Abstract
Expression of a viral interleukin-6 (vIL-6) has been detected in certain Kaposi sarcoma (KS)–associated herpesvirus positive (KSHV+) lesions. The release of vIL-6 systemically and its contribution to the pathogenesis of HIV-related malignancies was studied. Serum vIL-6 was detected in 13 (38.2%) of 34 HIV+ patients with KS, in 6 (85.7%) of 7 HIV+patients with primary effusion lymphoma (PEL) and/or multicentric Castleman disease (MCD), and in 18 (60.0%) of 30 HIV+, mostly homosexual, individuals without KS, MCD, or PEL. By contrast, serum vIL-6 was detected in only 3 (23.1%) of 13 patients with classic KS, 1 (2.5%) of 40 blood donors from the United States, and 4 (19.0%) of 21 blood donors from Italy. Circulating vIL-6 levels were associated with HIV+ status (P < .0001). However, within the HIV+ cohort, serum vIL-6 levels were not associated with the occurrence of KSHV-associated malignancies (P = .43).
Introduction
Kaposi sarcoma (KS)–associated herpesvirus (KSHV; also known as human herpesvirus 8) has been linked to 3 AIDS-related disorders: KS, primary effusion lymphoma (PEL), and multicentric Castleman disease (MCD).1,2 Molecular piracy of potentially useful cellular genes has emerged as a characteristic feature of this virus.3 Viral interleukin-6 (vIL-6), produced predominantly during lytic viral replication, exhibits approximately 25% amino acid identity to cellular IL-6.3-5 Similar to human IL-6 (hIL-6), vIL-6 supports the growth and survival of certain mouse and human cell lines.4-7 When expressed in mice, recombinant vIL-6 accelerated the hematopoiesis and induced vascular endothelial growth factor, which in turn has been implicated in the pathogenesis of KS, MCD, and PEL.8-12 Constitutive expression of vIL-6 has been identified in PEL cells and in the immunoblastic cells within the mantle zone of MCD lymph nodes.13-15 By contrast, expression of vIL-6 has been undetectable or restricted to few lytically infected cells in KS lesions.15 16 To investigate if vIL-6 is released into the circulation and if, through systemic distribution, vIL-6 contributes to the development of KSHV-associated disorders, we assayed vIL-6 in sera from HIV-infected and noninfected individuals.
Study design
Sera from blood donors, HIV-infected individuals, and patients with KS, PEL, or MCD were collected with consent from blood banks and clinical centers in the United States, Italy, and Japan. Samples were stored at −70°C prior to testing.
Enzyme-linked immunosorbent assay (ELISA) for vIL-6 (assay sensitivity, 30 pg/mL) was performed as described elsewhere.14 All samples were diluted 1:10 prior to assay, and the lower limit of ELISA sensitivity for serum vIL-6 was thus 300 pg/mL. hIL-6 was measured using an hIL-6 Quantikine kit (R&D Systems, Minneapolis, MN) that does not detect vIL-6.14 HIV RNA load was determined by Amplicor HIV test (Roche Diagnostic Systems, Basel, Switzerland). Counts for CD4+ and CD8+cells were determined by flow cytometry. Severity of KS in patients was assessed by both the TIS staging system17 and counting the total number of lesions. Serum antibodies to KSHV were detected using the whole-virus lysate ELISA kit18 (Advanced Biotechnologies, Columbia, MD) according to the manufacturer's instructions. All statistical analyses were performed with StatView (version 5.0.1) software.
Results and discussion
Using a recently established vIL-6–specific ELISA that does not detect hIL-6,14 serum vIL-6 was detected in 1 (2.5%) of 40 blood donors from the United States (range, < 300-927 pg/mL; median, < 300 pg/mL; and 75th percentile, < 300 pg/mL) and 4 (19.0%) of 21 blood donors from Italy (range, < 300-2284 pg/mL; median, < 300 pg/mL; and 75thpercentile, < 300 pg/mL) (Figure 1A). In control experiments, all positive sera tested negative when the plates were not coated with antibody, demonstrating that positive reactions were not attributable to nonspecific binding (data not shown). Immunoglobulin G (IgG) antibodies against KSHV were detected in 4 (19.0%) of 21 Italian and 0 (0%) of 40 US blood donors (Figure 1B).
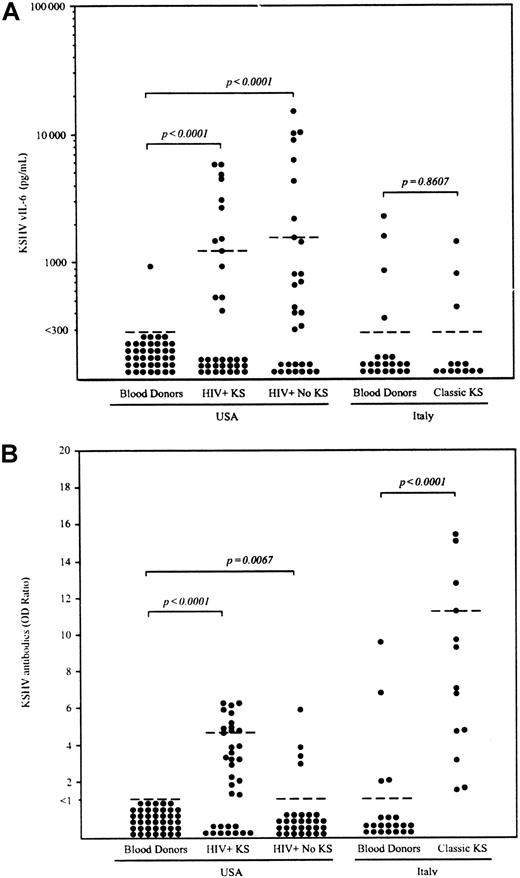
Detection of KSHV vIL-6 and anti-KSHV antibody in sera from blood donors, HIV+ patients with or without KS, and patients with classic KS.
(A) The lower limit of ELISA sensitivity in serum samples was set at 300 pg/mL vIL-6. (B) IgG antibody for the whole KSHV lysate was determined by ELISA. Optical density (OD) ratios were determined by dividing the OD reading of each sample by cut-off value (average of negative control × 3). Samples with OD ratio of at least 1.0 were interpreted as positive. Dotted bars indicate the 75thpercentile for each group. Statistical significance of group differences was determined by the Mann-Whitney test.
Detection of KSHV vIL-6 and anti-KSHV antibody in sera from blood donors, HIV+ patients with or without KS, and patients with classic KS.
(A) The lower limit of ELISA sensitivity in serum samples was set at 300 pg/mL vIL-6. (B) IgG antibody for the whole KSHV lysate was determined by ELISA. Optical density (OD) ratios were determined by dividing the OD reading of each sample by cut-off value (average of negative control × 3). Samples with OD ratio of at least 1.0 were interpreted as positive. Dotted bars indicate the 75thpercentile for each group. Statistical significance of group differences was determined by the Mann-Whitney test.
We measured circulating vIL-6 levels in patients with classic (HIV−) KS and HIV-associated KS (Figure 1A). Serum vIL-6 was detectable in 3 (23.1%) of 13 patients with classic KS (range, < 300-1445 pg/mL; median, < 300 pg/mL; and 75th percentile, < 300 pg/mL) and in 13 (38.2%) of 34 HIV+ patients with KS (range, < 300-7460 pg/mL; median, < 300 pg/mL; and 75th percentile, 1233 pg/mL). Compared with US blood donors, vIL-6 levels were significantly elevated in US HIV+ patients with KS (P < .0001, as measured by Mann-Whitney test), but not in patients with classic KS (P = .86). By contrast, KSHV antibody titers were markedly elevated in patients with both types of KS compared with blood donors (P < .0001). To better evaluate the results in HIV+ patients with KS, we measured serum vIL-6 in 30 HIV+, mostly (90.6%) homosexual, individuals without clinically apparent KS, PEL, or MCD. Serum vIL-6 was detected in 18 (60.0%) of these 30 HIV+ individuals (range, < 300-15 060 pg/mL; median, 406 pg/mL; and 75thpercentile, 1564 pg/mL). There was no significant difference in the frequency (P = .13, Fisher exact test) and levels of vIL-6 (P = .15, Mann-Whitney test) between these HIV+ patients with KS and those without KS, PEL, or MCD. By contrast, antibody titers to KSHV were detected more frequently in HIV+ patients with KS (23 [67.6%] of 34 patients) compared to those patients without KS, PEL, or MCD (4 [13.3%] of 30 patients) (P < .0001, Fisher exact test).
Serum samples were available from more than one time-point in 10 of 30 HIV+ patients. In most cases, serum levels of vIL-6 remained relatively stable for a period of months (Table1). hIL-6 was undetectable or was detected at low levels (range, < 1.0-116 pg/mL, and median, 2.4 pg/mL). We evaluated correlations between serum vIL-6 detection and a number of parameters in HIV infection. Our analysis extended to all 64 HIV+ patients with (n = 34) or without (n = 30) KS, where serum vIL-6 was either detected (n = 31) or not detected (n = 33). There was no direct association noted between vIL-6 levels and numbers of KS lesions (P = .13, Fisher exact test [n = 64]); KS severity by the TIS staging system17(v = 0.307, Cramer test [n = 64]); treatment with antiretroviral agents (P > .99, Fisher exact test [n = 64]); or antiherpesvirus agents (P = .27, Fisher exact test [n = 64]), HIV RNA load (P = .95, Mann-Whitney test [n = 35]), CD4 cell counts (P = .38, Mann-Whitney test [n = 64]), or lymphadenopathy (P = .15, Fisher exact test [n = 64]). These results are consistent with previous studies showing that KSHV infects a substantial subset of homosexual HIV+ patients19,20 and that KSHV replicates in only a minority of spindle cells within KS lesions.16,21 A more recent study demonstrated cell-free KSHV DNA in the circulation of most HIV-1/KSHV–coinfected subjects regardless of KS disease status.22 These results further suggest that KSHV frequently replicates and expresses vIL-6 in some tissue other than the KS lesions in KSHV-infected HIV+ patients.
Clinical events and laboratory findings in patients with HIV infection
| Case no. . | Sampling mo/y . | KS . | vIL-6 pg/mL . | hIL-6 pg/mL . | Anti-KSHV IgG OD ratio* . | CD4/CD8 cells/μL . | Antiretroviral therapy . | Anti-KS treatment . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| NRTI . | NNRTI . | PI . | ||||||||
| 1 | 12/87 | − | < 300 | < 1.0 | < 1.0 | 19/477 | AZT, ddC | — | — | — |
| 4/88 | + | 370 | 60.7 | < 1.0 | 2/317 | AZT | — | — | Irradiation | |
| 2 | 9/93 | + | < 300 | 3.8 | 5.92 | 242/591 | AZT | — | — | — |
| 4/99 | + | 5361 | 23.3 | 4.62 | 122/514 | ABV | NVP | IDV | ADM | |
| 6/99 | + | 7460 | < 1.0 | 4.95 | 32/255 | ABV | NVP | IDV | ADM | |
| 3 | 6/87 | + | < 300 | < 1.0 | 3.86 | 875/410 | AZT | — | — | — |
| 7/87 | + | < 300 | < 1.0 | 3.71 | 972/1458 | AZT | — | — | — | |
| 4 | 5/89 | + | < 300 | 4.6 | < 1.0 | 441/2001 | AZT | — | — | — |
| 8/89 | + | 470 | 25.0 | < 1.0 | 502/2279 | — | — | — | — | |
| 5 | 3/98 | + | 530 | 6.2 | 6.26 | 601/1328 | AZT, 3TC | — | NFV | Thalidomide |
| 4/99 | + | 714 | 3.7 | 6.61 | 714/1006 | AZT, 3TC | — | NFV | IL-12 | |
| 6/99 | + | 671 | 3.8 | 7.41 | 505/1173 | AZT, 3TC | — | NFV | IL-12 | |
| 6 | 8/98 | + | < 300 | 65.9 | 5.74 | 275/1132 | D4T, 3TC | — | IDV | — |
| 4/99 | + | < 300 | < 1.0 | 4.78 | 287/670 | D4T, 3TC | — | IDV | Thalidomide | |
| 5/99 | + | < 300 | 7.6 | 3.32 | 345/782 | D4T, 3TC | — | IDV | ADM | |
| 7 | 4/99 | + | < 300 | < 1.0 | 6.16 | 144/883 | D4T, 3TC | — | NFV | — |
| 6/99 | + | < 300 | 3.8 | 6.00 | 137/906 | D4T, 3TC | — | NFV | ADM | |
| 8 | 4/99 | + | 4507 | < 1.0 | 4.91 | 260/398 | AZT, 3TC | — | NFV | CDV |
| 6/99 | + | 4753 | < 1.0 | 4.03 | 325/340 | ABV | NVP | SQV, RTV | ADM | |
| 9 | 4/99 | − | 10 384 | < 1.0 | 5.91 | 441/2001 | F-ddA, D4T | — | NFV | — |
| 6/99 | − | 11 311 | < 1.0 | 5.69 | 502/2279 | ddI, ABV | — | SQV, RTV | — | |
| 10 | 4/99 | − | < 300 | < 1.0 | < 1.0 | 28/936 | F-ddA, D4T | — | — | — |
| 6/99 | − | < 300 | 2.4 | < 1.0 | 32/1116 | — | — | — | — | |
| Case no. . | Sampling mo/y . | KS . | vIL-6 pg/mL . | hIL-6 pg/mL . | Anti-KSHV IgG OD ratio* . | CD4/CD8 cells/μL . | Antiretroviral therapy . | Anti-KS treatment . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| NRTI . | NNRTI . | PI . | ||||||||
| 1 | 12/87 | − | < 300 | < 1.0 | < 1.0 | 19/477 | AZT, ddC | — | — | — |
| 4/88 | + | 370 | 60.7 | < 1.0 | 2/317 | AZT | — | — | Irradiation | |
| 2 | 9/93 | + | < 300 | 3.8 | 5.92 | 242/591 | AZT | — | — | — |
| 4/99 | + | 5361 | 23.3 | 4.62 | 122/514 | ABV | NVP | IDV | ADM | |
| 6/99 | + | 7460 | < 1.0 | 4.95 | 32/255 | ABV | NVP | IDV | ADM | |
| 3 | 6/87 | + | < 300 | < 1.0 | 3.86 | 875/410 | AZT | — | — | — |
| 7/87 | + | < 300 | < 1.0 | 3.71 | 972/1458 | AZT | — | — | — | |
| 4 | 5/89 | + | < 300 | 4.6 | < 1.0 | 441/2001 | AZT | — | — | — |
| 8/89 | + | 470 | 25.0 | < 1.0 | 502/2279 | — | — | — | — | |
| 5 | 3/98 | + | 530 | 6.2 | 6.26 | 601/1328 | AZT, 3TC | — | NFV | Thalidomide |
| 4/99 | + | 714 | 3.7 | 6.61 | 714/1006 | AZT, 3TC | — | NFV | IL-12 | |
| 6/99 | + | 671 | 3.8 | 7.41 | 505/1173 | AZT, 3TC | — | NFV | IL-12 | |
| 6 | 8/98 | + | < 300 | 65.9 | 5.74 | 275/1132 | D4T, 3TC | — | IDV | — |
| 4/99 | + | < 300 | < 1.0 | 4.78 | 287/670 | D4T, 3TC | — | IDV | Thalidomide | |
| 5/99 | + | < 300 | 7.6 | 3.32 | 345/782 | D4T, 3TC | — | IDV | ADM | |
| 7 | 4/99 | + | < 300 | < 1.0 | 6.16 | 144/883 | D4T, 3TC | — | NFV | — |
| 6/99 | + | < 300 | 3.8 | 6.00 | 137/906 | D4T, 3TC | — | NFV | ADM | |
| 8 | 4/99 | + | 4507 | < 1.0 | 4.91 | 260/398 | AZT, 3TC | — | NFV | CDV |
| 6/99 | + | 4753 | < 1.0 | 4.03 | 325/340 | ABV | NVP | SQV, RTV | ADM | |
| 9 | 4/99 | − | 10 384 | < 1.0 | 5.91 | 441/2001 | F-ddA, D4T | — | NFV | — |
| 6/99 | − | 11 311 | < 1.0 | 5.69 | 502/2279 | ddI, ABV | — | SQV, RTV | — | |
| 10 | 4/99 | − | < 300 | < 1.0 | < 1.0 | 28/936 | F-ddA, D4T | — | — | — |
| 6/99 | − | < 300 | 2.4 | < 1.0 | 32/1116 | — | — | — | — | |
OD ratios were determined by dividing the OD reading of each sample by cut-off value (average of negative control) × 3. Samples with OD ratio 1.0 were interpreted as positive.
KS indicates Kaposi sarcoma; vIL-6, viral interleukin-6; hIL-6, human IL-6; KSHV, Kaposi sarcoma–associated herpesvirus; IgG, immunoglobulin G; OD, optical density; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-NRTI; PI, protease inhibitor; AZT, zidovudine; ddC, zalcitabine; ABV, abacavir; NVP, nevirapine; IDV, indinavir; ADM, liposomed adriamycin; NFV, nelfinavir; 3TC, lamivudine; D4T, stavudine; CDV, cidofovir; SQV, saquinavir; RTV, ritonavir; F-ddA, lodenosine; ddI, didanosine.
Unlike KS lesions, hIL-6 and vIL-6 are commonly expressed in MCD and PEL tissues.13 By immunohistochemistry, the vIL-6+ cells are confined to the mantle zones,13,15 whereas the hIL-6+ cells are in the germinal centers.15 Additionally (Table2), vIL-6 was detected in 3 of 7 HIV+ patients with MCD and/or PEL at first sampling (range, < 300-11 833 pg/mL). On repeated sampling, 6 (85.7%) of 7 patients had circulating vIL-6 at least once. In contrast to KS, vIL-6 serum levels fluctuated widely in HIV+ patients with MCD. This finding is consistent with recent studies showing that KSHV load fluctuates markedly over short time periods in AIDS-MCD patients,23 24 suggesting that KSHV can undergo frequent bursts of replication in this disease. hIL-6 was detected at high (> 1000 pg/mL) levels in 3 of 11 MCD patients on at least one occasion, but was otherwise absent or present at low levels (Table 2). There was no correlation noted between serum levels of hIL-6 and vIL-6 in this group (P = .94, Spearman rank correlation). These results suggest that cellular IL-6 and vIL-6 are differently regulated and perhaps play different roles in the pathogenesis of MCD.
Human and viral interleukin-6 in sera from patients with multicentric Castleman disease and/or primary effusion lymphoma
| Case no. . | HIV status . | MCD . | PEL . | Related diseases . | Time of 2nd/3rd sampling . | vIL-6 pg/mL . | hIL-6 pg/mL . | Anti-KSHV IgG OD ratio* . |
|---|---|---|---|---|---|---|---|---|
| 11 | + | + | − | — | — | < 300 | 11.6 | 8.23 |
| 2 days later | 1076 | 5.8 | 9.01 | |||||
| 13 days later | 2628 | 1964.4 | 9.63 | |||||
| 12 | + | + | − | KS | — | 5795 | < 1.0 | 6.24 |
| 13 | + | + | − | AIHA | — | < 300 | 43.4 | 6.38 |
| 14 | + | + | − | AIHA | — | 766 | 6096.7 | 2.36 |
| 10 days later | 2549 | 6432.8 | 2.38 | |||||
| 15 | + | + | + | — | — | < 300 | 18 113.0 | 10.62 |
| 40 days later | 17 804 | 134.0 | 9.87 | |||||
| 16 | + | − | + | NHL in CNS | — | < 300 | 54.9 | < 1.0 |
| 17 | + | − | + | — | — | 11 833 | < 1.0 | 8.49 |
| 7 months later | 448 | < 1.0 | 9.73 | |||||
| 18 | − | + | − | — | — | < 300 | 31.7 | < 1.0 |
| 19 | − | + | − | — | — | < 300 | 38.8 | < 1.0 |
| 20 | − | + | − | — | — | < 300 | < 1.0 | < 1.0 |
| 21 | − | + | − | — | — | < 300 | < 1.0 | < 1.0 |
| Case no. . | HIV status . | MCD . | PEL . | Related diseases . | Time of 2nd/3rd sampling . | vIL-6 pg/mL . | hIL-6 pg/mL . | Anti-KSHV IgG OD ratio* . |
|---|---|---|---|---|---|---|---|---|
| 11 | + | + | − | — | — | < 300 | 11.6 | 8.23 |
| 2 days later | 1076 | 5.8 | 9.01 | |||||
| 13 days later | 2628 | 1964.4 | 9.63 | |||||
| 12 | + | + | − | KS | — | 5795 | < 1.0 | 6.24 |
| 13 | + | + | − | AIHA | — | < 300 | 43.4 | 6.38 |
| 14 | + | + | − | AIHA | — | 766 | 6096.7 | 2.36 |
| 10 days later | 2549 | 6432.8 | 2.38 | |||||
| 15 | + | + | + | — | — | < 300 | 18 113.0 | 10.62 |
| 40 days later | 17 804 | 134.0 | 9.87 | |||||
| 16 | + | − | + | NHL in CNS | — | < 300 | 54.9 | < 1.0 |
| 17 | + | − | + | — | — | 11 833 | < 1.0 | 8.49 |
| 7 months later | 448 | < 1.0 | 9.73 | |||||
| 18 | − | + | − | — | — | < 300 | 31.7 | < 1.0 |
| 19 | − | + | − | — | — | < 300 | 38.8 | < 1.0 |
| 20 | − | + | − | — | — | < 300 | < 1.0 | < 1.0 |
| 21 | − | + | − | — | — | < 300 | < 1.0 | < 1.0 |
Samples with OD ratio 1.0 were interpreted as positive.
MCD indicates multicentric Castleman disease; PEL, primary effusion lymphoma; AIHA, autoimmune hemolytic anemia; NHL, non-Hodgkin lymphoma; CNS, central nervous system; for other abbreviations, see Table 1.
Overall, serum vIL-6 was detected in 46 (52.3%) of 88 serum samples from 71 HIV+ patients, as opposed to 8 (10.3%) of 78 serum samples from 78 HIV− individuals. Circulating vIL-6 levels were associated with HIV+ status (P < .0001, Mann-Whitney test) and the presence of anti-KSHV IgG (P = .013, Mann-Whitney test). However, within the HIV+ cohort, serum vIL-6 levels were not associated with the occurrence of KSHV-associated malignancies (P = .43, Mann-Whitney test). In addition to KSHV-mediated immune evasion mechanisms,25 HIV-induced immunodeficiency may allow for increased KSHV replication and vIL-6 expression in KSHV-infected patients with AIDS. In spite of considerable effort, the sites of initial KSHV infection and replication and the sites of viral latency and subsequent reactivation are incompletely characterized. Many of the currently available serological assays for the detection of KSHV antibodies have inadequate concordance with each other.18 19 Thus, when suspecting KSHV infection, vIL-6 testing may provide useful and complementary information on the occurrence of KSHV replication.
We thank Drs Elaine S. Jaffe and Stefania Pittaluga for reviewing lymph node sections of MCD cases; Dr James Pluda for his assistance in caring for the patients; Drs Michael W. Baseler, David J. Waters, and Randy A. Stevens for coordinating the fluorescence-activated cell sorter (FACS) and HIV RNA viral load testing; the staff of the Medicine Branch and the HIV and AIDS Malignancy Branch, National Institutes of Health (NIH) Clinical Center, Bethesda, MD, for their help with patients on NIH protocols; Drs Aikichi Iwamoto, Carlo Parravicini, and James Braun for providing serum samples; Dr Ghanshyam Gupta for his help in statistical analysis; and Drs Yuan Chang and Patrick Moore for helpful discussions.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yoshiyasu Aoki, Medicine Branch, National Cancer Institute, National Institutes of Health, Bldg 10 Rm 12C207, 9000 Rockville Pike, Bethesda, MD 20892; e-mail: aokiy@mail.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal