could depend on one or more of the 4 calcium-binding regions on αIIb or to the cation-dependent molecular relations between αIIb and β3 required for complex formation. Future studies, using CHO cells expressing chimeric αIIb-αvβ3 molecules, should help us to further localize these auto-epitopes.
Supported by National Institutes of Health grants HL61809 (R.M.) and HL42977 (J.C.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert McMillan, the Department of Molecular and Experimental Medicine, Rm 215, The Scripps Research Institute, 10550 North Torrey Pines Rd, La Jolla, CA 92037; e-mail:mcmillan@scripps.edu.