Nontransferrin-bound iron (NTBI) appears in the serum of individuals with iron overload and in a variety of other pathologic conditions. Because NTBI constitutes a labile form of iron, it might underlie some of the biologic damage associated with iron overload. We have developed a simple method for NTBI determination, which operates in a 96-well enzyme-linked immunosorbent assay format with sensitivity comparable to that of previous assays. A weak ligand, oxalic acid, mobilizes the NTBI and mediates its transfer to the iron chelator deferoxamine (DFO) immobilized on the plate. The amount of DFO-bound iron, originating from NTBI, is quantitatively revealed in a fluorescence plate reader by the fluorescent metallosensor calcein. No NTBI is found in normal sera because transferrin-bound iron is not detected in the assay. Thalassemic sera contained NTBI in 80% of the cases (range, 0.9-12.8 μmol/L). In patients given intravenous infusions of DFO, NTBI initially became undetectable due to the presence of DFO in the sera, but reappeared in 55% of the cases within an hour of cessation of the DFO infusion. This apparent rebound was attributable to the loss of DFO from the circulation and the possibility that a major portion of NTBI was not mobilized by DFO. NTBI was also found in patients with end-stage renal disease who were treated for anemia with intravenous iron supplements and in patients with hereditary hemochromatosis, at respective frequencies of 22% and 69%. The availability of a simple assay for monitoring NTBI could provide a useful index of iron status during chelation and supplementation treatments.
The presence of nontransferrin-bound iron (NTBI) in the circulation is a pathologic phenomenon, which generally occurs in patients with iron-overload conditions1-3 and is thought to be absent from healthy individuals in whom virtually all the serum iron is bound to transferrin (Tf). Until recently, NTBI has been thought to arise from either repeated transfusions, which are required by patients with various hemolytic diseases, hemoglobinopathies such as thalassemia, or excessive iron absorption associated with hereditary hemochromatosis (HHC). However, NTBI can also appear in the circulation of patients undergoing chemotherapy,4,5 heart bypass operations,6 and other conditions in which large amounts of iron from hemoglobin catabolism are suddenly released into the circulation. Recently, NTBI was also found in dialysis patients treated for anemia with erythropoietin and iron supplements (personal communication, Prof J.J.M. Marx, University Hospital, Uttrecht, Holland).
The clinical significance of NTBI is threefold: it is a potential target of iron chelators; it could serve as an indicator of the iron status of an individual who is already iron overloaded or at risk; and it may participate as one of the causative factors in the tissue loading with iron, which can lead to cardiac, hepatic, and endocrine dysfunction. The first two points highlight the potential importance of a convenient and reliable method for the detection of NTBI. In the first case, it would allow the physician to assess the efficacy of an iron-overloaded patient's chelation regimen and modify it accordingly. In the second case, it could be used to help diagnose individuals who already have or are predisposed to iron overload. This paper introduces a novel assay for NTBI and shows its application to monitoring chelator activity in patients with thalassemia and detecting NTBI in a high-risk population such as patients with HHC and a lower risk population such as patients on dialysis who receive iron supplementation. Results also show, in accordance with previous reports,5 that NTBI can occur in sera with less than full transferrin saturation.
Materials and methods
NTBI assay
The assay for NTBI is schematized in Figure1.
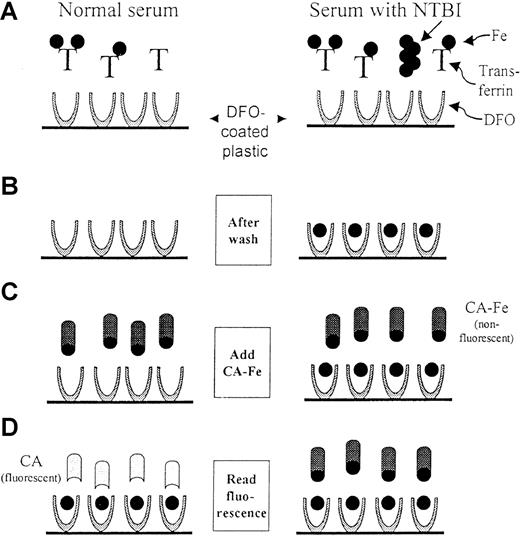
Scheme of the NTBI assay.
The scheme depicts the 4 basic steps of the assay for both normal (left) and NTBI-containing (right) sera. The iron is depicted as a full circle and the transferrin molecules denoted by T. Step 1: A serum sample, which has been premixed with 100 mmol/L oxalate, 2.5 mmol/L MnCl2, and 20 mmol/L HEPES pH 7.4, is added to DFO-coated wells. The mobilized NTBI binds to the DFO on the plastic during a 2-hour incubation. Step 2: Only the DFO-bound iron remains after the wells are washed. Step 3: Calcein-Fe complex (CA-Fe) is added to the wells. CA-Fe is nonfluorescent because of quenching of calcein by the bound iron. Step 4: The fluorescence is measured after a 2-hour incubation, during which the remaining available DFO molecules withdraw iron from CA-Fe, causing the released calcein to become fluorescent. For normal serum, a maximum level of fluorescence is attained, whereas for NTBI-containing serum, fluorescence is relatively lower. The fluorescence generated is inversely proportional to the concentration of NTBI in the original sample.
Scheme of the NTBI assay.
The scheme depicts the 4 basic steps of the assay for both normal (left) and NTBI-containing (right) sera. The iron is depicted as a full circle and the transferrin molecules denoted by T. Step 1: A serum sample, which has been premixed with 100 mmol/L oxalate, 2.5 mmol/L MnCl2, and 20 mmol/L HEPES pH 7.4, is added to DFO-coated wells. The mobilized NTBI binds to the DFO on the plastic during a 2-hour incubation. Step 2: Only the DFO-bound iron remains after the wells are washed. Step 3: Calcein-Fe complex (CA-Fe) is added to the wells. CA-Fe is nonfluorescent because of quenching of calcein by the bound iron. Step 4: The fluorescence is measured after a 2-hour incubation, during which the remaining available DFO molecules withdraw iron from CA-Fe, causing the released calcein to become fluorescent. For normal serum, a maximum level of fluorescence is attained, whereas for NTBI-containing serum, fluorescence is relatively lower. The fluorescence generated is inversely proportional to the concentration of NTBI in the original sample.
1. Samples of 20 μL of serum (defined as “input sample”) are placed in disposable plastic 96-well plates (“mixing plate”). All samples are prepared in duplicate.
2. To each sample is added 230 μL of a solution containing 100 mmol/L sodium oxalate (BDH Chemicals Ltd, Poole, UK), 20 mmol/L HEPES (Biological Industries, Kibbutz Beit Haemek, Israel), pH 7.4 containing 2.5 mmol/L MnCl2 (Aldrich Chemical Co, Milwaukee, WI). The oxalate-HEPES solution and the MnCl2 (1 mol/L stock) are kept as frozen aliquots and mixed just before use. The iron content of the sodium oxalate is less than 0.001% according to the manufacturer. We have determined the iron concentration in the 100 mmol/L sodium oxalate solution as 0.7 μmol/L, using the bathophenanthroline sulfonate method (data not shown).
3. From the diluted samples, 100 μL is transferred in duplicate to 96-well plates coated with deferoxamine (DFO). For preparation of plates see below.
4. After 2 hours of incubation at 37°C, the plates are washed twice with distilled water, once with 5 mmol/L EDTA (pH 8.0), then twice with distilled water.
5. To each well is added 0.1 mL of a preformed calcein-iron complex (CA-Fe) consisting of 600 nmol/L calcein (Sigma Chemical, St Louis, MO), 540 nmol/L ferrous ammonium sulfate (FAS) in 20 mmol/L HEPES, 150 mmol/L NaCl, pH 7.3 (HBS), and the plate is incubated for 2 hours at 37°C.
6. The fluorescence in the wells is determined in a multiwell plate reader (BMG Lab Technologies, Offenburg, Germany) with excitation/emission filters of 485/538 nm and gain of 25.
Preparation of DFO-coated plates
1. A solution is prepared containing hydroxyethyl-starch-DFO, 0.5 mg/mL (Biomedical Frontiers, Minneapolis, MN), in 10 mmol/L sodium phosphate (dibasic), pH 8.6.
2. From this solution, 100 μL/well is added to 96-well polystyrene plates (F96 Maxisorp; Nunc, Roskilde, Denmark) and the plates are incubated at 4°C for 72 hours.
3. The plates are washed twice with distilled water and, after shaking off all excess water, can be used immediately, or stored at 4°C for at least 2 months. The degree of variability in DFO activity between wells of a plate, as determined by using CA-Fe de-quenching in blank plates, is less than 4%. The degree of variability between different plates is less than 11%.
Calibration
Calibration is shown in Figure 2.
Calibration of iron concentration versus fluorescence.
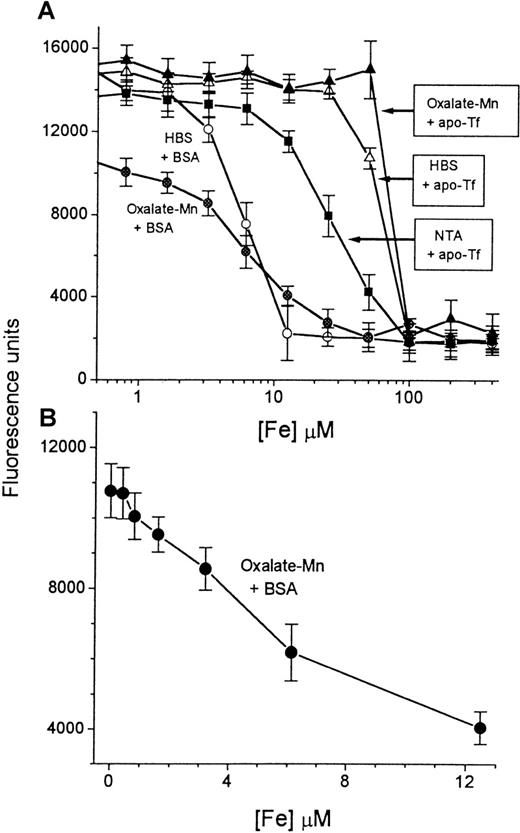
A series of concentrations of iron, ranging from 0 to 400 μmol/L (described in “Materials and methods”), was prepared in HBS buffer containing either BSA (circles) or apo-Tf (triangles). A 20-μL sample from each solution (“input sample”) was mixed with either HBS containing 2 mmol/L NaHCO3 (empty symbols) or oxalate-Mn reagent (filled symbols) (final volume, 250 μL). Aliquots of 100 μL were transferred to DFO-coated plates and processed in the NTBI assay as described in “Materials and methods.” The fluorescence was read after 2 hours of incubation with CA-Fe and plotted semilogarithmically against the concentration of iron in the original 20-μL input sample (A). The most sensitive region of the calibration curve (0-12.5 μmol/L Fe) was plotted separately as a linear graph (B). Bars indicate standard deviation of the mean of 4 individual samples.
Calibration of iron concentration versus fluorescence.
A series of concentrations of iron, ranging from 0 to 400 μmol/L (described in “Materials and methods”), was prepared in HBS buffer containing either BSA (circles) or apo-Tf (triangles). A 20-μL sample from each solution (“input sample”) was mixed with either HBS containing 2 mmol/L NaHCO3 (empty symbols) or oxalate-Mn reagent (filled symbols) (final volume, 250 μL). Aliquots of 100 μL were transferred to DFO-coated plates and processed in the NTBI assay as described in “Materials and methods.” The fluorescence was read after 2 hours of incubation with CA-Fe and plotted semilogarithmically against the concentration of iron in the original 20-μL input sample (A). The most sensitive region of the calibration curve (0-12.5 μmol/L Fe) was plotted separately as a linear graph (B). Bars indicate standard deviation of the mean of 4 individual samples.
1. To 0.1 mL of 10 mmol/L FAS (freshly prepared; Sigma Chemical) in distilled water in a polyethylene tube is added 0.1 mL of 70 mmol/L nitrilotriacetate, sodium form, pH 7.0. After 5 minutes, 0.3 mL water is added to give 2 mmol/L Fe+++.
2. A serial dilution of 1:1 in water, using 50 μL for each dilution step, is performed for up to 12 steps in polyethylene tubes, giving 50 μL of iron at concentrations from 400 down to 0.2 μmol/L.
3. To each tube is added 0.2 mL bovine serum albumin (BSA, Fraction V; Sigma Chemical), 10 mg/mL in HBS.
4. A sample of 20 μL is transferred to the “mixing plate” and subsequently processed in the same way as serum samples.
Preparation of CA-Fe
1. A stock solution of the CA-Fe complex is produced by mixing 20 mmol/L FAS (prepared fresh in deionized water) with 40 μmol/L calcein (Sigma Chemical) to give final concentrations of 40 μmol/L calcein and 36 μmol/L iron (CA-Fe ratio is 1.0:0.9).
2. The mixture is incubated in the dark at 37°C for 30 minutes.
3. The CA-Fe complex is divided into aliquots and stored frozen. The solution is stable for up to 3 months in the freezer, but thawed solutions are not reused.
4. For use in the assay, the concentrated stock CA-Fe (40 μmol/L:36 μmol/L) is freshly diluted 1:67 in HBS 150 mmol/L NaCl, 20 mmol/L HEPES, pH 7.3 to produce a final concentration of 0.6 μmol/L CA-Fe.
Modification for measurements of NTBI in sera with transferrin subsaturation values
The previously described method was modified for sera with transferrin saturation values in the range of 30% to 60%, to minimize apo-Tf–mediated sequestration of NTBI, which had been mobilized by the oxalate-manganese reagent. The NTBI solubilizing reagent used in step 2 of the method was changed from 100 mmol/L oxalate, 20 mmol/L HEPES, 2.5 mmol/L MnCl2, pH 7.4, to 200 mmol/L oxalate, 20 mmol/L HEPES, 20 mmol/L MnCl2, 1 μmol/L FeCl3, pH 7.4. The addition of FeCl3 (BDH Chemicals, Ltd), prepared as a 1 mmol/L stock in 0.1 mol/L HCl from anhydrous powder, was required for lowering the concentration of available apo-Tf. The Mn++ concentration was increased from 10 to 20 mmol/L and the oxalate concentration was doubled from 100 to 200 mmol/L to ensure a high oxalate–Mn ratio and to preclude the possible formation of oxalate-metal precipitates.
The basis for the use of increased Mn++ concentration was empirical. A series of transition metals were tested at different concentrations for the capacity to enhance the detection of NTBI in thalassemic sera. The rationale behind this approach was to find a metal that would block both specific (ie, apo-Tf) and nonspecific iron-binding sites that might be present in serum samples. Among the metals tested, Mn++ was the most suitable because it enhanced the relative changes in fluorescence and did not interfere with the assay. Subsequently, we found that Mn++facilitates the binding of Fe+++ by DFO, in the presence of high oxalate concentrations. This was determined by using the iron probe N-(fluorescein-5-thiocarbamoyl) desferrioxamine (Fl-DFO; Molecular Probes, Eugene, OR), whose fluorescence is quenched stoichiometrically by Fe+++ under conditions that mimic those in the assay. The rate of quenching of Fl-DFO (2.5 μmol/L) by ferric ammonium citrate (20 μmol/L) was 5-fold slower in the presence (t1/2 = 12.5 minutes) than in the absence of 200 mmol/L oxalate (t1/2 = 2.5 minutes), but was only 2.6-fold slower when 20 mmol/L MnCl2 was added to the oxalate (t1/2 = 6.5 minutes). The effect of Mn++ was concentration dependent and was maximal at 20 mmol/L (Breuer et al, unpublished data). Hence, we attribute the enhancement of the sensitivity of the assay by Mn++ to its facilitation of DFO binding of iron. Thalassemic sera, which generally have very low iron-binding activity, showed detectable NTBI in the presence of 2.5 mmol/L Mn++; however, NTBI was detectable in sera with higher iron-binding activity (low transferrin saturation) only when 20 mmol/L Mn++ was used.
The above modification results in a 20% decrease in the fluorescence signals obtained in the reagent blanks (bovine serum albumin [BSA] 10 mg/mL in HBS) and iron standards and, therefore, requires the establishment of an appropriate calibration curve using the modified oxalate-Mn-Fe reagent. This curve (Figure 5) is parallel to the one shown in Figure 2. To preclude the possibility of false-positive values, each assay plate contains 4 reagent-blank wells with 20 μL BSA 10 mg/mL in HBS. These 4 wells provide the zero NTBI value for each particular plate and are used to adjust the values obtained in each test plate to the iron standard curve, which is generated in a different plate. This adjustment depends on the variability in DFO content between plates, which fluctuates between 0% and 11%.
Patient treatments
The study included 12 patients, aged 10 to 16, all with transfusional iron overload. Seven had thalassemia major, 3 had thalassemia intermedia, and 2 had aplasia. All the drug treatments carried out in this study were within the framework of their routine therapeutic regimens.
The 9 patients undergoing DFO infusion kinetics arrived at the clinic in the morning, having been instructed not to take any medications before their arrival. A serum sample was taken (time 0′) and, immediately afterward, the patient began to receive an infusion of DFO. The total DFO dose was usually 1 g; in a few cases it was only 0.5 g. Duration of the infusion was between 30 and 60 minutes.
Sera routinely taken for ferritin estimation were used for serum sampling for NTBI in patients with end-stage renal disease (ESRD).
Results
Configuration of the NTBI assay
The procedure of the NTBI assay (schematized in Figure 1) is based on the binding of mobilized NTBI to DFO immobilized in 96-well plates. The amount of DFO-bound iron is quantified by use of the metal probe calcein complexed to iron, which becomes fluorescent following the withdrawal of the iron by DFO.7-9 We found that to obtain optimal detection with the immobilized DFO, it was necessary to mobilize the NTBI using a charged low-molecular-weight chelator, oxalate. Nitrilotriacetate, which is used as a mobilizing agent in the assay developed by Singh et al,3 is not compatible with our method, because it can remove iron from transferrin when present together with DFO11 (Figure 2). Oxalate does not appear to share this property with nitrilotriacetate in our system, although it can still mobilize NTBI. In preliminary experiments with thalassemic sera, we found that the presence of oxalate was necessary; NTBI was detected in only 3 of 15 cases when sera were tested without oxalate, but in 12 of 15 cases when tested with oxalate (data not shown). This finding suggests that NTBI may have 2 distinct chemical forms, the first directly chelatable by DFO and the second that requires prior mobilization by a charged low-molecular-weight chelator. Only the results obtained with oxalate are reported here.
Calibration of the NTBI assay
The sensitivity of the assay was examined by preparing a series of iron concentrations in HBS solution containing BSA, 10 mg/mL (Figure2A). The titration curve performed with HBS-BSA shows that the iron-binding capacity of the wells is saturated when the iron concentration of the input sample (defined in “Materials and methods”) is 12.5 μmol/L. The calculated total iron-binding capacity of each well, based on this value, is 100 pmol. This was confirmed by exposing the wells to increasing concentrations of CA-Fe complex and determining the fluorescence after 2 hours of incubation. The curve of fluorescence units (FUs) generated versus picomoles iron added (in the form of CA-Fe) leveled off at 112 pmol Fe/well (data not shown), indicating that saturation of the DFO sites in the well occurs at this value. The titration curve shown in Figure 2A for samples with BSA was altered by the presence of oxalate-Mn. We attribute the decrease in slope to the action of oxalate as a competing ligand, which causes an apparent change in the affinity of the DFO in the well. The failure to reach the maximal level of fluorescence (15 000 U) in the presence of oxalate is attributable to contaminating iron in different components (100 mmol/L oxalate contains 0.7 μmol/L Fe, see “Materials and methods”). The useful range of the titration curve carried out under standard assay conditions (ie, in the presence of oxalate-Mn) is shown in Figure 2B, corresponding to iron concentrations of 0 to 12.5 μmol/L in the input sample. The relationship between FUs obtained and iron concentration is linear down to about 8 μmol/L, with a lower limit of detection of 1 μmol/L. A standard curve of the type shown in Figure 2B is routinely used for calibrating NTBI in serum samples.
To show that transferrin-bound iron is not detected by the assay, we generated Tf-Fe by incubating a fixed concentration of apo-Tf with increasing concentrations of iron and determining the iron concentration in the samples by the assay (Figure 2A). At the concentration of apo-Tf used (3.2 mg/mL), the total theoretical iron-binding capacity was approximately 80 μmol/L. In the presence of oxalate-Mn, iron was not detectable at concentrations below 80 μmol/L. This indicates that transferrin-bound iron is spared by the assay. We have found that oxalate-Mn appears to be slightly superior to HBS-NaHCO3 at sparing transferrin-bound iron. In contrast, nitrilotriacetate (5 mmol/L) causes iron to be detectable at concentrations less than 80 μmol/L, indicating removal of iron from transferrin.
Application of the assay to serum samples from iron-overloaded patients
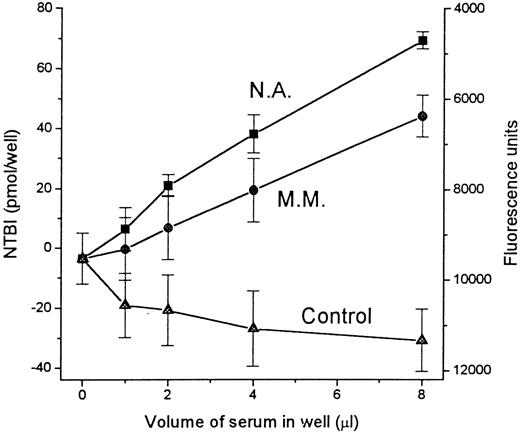
We initially examined the working range of the assay as a function of the serum sample volume (Figure 3). For most thalassemic patients, the highest sensitivity was obtained with serum samples of 8 μL applied per well. Under these experimental conditions, the amounts of iron detected were within the iron-binding capacity of the wells. Two thalassemic sera and 1 control serum were serially diluted in HBS containing 10 mg/mL BSA and equivalent sample volumes (20 μL) from each dilution were processed as described in “Materials and methods.” A linear correlation was observed between the volume of serum in the wells, the fluorescent signal generated, and NTBI in pmol/well (obtained from the standard curve in Figure 2B) for both thalassemic sera. In Figure 3, the NTBI values are expressed in terms of pmol/well. To convert pmol/well to pmol/μL serum (= μmol/L NTBI in serum), we corrected for the volumes and dilutions made. Thus, for the input sample of 20 μL serum, only 8 μL was finally applied to each well. Hence, to obtain the actual NTBI concentration in the serum in μmol/L, the pmol/well value was multiplied by the factor 2.5 (= 20/8). In this way, we calculated the NTBI concentrations of the 2 thalassemic sera to be 8.7 and 5.5 μmol/L. The control serum sample showed virtually no change in signal with increasing input volume of the original serum sample. The apparently negative values obtained for the control are attributed to the sequestration of contaminant iron in the oxalate-Mn reagent by apo-Tf. This effect of apo-Tf on samples with oxalate-Mn was also observed in Figure 2, where samples containing undetectable iron showed an apparent increase in the fluorescence generated with apo-Tf, but not with BSA. As shown in Figure 4, the contaminant iron is not a drawback, but in fact, an asset, because it provides an indication of the iron-binding capacity of a given serum sample.
NTBI as a function of the volume of serum added to the test wells.
Aliquots of serum from 2 thalassemics (N.A. and M.M.) and a healthy individual (Control) were serially diluted in HBS containing 10 mg/mL BSA, and assayed for NTBI as described in “Materials and methods” and in Figure 2. Depicted on the abscissa is the volume of the aliquot of original serum in the 100-μL sample applied to each well. The fluorescence intensity obtained for each sample is indicated on the right scale in terms of raw fluorescence units (descending scale). The fluorescence units were converted to iron concentrations (IC, in μmol/L), using the calibration curve depicted in Figure 2B and then to pmol/well by multiplying IC by the volume of original serum in each well. Negative values are due to chelation of contaminant iron in the assay reagent by serum transferrin. Bars indicate standard deviation of the mean of 4 individual samples.
NTBI as a function of the volume of serum added to the test wells.
Aliquots of serum from 2 thalassemics (N.A. and M.M.) and a healthy individual (Control) were serially diluted in HBS containing 10 mg/mL BSA, and assayed for NTBI as described in “Materials and methods” and in Figure 2. Depicted on the abscissa is the volume of the aliquot of original serum in the 100-μL sample applied to each well. The fluorescence intensity obtained for each sample is indicated on the right scale in terms of raw fluorescence units (descending scale). The fluorescence units were converted to iron concentrations (IC, in μmol/L), using the calibration curve depicted in Figure 2B and then to pmol/well by multiplying IC by the volume of original serum in each well. Negative values are due to chelation of contaminant iron in the assay reagent by serum transferrin. Bars indicate standard deviation of the mean of 4 individual samples.
Monitoring the dynamics of chelation by DFO with the NTBI assay.
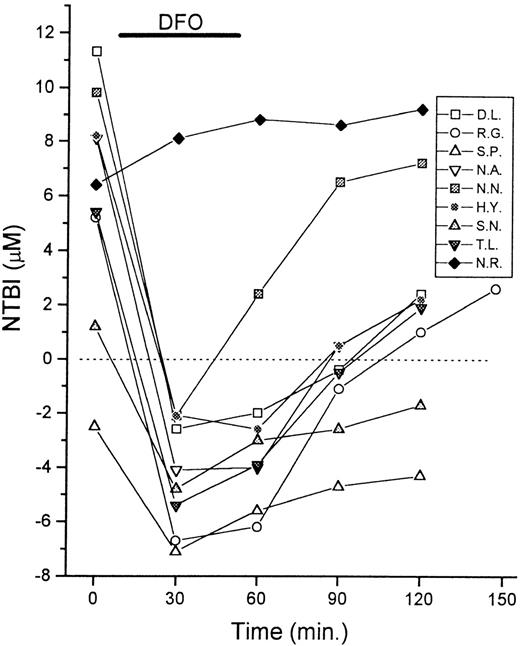
Serum samples of 9 patients with thalassemia were taken for NTBI measurements immediately before (0 minute) and at intervals of 30 minutes during and after infusion of DFO (0.5-1.0 g, IV). The duration of the treatment was 30 to 60 minutes, as indicated in the graph. The NTBI levels are shown in μmol/L as mean values of quadruplicates (SD < 10%). Negative values indicate chelation of contaminant iron present in reagents by serum components.
Monitoring the dynamics of chelation by DFO with the NTBI assay.
Serum samples of 9 patients with thalassemia were taken for NTBI measurements immediately before (0 minute) and at intervals of 30 minutes during and after infusion of DFO (0.5-1.0 g, IV). The duration of the treatment was 30 to 60 minutes, as indicated in the graph. The NTBI levels are shown in μmol/L as mean values of quadruplicates (SD < 10%). Negative values indicate chelation of contaminant iron present in reagents by serum components.
Reliability of the NTBI measurements
To assess the day-to-day reproducibility of the assay, we chose 16 thalassemic serum samples taken from 5 different patients and tested them on 3 different occasions, while freezing the sera in the intervals. The deviation of the individual NTBI measurements varied from 0.8% to 26%; the average deviation for all 16 sera was 8.3%. We also tested 9 patients repetitively at monthly intervals (Table1). Six of the patients showed relatively steady NTBI levels with less than 25% variability among samples. The 3 patients who showed significant fluctuations of more than 50% had lower NTBI values than the others. Two of them (S.N. and A.A.) have thalassemia intermedia; the third (S.P.) has thalassemia major, but is under aggressive combined chelation therapy of defferiprone (L1) daily and DFO once monthly. Therefore, we attribute the large fluctuations in these cases to individual variability rather than to assay-based errors.
Among 47 control sera from individuals without known iron-overload, no NTBI positives were found (under the assay conditions using 100 mmol/L oxalate, 2.5 mmol/L MnCl2), with values ranging from −1.8 to −5.2, indicating that the test does not give false-positive values.
Application of the test to monitoring the course of chelation therapy
We investigated the possible usefulness of the assay in monitoring the effectiveness of chelator treatment. Patients were sampled periodically during the course of intravenous (IV) infusion of DFO and their serum NTBI was measured (Figure 4). The NTBI became undetectable immediately after DFO infusion in 8 of 9 cases and reached apparently negative values. Cessation of DFO infusion (at around 60 minutes) was followed by a gradual return toward higher apparent NTBI values. The phenomenon of gradual NTBI rebound after DFO treatment has been documented previously by Porter et al,13 using a different NTBI assay, based on the mobilization of NTBI with nitrilotriacetate and its detection with the chelator L1. The NTBI rebound effect would be consistent with the facts that DFO has a short plasma half-life11 and that the plasma iron turnover in patients with thalassemia is 15-fold greater than normal.1,2,13 14
It should be emphasized that our results do not necessarily indicate the transient disappearance of NTBI from the serum during DFO infusion, but rather the presence of excess free DFO in the serum, which competes with the DFO immobilized on the assay plates for the soluble iron in the sample. The negative values are obtained due to binding of the contaminant iron in the oxalate-Mn reagent (see “Materials and methods”) by the free DFO in the serum sample, similar to the binding by apo-Tf in control serum samples. Therefore, although the chelator is present in the circulation, the present assay gives no indication of NTBI status, but rather monitors the chelator activity within a given serum sample. Because chelator kinetics can be variable,11 14 the assay could be a valuable indicator of chelator handling and activity in individual patients. This is illustrated by the patient N.R., who was known to be severely iron overloaded at the start of the treatment and, unlike the other patients, had never previously been treated with chelators (Figure 4). In this case, a slight increase rather than decrease in the apparent NTBI was obtained, presumably because the patient's iron load was in excess of the DFO infused. A subsequent infusion of DFO (1 week later) gave NTBI kinetics similar to those of the other patients (not shown).
Detection of NTBI in patients with transferrin saturation of 30% to 60%
It has been shown previously that NTBI can occur concurrently with transferrin saturation levels of less than 60% in patients with perturbations in iron metabolism.5 This feature necessitated a modification of the present method in an attempt to overcome the sequestration by apo-Tf of iron that had been mobilized by oxalate. Normal sera contain 20 to 30 μmol/L apo-Tf, or 40 to 60 μmol/L iron-binding activity, which is in excess of the observed NTBI concentrations (< 17 μmol/L). In preliminary experiments, using 1:1 mixtures of normal and thalassemic sera, we found that NTBI was not detectable in these mixtures when the oxalate-Mn reagent used for thalassemic sera (100 mmol/L oxalate, 2.5 mmol/L MnCl2) was used. However, NTBI was detectable when the oxalate-Mn reagent was modified to 200 mmol/L oxalate, 20 mmol/L MnCl2, 1 μmol/L FeCl3 (see “Materials and methods”). Although these maneuvers caused a decrease in the fluorescence signals obtained in the reagent blanks (BSA 10 mg/mL in HBS) and required the establishment of new standard curves for the oxalate-Mn-Fe reagent, they significantly increased the sensitivity of the assay in sera with low transferrin saturation. It is unlikely that these measures contributed to the generation of false-positive results, because the zero NTBI value in each assay plate is based on the signal obtained with the reagent blank solution in that particular plate (Figure5). The presence of 1 μmol/L FeCl3, in addition to the contaminant iron in the oxalate-Mn reagent (see “Materials and methods”), gave rise to apparently negative NTBI values in NTBI-free samples (from −2 to −16 μmol/L). Because these values generally correlated well with the transferrin saturation, they are considered to reflect the iron-binding capacity in the sera and, as such, do not detract from the reliability of the assay. Because the measures mentioned above do not completely block apo-Tf in sera with low transferrin saturation, this aspect of the assay will necessitate further optimization, possibly by using a Co+++-bicarbonate complex to block transferrin, as described by Gosriwatana et al.22
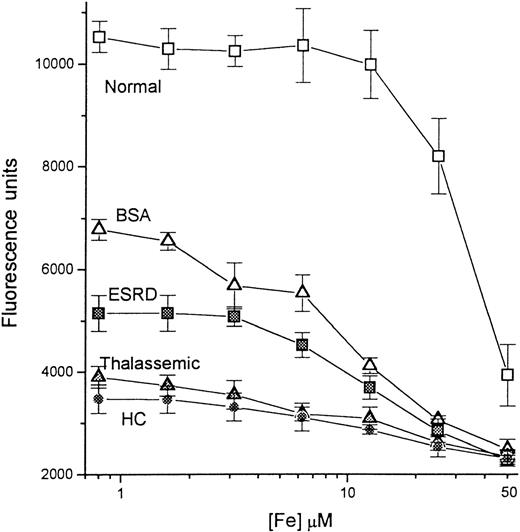
Calibration of iron concentration in the presence of normal and pathologic sera.
A series of concentrations of iron, ranging from 0 to 50 μmol/L, was prepared as described in “Materials and methods.” A 20-μL sample from each iron solution was mixed with 20 μL of either 10 mg/mL BSA in HBS buffer (BSA; empty triangles) or sera from a healthy individual (Normal, empty squares), a patient with end-stage renal disease (ESRD, filled squares), a thalassemic patient (Thalassemic; filled triangles), and a hemochromatosis patient (HC; filled circles), and incubated for 20 minutes. To each mixture was then added 210 μL oxalate-Mn-Fe reagent (200 mmol/L Na-oxalate, 20 mmol/L MnCl2, 1 μmol/L FeCl3) to give a final volume of 250 μL; aliquots of 100 μL were transferred to DFO-coated plates and processed as before. The fluorescence was read after 2 hours of incubation with CA-Fe and plotted semilogarithmically against the concentration of iron in the original 20-μL input sample.
Calibration of iron concentration in the presence of normal and pathologic sera.
A series of concentrations of iron, ranging from 0 to 50 μmol/L, was prepared as described in “Materials and methods.” A 20-μL sample from each iron solution was mixed with 20 μL of either 10 mg/mL BSA in HBS buffer (BSA; empty triangles) or sera from a healthy individual (Normal, empty squares), a patient with end-stage renal disease (ESRD, filled squares), a thalassemic patient (Thalassemic; filled triangles), and a hemochromatosis patient (HC; filled circles), and incubated for 20 minutes. To each mixture was then added 210 μL oxalate-Mn-Fe reagent (200 mmol/L Na-oxalate, 20 mmol/L MnCl2, 1 μmol/L FeCl3) to give a final volume of 250 μL; aliquots of 100 μL were transferred to DFO-coated plates and processed as before. The fluorescence was read after 2 hours of incubation with CA-Fe and plotted semilogarithmically against the concentration of iron in the original 20-μL input sample.
The detection of exogenous NTBI added to various serum types was examined (Figure 5). Iron in the form of Fe-nitrilotriacetate was mixed with the sera, and NTBI was assayed using the oxalate-Mn-Fe reagent. Four different sera were examined, 1 from a healthy individual and 3 from patients. The respective NTBI contents of the normal, the ESRD, thalassemic, and HHC sera were determined previously as 0, 6.2, 9.6, and 11.5 μmol/L. Parallel samples were prepared containing 10 mg/mL BSA. The BSA curve is defined as representative of a sample with neither NTBI nor iron-binding capacity and serves as the calibration curve. Based on this curve, fluorescence of more than 6600 U indicates an NTBI-negative sample, whereas fluorescence of less than 6600 U indicates an NTBI-positive sample. As expected, the normal serum, with no NTBI and a high iron-binding capacity, produced much higher fluorescence than BSA, and NTBI was detectable only when the concentration of added Fe-nitrilotriacetate was more than 30 μmol/L. This may not reflect the full iron-binding capacity of the serum, because the binding conditions were suboptimal due to the absence of added HCO3. The NTBI-containing sera showed a gradual increase in NTBI content (manifested as decreasing fluorescence) with increasing Fe-nitrilotriacetate input, although the relationship between the input iron and the signal obtained was also influenced by the initial NTBI contents and iron-binding capacities of the sera.
Using the modified assay, 3 groups of patients were tested (Table2): controls, a heterogeneous group without any known iron overload, some of whom were receiving iron supplements; HHC, with various levels of iron overload; and ESRD, among whom approximately 80% were receiving erythropoietin and IV iron supplements (none of the patients had received IV iron in the week before sampling). The respective frequencies of NTBI-positive patients in the 3 groups were 4.3%, 69%, and 22%. The presence of NTBI in the control group, who were assumed to be at low risk, was surprising. One of the 2 NTBI-positive individuals had a transferrin saturation of 75% and, as was subsequently discovered, had taken a daily dose of oral iron supplement shortly before serum sampling. The reason for the presence of NTBI in the second control sample was not determined. Based on this finding, it is conceivable that some NTBI-positive samples in the other groups may also be due to a transient rise in serum iron, which indicates that repeated measurements are mandatory for establishing the persistent presence of NTBI.
Discussion
The occurrence of NTBI in the sera of thalassemic patients was originally thought to arise exclusively in cases of severe iron overload. It was reasoned that the iron-binding capacity of serum transferrin was overwhelmed, leading to a spillover of iron to other low-affinity ligands.1 This was consistent with the relatively high (> 80%) transferrin saturation found in such patients. However, more recently, NTBI was also detected in the sera of patients without full transferrin saturation,5 which indicates that a fraction of NTBI may exist in a form that is not accessible to apo-Tf. Because NTBI has now been found to occur in a variety of pathologic conditions in the absence of chronic iron overload,4-6 we have sought a means to identify NTBI also in populations of patients who are at relatively lower risk of iron overload. This paper describes a novel approach for assessing NTBI, which led to the development of an assay that is both sensitive and convenient for handling large numbers of samples. The assay was applied to a variety of sera for monitoring chelation kinetics in patients with thalassemia and for detecting NTBI in patients with ESRD.
There are presently 2 main approaches for determining NTBI in biologic fluids. One approach is based on mobilization of NTBI with an anionic ligand (EDTA, citrate, or nitrilotriacetate) followed by ultrafiltration and quantitation of the filterable iron by either established methods1 or by subsequent complexation with the chelator L1 and separation/identification by high-performance liquid chromatography (HPLC).3 An alternate approach uses the antibiotic bleomycin, which combines with NTBI, but apparently not with iron bound to transferrin or other proteins, to form highly reactive complexes that are quantified by the amount of DNA cleavage products that they generate.15 Although both these methods have been used successfully for some time, the present assay offers the advantages of technical simplicity and low labor intensity, without loss of sensitivity or generation of false-positive values. The NTBI concentrations obtained with the present assay are roughly comparable to those obtained previously, though slightly higher. The reported NTBI values in thalassemic sera obtained by various methods are in the range of 0 to 9 μmol/L,1 14 which is roughly similar to the values found for thalassemic patients in this paper, 0 to 10.6 μmol/L.
The chemistry of NTBI is probably more complex than was originally thought,1 because it might be comprised of a heterogeneous mixture of complexes whose composition might vary with the degree and type of iron overload or iron supplementation. Evidently, understanding its nature in different disease states is essential for preventing its occurrence. Analysis of NTBI by HPLC and high-resolution nuclear magnetic resonance indicated the presence of citrate-iron and ternary citrate-acetate-iron complexes. Interestingly, the chelation of NTBI by DFO appeared to be a very slow process, requiring a number of hours even at 1 mmol/L concentration of the chelator.23 The presence of NTBI in sera without full transferrin saturation5 indicates that a fraction of the iron may exist in a form that cannot be bound directly by apo-Tf. Similarly, if NTBI is not fully accessible to chelators such as DFO, this may explain its apparent rebound following cessation of DFO infusion13(Figure 4). This possibility is reinforced by the requirement for NTBI-mobilizing agents such as nitriloacetate or oxalate in the assays based on binding to L13 or DFO (present paper). Indeed, we found that the presence of oxalate in our assay enabled the detection of NTBI in 12 of 15 thalassemic patients as opposed to only in 3 of 15 patients in its absence. This finding further supports our view regarding the heterogeneity of NTBI. Although the bleomycin-based NTBI assay does not use a mobilizing agent,15 ascorbate, which is a required component, most likely fulfills this function while bleomycin acts as the iron-binding moiety. At present it is not clear why DFO, L1, or apo-Tf fails to mobilize some forms of NTBI, whereas small, negatively charged molecules such as oxalate (this article), EDTA,1 citrate,1 or nitrilotriacetate3 can mobilize and shuttle it to these high-affinity ligands. Conceivably, the latter ligands fail to access the presumed polynuclear forms of the metal in NTBI and form the required multiple coordination points for stable binding. Thus, iron-mobilizing agents or shuttles as supplementary tools for elimination of NTBI by chelators could have an important function in all those cases where iron chelators alone might have failed. This has therapeutic implications both in terms of novel chelator design and for supplementing current chelation regimens with agents targeted to mobilize NTBI. Previously, we have implemented a similar shuttle-chelator combination strategy in iron chelation protocols for the treatment of malaria infections.16
As to the origin of NTBI, one may infer that it represents a fraction of iron that entered the circulation, either directly from the site of intestinal absorption or from macrophages. That fraction presumably failed to be absorbed by apo-Tf and, hence, aggregated into insoluble deposits or was adsorbed to negatively charged pockets of serum proteins. It is also conceivable that some iron aggregates may have been formed intracellularly, before their entry into the circulation, whether by loss or extrusion from iron-overloaded cells as hemosiderin-like complexes or as polymer-iron complexes used for iron supplementation of patients.
A major advantage of the present NTBI assay is its potential usefulness for conveniently screening large groups of patients. A limited example of such an application is shown in Table 2. The presence of NTBI in 69% of the patients with HHC was expected and observed accordingly. However, the finding of 4.3% NTBI-positive patients in the control group was not anticipated. It should be emphasized that only 15 of these samples were from healthy individuals, whereas the remaining 31 were from patients with a variety of non–iron-overload conditions, who were referred for tests of transferrin saturation or serum ferritin. We are aware of the possibility that NTBI might occur only transiently in these patients, perhaps as an immediate and short-term result of iron supplementation, and might not be obtained in successive samplings.
With respect to patients with ESRD, the 22% frequency of NTBI is clearly significant, yet it may also represent a transient form of NTBI. Studies are under way to determine the persistence of NTBI in these patients over time and its correlation with iron supplementation and with other diagnostic indicators of iron status (Slotki et al, in preparation). We have also considered the possibility that patients with ESRD may have elevated serum Al+++ levels, which could masquerade as NTBI in our method, because DFO also binds Al+++. This possibility is not likely for 2 reasons. First, serum Al+++ levels rarely exceed 40 μg/L (= 1.5 μmol/L),17,18 which is considerably below the binding capacity of apo-Tf (about 50 μmol/L), the major Al+++-binding protein in the serum.19 Second, the assay is relatively insensitive to Al+++ due to the lower affinity of DFO for Al+++ than for Fe+++(respective stability constants 1022 and 1032). Nonetheless, we examined the question directly by premixing serum samples from NTBI-positive and NTBI-negative ESRD patients with increasing concentrations of AlCl3, and subjecting the samples to the NTBI assay. No additional NTBI was detectable at Al+++ concentrations up to 50 μmol/L in any of the sera.
The immediate potential applications of the assay presented here include routine monitoring of NTBI status in iron overload, assessment of chelator activity and handling during chelator therapy, and screening of selected patient populations for NTBI. The screening application may be instrumental in uncovering the possible connection between NTBI and various potentially iron-associated pathologies, such as cardiovascular disease.20 21
Note added in proof. The present assay was simplified by using fluorescent-DFO analogs that undergo quenching upon stoichiometric binding of the metal (this work and also Breuer et al, manuscript submitted). Both the present and the modified assays can be applied either in the presence (indirect assay [IA]) or absence (direct assay [DA]) of shuttle agents such as oxalate. The DA detected virtually no NTBI in serum samples of normal individuals, whether or not they were supplemented with more than 95% saturated Tf. The IA, however, detected 1-5% iron mobilized from more than 60% iron saturated Tf. This indicates that NTBI values obtained by the IA from samples with relatively high (more than 60%) Tf saturation might include a substantial component derived from Tf itself. Indeed, we found that among 14 βE Hb patients from Thailand (all with Tf saturations of more than 95%) that were not under chelation treatment, 86% of them had NTBI levels of 1 to 8 μM (mean 2.9 ± 1.7 μM) as detected by the DA and 2.4 to 11 μM (mean 3.7 ± 2.7 μM) by the IA. In the same type of patients orally chelated with L1 for up to 2 years, NTBI values (μM) dropped significantly (P < 0.05, pairedt-test) from 3.98 ± 1.31 to 1.35 ± 0.48 by the DA and from 8.8 ± 0.3 to 7.1 ± 0.4 by the IA. The DA also detected NTBI (0.4 to 1.1 μM; 0.6 ± μM) in 9% of samples from primary hemochromatotic patients (n = 31; 92 samples), but in none of the ESRD patients (Israel). However, because most HC and ESRD sera had Tf saturations much less than 70%, the IA shown in this article provided a minimum estimate of their NTBI levels.
Acknowledgments
We would like to thank the staff and patients of the Children's Day Hospital at Shaare Zedek Medical Center, Jerusalem, for their cooperation; Ms Hava Glickstein for excellent technical assistance; and the partners of the Biomed II group, Profs J. J. M. Marx, R. C. Hider, and P. Brissot, for advice and encouragement. The method was filed for intellectual rights on December 17, 1998, application no. 127621.
Supported by a grant from the Israel Science Foundation and by Biomed II Programme of the EEC.
Reprints:William Breuer, Department of Biological Chemistry, Institute of Life Sciences, Hebrew University of Jerusalem, Jerusalem 91904, Israel.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.