Eosinophils are major effector cells in cellular inflammatory conditions such as parasitic infections, atopic diseases, bullous dermatoses, and vasculitis. Biological activities of adenosine triphosphate (ATP) were characterized in human eosinophils and compared with those of other eosinophil activators such as complement fragment product C5a, platelet-activating factor (PAF), and eotaxin. ATP initiated production of reactive oxygen metabolites, as demonstrated by lucigenin-dependent chemiluminescence. Furthermore, ATP caused up-regulation of the integrin CD11b. In addition, fluorescence microscope measurements labeled with fura-2 (1-[2-(5-carboxy-oxazol-2-yl)-6-aminobenzofuran-5-oxy]-2-(2′-amino-5′-methyl-phenoxy)-ethane-N, N, N, N′-tetraacetic acid, pentaacetoxymethyl ester) eosinophils in the presence or absence of ethyleneglycotetraacetic acid (EGTA) indicated that there was Ca++ mobilization from intracellular stores by ATP. Flow cytometric studies showed transient actin polymerization upon stimulation with ATP and its stable analogues adenosine 5′-0-(3-thiotriphosphate) and 2-methylthioadenosine triphosphate tetrasodium (met-ATP). The reactions induced by ATP were comparable to those obtained by C5a, PAF, and eotaxin. Production of reactive oxygen metabolites and actin polymerization after stimulation with ATP was inhibited by pertussis toxin, which indicated involvement of receptor-coupled guanine nucleotide–binding proteins (Gi proteins). In addition, experiments with oxidized ATP also suggest involvement of P2X receptors in this activation process. The results show that ATP is a strong activator of eosinophils and has biological activity comparable to those of the eosinophil chemotaxins C5a, PAF, and eotaxin. The findings strongly suggest a role of ATP in the pathogenesis of eosinophilic inflammation as an activator of proinflammatory effector functions.
Human eosinophils are considered major effector cells in a number of inflammatory conditions such as parasitic infections, atopic diseases, bullous dermatoses, or vasculitis.1-3 The recruitment of eosinophils to sites of inflammation is caused by various specific and nonspecific agents. The complement fragment product C5a, the phosphatidylcholin-related molecule platelet-activating factor (PAF), and the CC-chemokines eotaxin and RANTES are well-characterized eosinophil activators.4-6 In addition to chemotaxis, C5a, PAF, eotaxin, and RANTES stimulate eosinophil effector functions such as the production of reactive oxygen metabolites and up-regulation of CD11b.7 8
Activation of eosinophilic granulocytes by chemotaxins requires binding to membrane-spanning ligand-specific receptors.9 These receptors interact at the intracellular site of the plasma membrane with pertussis toxin–sensitive or cholera toxin–sensitive heterotrimeric guanine nucleotide–binding proteins (G proteins).10,11 Activated G proteins (GTP-form) dissociate into the GTP-α subunit and free βγ-dimers. The latter may activate phospholipase Cβ2.12 This enzyme cleaves phosphatidylinositol4,5 bisphosphate into diacylglycerol and inositol trisphosphate, which mobilize Ca++ from intracellular stores.9 In leukocytes, G proteins regulate the reorganization of the actin cytoskeleton independently of activated phospholipase C.13 However, the single steps of this regulatory mechanism are poorly understood. In concert, intracellular Ca++ transients and actin reorganization regulate cell responses such as migration, production of reactive oxygen species, and up-regulation of integrin.14 15
ATP is an important mediator in the nervous and cardiovascular systems.16,17 Neurons, platelets, macrophages, T lymphocytes, and epithelial and endothelial cells release ATP via nonlytic pathways.18-25 Since the average cytoplasmatic concentration fluctuates around 10 mmol/L, any agent causing plasma membrane damage might also enhance the extracellular concentration of this nucleotide.26 Moreover, the extracellular ATP concentration is tightly regulated by hydrolysis through ubiquitous ecto-ATPases.24 Since inflammatory reactions inhibit ATP diphosphohydrolase activity, accumulation of extracellular ATP can be assumed under such conditions.27 In addition to acting on the nervous and cardiovascular systems, modulating effects of ATP on immune cell responses have been well documented. ATP modulates responses of B and T lymphocytes28 and mast cells.29 Furthermore it causes release of interleukin-1β (IL-1β) from macrophages.30 The biological activities of ATP include stimulation of cell proliferation31 and differentiation32 as well as modulation of proinflammatory activities of neutrophils.33 In addition, ATP displays chemotactic activity for eosinophils.34 Today, 2 different P2 receptor subtypes, P2X and P2Y are recognized. The P2X receptors are coupled to plasma membrane ion channels, whereas P2Y receptors are 7 membrane-spanning G protein–coupled receptors.35 36 In the present study, the signal pathways and biological activities of ATP in human eosinophils were characterized and compared to the activation profile of other well-defined stimuli.
Materials and methods
Materials
We used the following materials: ATP, recombinant human C5a, phosphatidylcholine-β-acetyl-O-hexadecyl (PAF), phorbol 12-myristate 13-acetate (PMA), lysophosphatidylcholine, Ficoll-Hypaque gradient, and lucigenin (Sigma, Deisenhofen, Germany); adenosine 5′-O-(3-thiotriphosphate) (ATPγS) (Boehringer Mannheim, Mannheim, Germany); 2-methylthioadenosine triphosphate tetrasodium (met-ATP) (Amersham-RBI, Deisenhofen, Germany); periodate-oxidized ATP (gift from Dr Hanau Ferrara, Italy); recombinant human RANTES and recombinant human eotaxin (PeproTech, Rocky Hill, NJ); N-(7-nitrobenz-2-oxa-1,3-diazol-4yl)–phallacidin (NBD-phallacidin) (Becton Dickinson, Heidelberg, Germany); 1-[2-(5-carboxy-oxazol-2-yl)-6-aminobenzofuran-5-oxy]-2-(2′-amino-5′-methyl-phenoxy)-ethane-N, N, N, N′-tetraacetic acid, pentaacetoxymethyl ester (fura-2) and pertussis toxin (Calbiochem, La Jolla, CA); monoclonal antibody (mAb) anti-CD16 (Immunotech, Marseilles, France); immunomagnetic beads (Dynabeads M-450; Dianova, Hamburg, Germany); and phycoerythrin-conjugated (PE-conjugated) CD11b (LEU 15, Becton Dickinson).
Isolation of eosinophils
Human eosinophil granulocytes of healthy nonatopic volunteers were isolated from heparin-anticoagulated (10 units/mL) blood by Ficoll-Hypaque separation and negative selection with anti-CD16 mAb-coated immunomagnetic beads (Dynabeads, Dianova).8 In brief, human granulocytes (2 × 107 cells) were incubated with 1 mL of 2 × 108 coated beads/mL for 1 hour at 4°C. Bead-coupled neutrophils were removed by repeated magnetic separation steps, and resulting eosinophils were resuspended. Pappenheim stain judged the purity of isolated eosinophils ≥ 96%.
Actin polymerization
The content of filamentous actin was analyzed by flow cytometry with NBD-phallacidin staining.8 Briefly, 50 μL aliquots of stimulated cell suspensions (5 × 105eosinophils/mL) were withdrawn at the indicated time intervals and fixed in a 7.4% formaldehyde buffer. After 1 hour, eosinophils were mixed with a staining cocktail containing 7.4% formaldehyde, 0.33 μmol/L NBD-phallacidin, and 1 mg/mL lysophosphatidylcholine. The fluorescence intensity was measured by flow cytometry.
Intracellular Ca++ measurements
Intracellular-free Ca++ was measured in fura-2–labeled cells with a digital fluorescence microscopy unit (Attofluor; Zeiss, Oberkochem, Germany). Briefly, eosinophils were incubated with 2 μmol/L fura-2 for 30 minutes at 37°C in buffer free of Ca++and Mg++. Cells were washed twice and finally resuspended in the same buffer containing 1.5 mmol/L calcium dichloride and magnesium dichloride. The fluorescence traces after stimulation were followed fluorospectrometrically, and the ratio between absorption at 340 nm and 380 nm was calculated.
Lucigenin-dependent chemiluminescence
Eosinophils were resuspended to a density of 5 × 104 cells/mL containing 200 μmol/L lucigenin. Measurements were performed in triplicate at 37°C.7Reactions over a 60-minute time period after cell stimulation were followed and expressed as intensity integral counts.
CD11b expression
The integrin CD11b was analyzed by flow cytometry with PE-conjugated anti-CD11b mAbs. Eosinophils were stimulated and then incubated for 30 minutes at 37°C. The reaction was stopped by diluting the sample with 100-fold ice-cold buffer. Samples were incubated on ice for 40 minutes with PE-conjugated anti-CD11b mAbs.
Results
Actin response and ATP
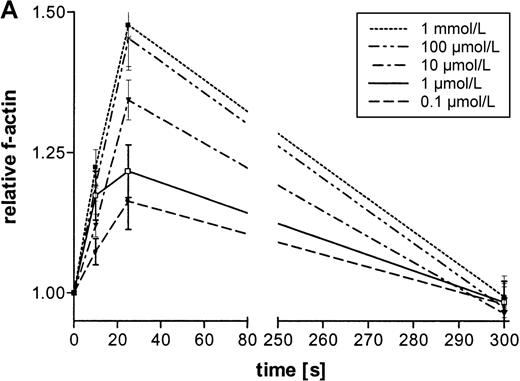
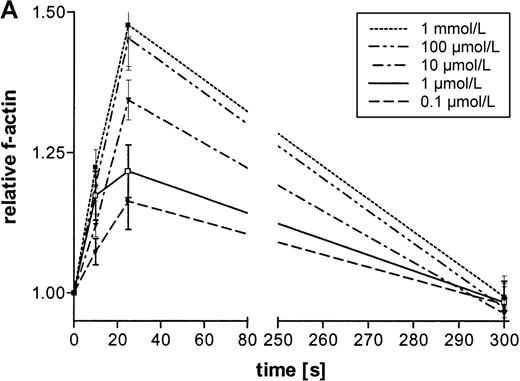
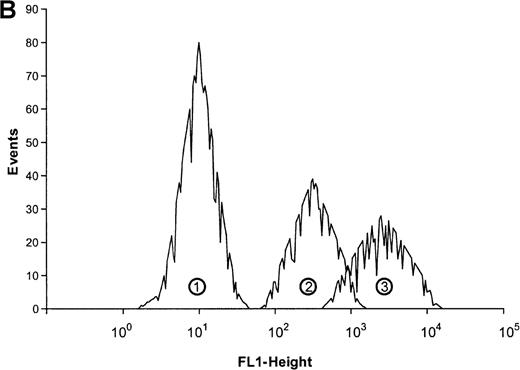
The influence of ATP on the actin network in eosinophils was analyzed by flow cytometry. This nucleotid caused a rapid and transient polymerization of actin molecules (Figure1A and B). There was a 50% transient increase of the filament-actin (f-actin) content within 30 seconds. Half-maximum and maximum effects were observed at 0.1 μmol/L and 100 μmol/L concentrations, respectively, and after 300 seconds, the actin content returned to control values. In tissue and cells, ATP can be easily metabolized into various products. To confirm that ATP and not its metabolic products produce this effect, experiments were conducted with ATP and its stable analogues ATPγS and met-ATP. Both ATP and the stable analogues induced a fast and transient actin polymerization in eosinophils (Table 1).
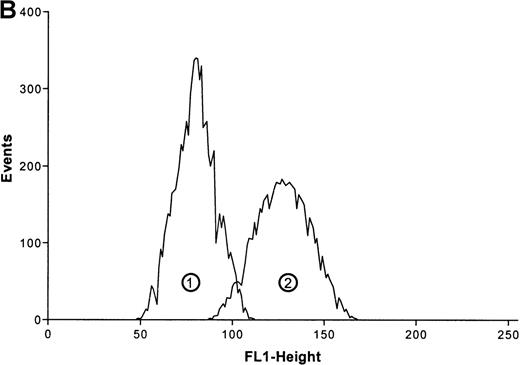
Effects of ATP on the actin polymerization in eosinophils.
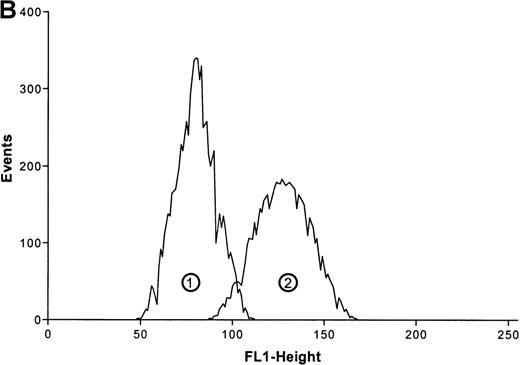
(A) Cells were stimulated with ATP in 5 concentrations: 1 mmol/L, 100 μmol/L, 10 μmol/L, 1 μmol/L, and 0.1 μmol/L. The relative f-actin content was determined at the indicated time points by flow cytometry. Data are means ± SEM (standard error of the mean; n = 8). (B) Representative histogram (25-second time point) of eosinophils stimulated with control buffer (➁) compared with eosinophils stimulated with 1 mmol/L of ATP (➀). Representative data of 1 experiment are shown. The experiments were repeated 8 times with identical results.
Effects of ATP on the actin polymerization in eosinophils.
(A) Cells were stimulated with ATP in 5 concentrations: 1 mmol/L, 100 μmol/L, 10 μmol/L, 1 μmol/L, and 0.1 μmol/L. The relative f-actin content was determined at the indicated time points by flow cytometry. Data are means ± SEM (standard error of the mean; n = 8). (B) Representative histogram (25-second time point) of eosinophils stimulated with control buffer (➁) compared with eosinophils stimulated with 1 mmol/L of ATP (➀). Representative data of 1 experiment are shown. The experiments were repeated 8 times with identical results.
Mobilization of intracellular Ca++ by ATP
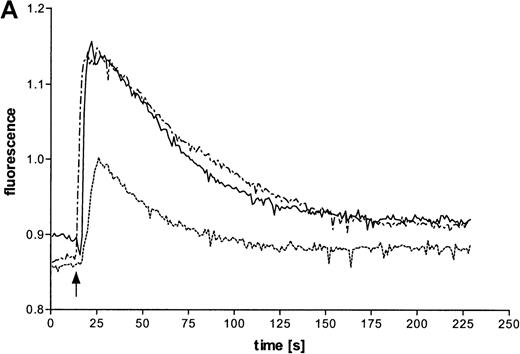
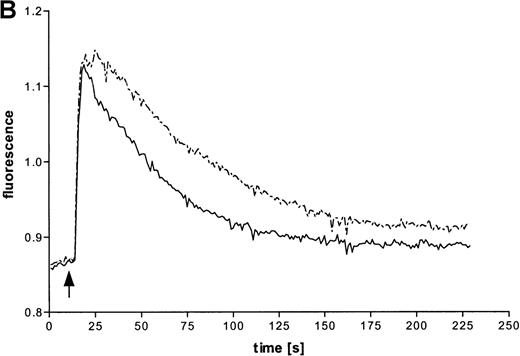
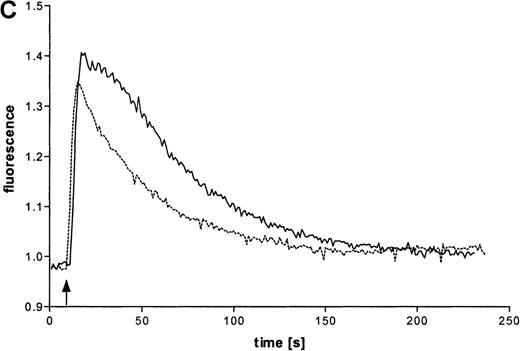
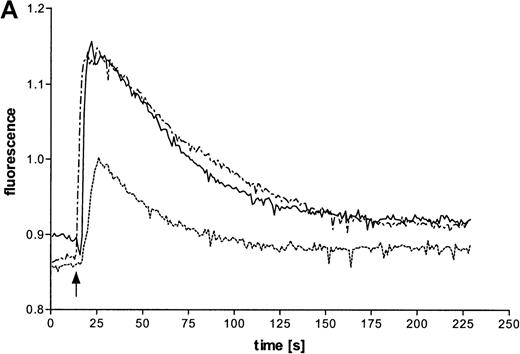
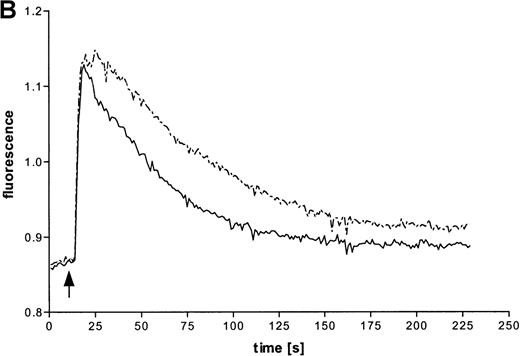
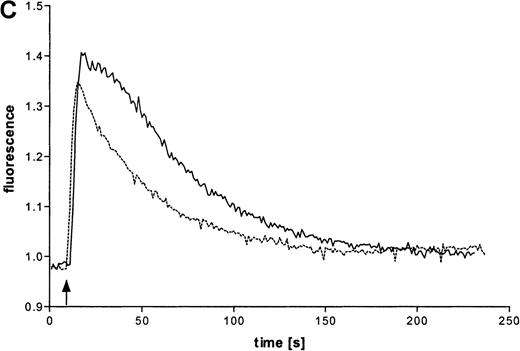
Intracellular Ca++ transients were followed in fura-2–labeled eosinophils by digital fluorescence microscopy. ATP induced a rapid and concentration-dependent intracellular response (Figure 2A). In order to analyze whether ATP stimulates mobilization of Ca++ from intracellular stores or influx across the plasma membrane from extracellular medium, measurements were performed in the presence of added EGTA (Figure 2B). Since this Ca++ chelator had no significant effect on the initial magnitude of the response, it appears that ATP mobilizes calcium from intracellular stores. However, EGTA accelerated the recovery to initial values, which also suggests involvement of transmembranous Ca++ fluxes in this response. To analyze participation of P2X receptors in the ATP-induced calcium response, experiments with oxidized ATP, a well-known P2X antagonist, were performed. This agent reduced the time course of the ATP-induced calcium response.
Effects of ATP on intracellular Ca++transients in eosinophils.
(A) Cells were labeled with fura-2 and stimulated in the absence of EGTA with ATP in three concentrations: 1 mmol/L (——), 100 μmol/L (-—-), and 10 μmol/L (—-). Representative data of 1 experiment are shown. The experiments were repeated 5 times with similar results. (B) Cells were labeled with fura-2 and stimulated in the absence (-—-) or presence (——) of 4 mmol/L EGTA with 100 μmol/L ATP. (C) Eosinophils were pretreated without (——) or with (—-) 300 μmol/L oxidized ATP for 2 hours at 37°C, labeled with fura-2, and stimulated with 100 μmol/L ATP.
Effects of ATP on intracellular Ca++transients in eosinophils.
(A) Cells were labeled with fura-2 and stimulated in the absence of EGTA with ATP in three concentrations: 1 mmol/L (——), 100 μmol/L (-—-), and 10 μmol/L (—-). Representative data of 1 experiment are shown. The experiments were repeated 5 times with similar results. (B) Cells were labeled with fura-2 and stimulated in the absence (-—-) or presence (——) of 4 mmol/L EGTA with 100 μmol/L ATP. (C) Eosinophils were pretreated without (——) or with (—-) 300 μmol/L oxidized ATP for 2 hours at 37°C, labeled with fura-2, and stimulated with 100 μmol/L ATP.
Activation of the respiratory burst
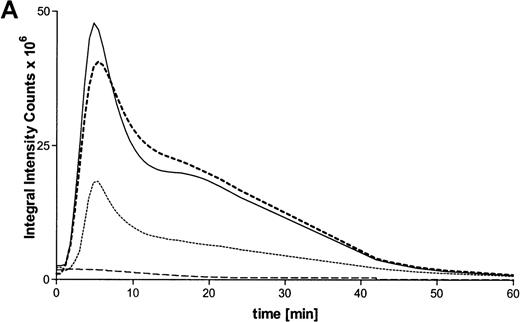
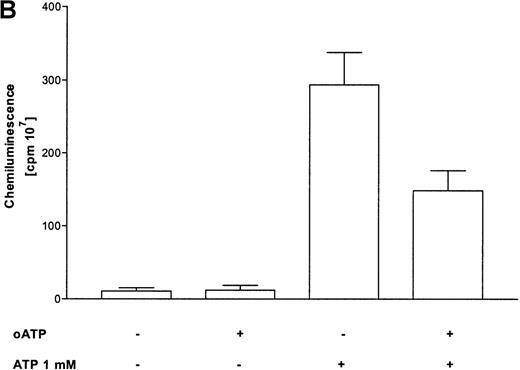
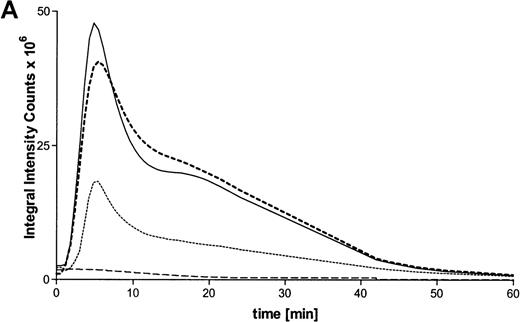
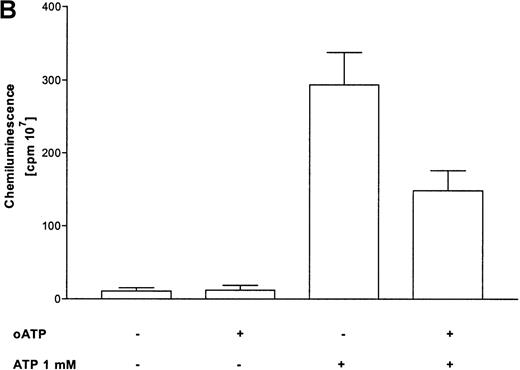
Activation of the respiratory burst by ATP was studied by lucigenin-dependent chemiluminescence. These experiments revealed ATP-dependent production of reactive oxygen metabolites in a concentration-dependent manner. Half-maximum and maximum effects were observed at 10 μmol/L and 100 μmol/L concentrations, respectively (Figure 3A). At optimum concentrations, continuous measurements indicated a rapid induction of the response, with maximum values after 5 minutes. In addition, the respiratory burst induced by the stable ATP analogues ATPγS and met-ATP showed similar results (Table 1). Again, to prove participation of P2X receptors in production of reactive oxygen metabolites, experiments with oxidized ATP were performed. Production of reactive oxygen metabolites was partially blocked by preincubation with oxidized ATP (Figure 3B).
Influence of ATP on the respiratory burst in eosinophils.
(A) Time courses of lucigenin-dependent chemiluminescence responses in eosinophils upon stimulation with medium (——) or ATP in three concentrations: 1000 μmol/L (–––), 100 μmol/L (——), and 10 μmol/L (· · · ·). Representative data of 1 experiment are shown. The experiments were repeated 5 times with similar results. (B) Eosinophils were pretreated with or without 300 μmol/L oATP for 2 hours at 37°C and then stimulated with 100 μmol/L ATP. Data are means ± SEM (n = 5). Global differences between groups:P ≤ .0001 (analysis of variance program, ANOVA);P ≤ .001 (**) compared with oxidized ATP-pretreated cells (Tukey multiple comparison test).
Influence of ATP on the respiratory burst in eosinophils.
(A) Time courses of lucigenin-dependent chemiluminescence responses in eosinophils upon stimulation with medium (——) or ATP in three concentrations: 1000 μmol/L (–––), 100 μmol/L (——), and 10 μmol/L (· · · ·). Representative data of 1 experiment are shown. The experiments were repeated 5 times with similar results. (B) Eosinophils were pretreated with or without 300 μmol/L oATP for 2 hours at 37°C and then stimulated with 100 μmol/L ATP. Data are means ± SEM (n = 5). Global differences between groups:P ≤ .0001 (analysis of variance program, ANOVA);P ≤ .001 (**) compared with oxidized ATP-pretreated cells (Tukey multiple comparison test).
CD11b up-regulation by ATP
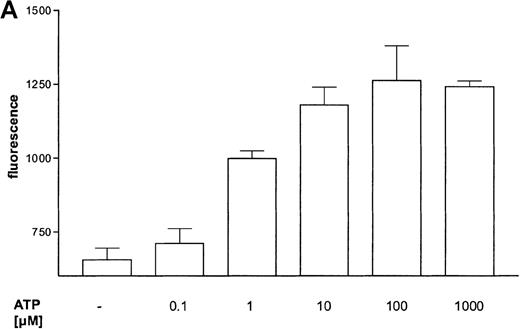
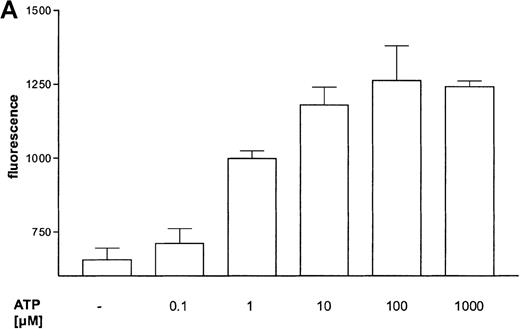
The influence of ATP on the expression of the integrin CD11b was measured by flow cytometry (Figure 4B). ATP induced a concentration-dependent response (Figure 4A). The time course of CD11b expression was very rapid, and half-maximum and maximum effects were seen after 30 seconds and 4 minutes, respectively (data not shown).
(A) Dose dependency of ATP-induced CD11b expression in eosinophils.
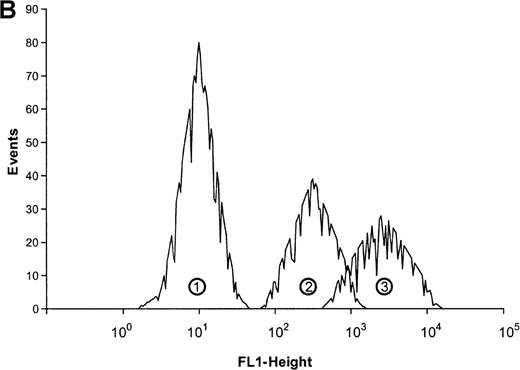
Eosinophils were stimulated with the indicated concentrations of ATP and then incubated for 30 minutes at 37°C. The reaction was stopped by diluting the sample with 100-fold ice-cold buffer. Samples were incubated on ice for 40 minutes with PE-conjugated anti-CD11b mAbs and then analyzed by flow cytometry. Data are means ± SEM (n = 5). (B) Representative histogram of anti-CD11b–stained eosinophils stimulated with control buffer (➀) compared to stimulation with 0.1 mmol/L ATP (③). Histogram ➁ represents the isotype control. Representative data of 1 experiment are shown. The experiments were repeated 5 times with identical results.
(A) Dose dependency of ATP-induced CD11b expression in eosinophils.
Eosinophils were stimulated with the indicated concentrations of ATP and then incubated for 30 minutes at 37°C. The reaction was stopped by diluting the sample with 100-fold ice-cold buffer. Samples were incubated on ice for 40 minutes with PE-conjugated anti-CD11b mAbs and then analyzed by flow cytometry. Data are means ± SEM (n = 5). (B) Representative histogram of anti-CD11b–stained eosinophils stimulated with control buffer (➀) compared to stimulation with 0.1 mmol/L ATP (③). Histogram ➁ represents the isotype control. Representative data of 1 experiment are shown. The experiments were repeated 5 times with identical results.
Comparison of the activation profiles of different eosinophil stimuli
The activation profile of ATP on eosinophils was compared to the responses provoked by other well-defined eosinophil activators such as C5a, PAF, eotaxin, and RANTES. All eosinophil activators that were tested stimulated intracellular Ca++ transients, actin reorganization, respiratory burst, and expression of CD11b in a concentration-dependent manner (data not shown). At optimal concentrations, ATP induced the strongest effects on each parameter (Table 2).
Inhibition of ATP-induced cell response by pertussis toxin
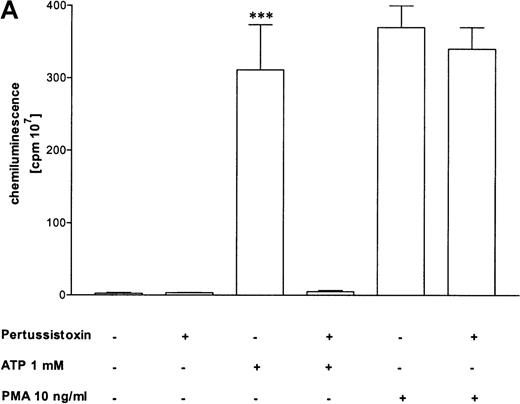
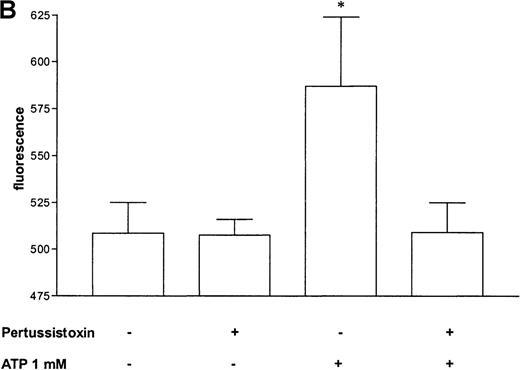
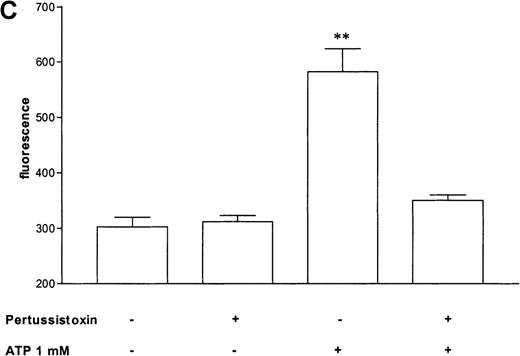
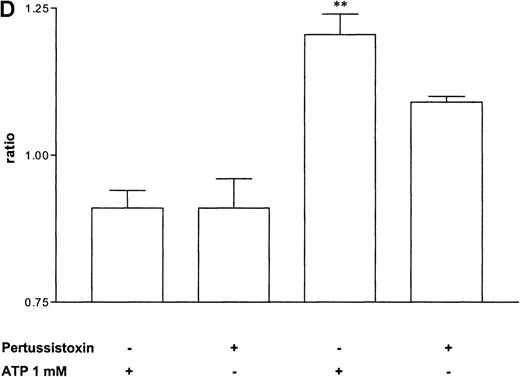
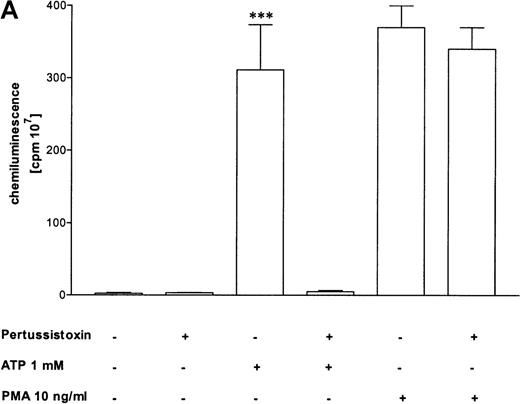
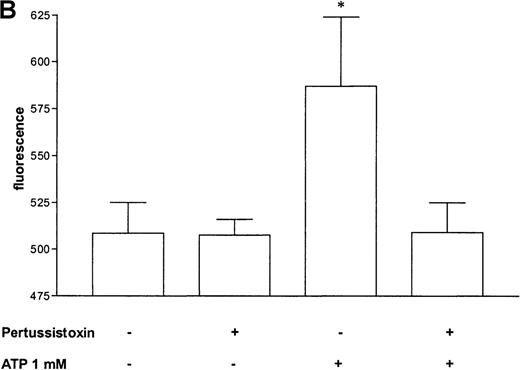
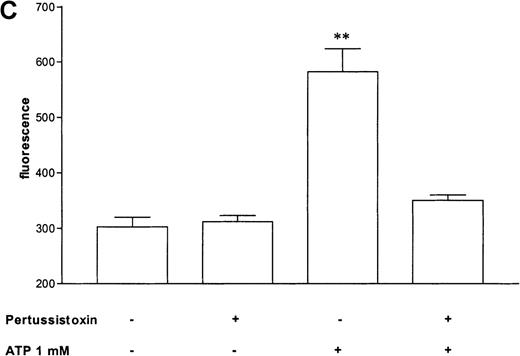
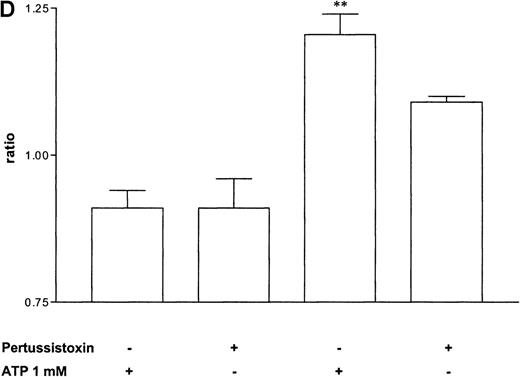
Pertussis toxin blocks cell activation that was induced by Gi-protein–coupled receptors.12 Pretreatment of eosinophils with pertussis toxin resulted in a complete block of the ATP-induced chemiluminescence (Figure 5A) and actin response (Figure 5B). In contrast, CD11b translocation (Figure 5C) was only blocked approximately 80%, and Ca++fluxes (Figure 5D) were inhibited approximately 43%. To demonstrate that eosinophil metabolic activity remained after pertussis toxin treatment, the chemiluminescence response was assumed with PMA. Toxin treatment did not significantly influence the phorbol ester-triggered response (Figure 5A).
Influence of pertussis toxin on the eosinophil functions.
Cells were incubated without or with 1 μg/mL pertussis toxin for 2 hours at 37°C and thereafter stimulated without or with 1 mmol/L ATP. (A) Lucigenin-dependent chemiluminescence in eosinophils was followed for 1 hour, and the integral counts were calculated. Data are means ± SEM (n = 5). (B) Actin polymerization after stimulation for 25 seconds was analyzed. Data are means ± SEM (n = 8). (C) Expression of CD11b was analyzed by flow cytometry after stimulation for 30 minutes. Data are means ± SEM (n = 6). (D) Ca++ transients were analyzed, and the ratio after stimulation for 10 seconds is given. Data are means ± SEM (n = 5). Global differences between groups: P ≤ .0001 (ANOVA); P ≤ .0001 (***) compared with pertussis toxin–pretreated cells (Tukey multiple comparison test);P ≤ .001 (**) compared with pertussis toxin–pretreated cells (Tukey test); P ≤ .01 (*) compared with pertussis toxin–pretreated cells (Tukey test).
Influence of pertussis toxin on the eosinophil functions.
Cells were incubated without or with 1 μg/mL pertussis toxin for 2 hours at 37°C and thereafter stimulated without or with 1 mmol/L ATP. (A) Lucigenin-dependent chemiluminescence in eosinophils was followed for 1 hour, and the integral counts were calculated. Data are means ± SEM (n = 5). (B) Actin polymerization after stimulation for 25 seconds was analyzed. Data are means ± SEM (n = 8). (C) Expression of CD11b was analyzed by flow cytometry after stimulation for 30 minutes. Data are means ± SEM (n = 6). (D) Ca++ transients were analyzed, and the ratio after stimulation for 10 seconds is given. Data are means ± SEM (n = 5). Global differences between groups: P ≤ .0001 (ANOVA); P ≤ .0001 (***) compared with pertussis toxin–pretreated cells (Tukey multiple comparison test);P ≤ .001 (**) compared with pertussis toxin–pretreated cells (Tukey test); P ≤ .01 (*) compared with pertussis toxin–pretreated cells (Tukey test).
Discussion
Eotaxin, PAF, C5a, and RANTES are well-defined chemotaxins for eosinophils.7 It can be assumed that all these agents are involved at different stages in the accumulation of eosinophils at inflammatory sites. In addition to migration, these chemotaxins stimulate eosinophil effector functions such as the production of reactive oxygen metabolites and the up-regulation of CD11b.7,8 Chemotactic activity of ATP for eosinophils has been described in a previous publication.34 To improve our understanding of the biological activities of ATP, we analyzed various intracellular signal mechanisms and cell effector functions in eosinophils. As could be expected from an agent with chemotactic activity, this study showed that ATP induces a concentration-dependent reorganization of the actin network. The precise mechanisms regulating the actin response are not fully understood; however, it is believed to involve interaction of phospholipids with actin-binding proteins.13 Half-maximum effects of ATP on the actin response were obtained in the low μmol/L range, whereas maximum actin polymerization required a 1000-fold higher concentration of ATP. This reaction is not surprising because it is understood that from receptor binding studies in neutrophils, early actin polymerization requires occupancy of only a couple of receptors, whereas maximum actin reactions require occupancy of almost all expressed receptors.37
To exclude the possibility that the effects induced by ATP are due to anionic charges, we performed actin measurements after exposure of eosinophils to heparin, dermatansulfate, and polyvinylsulfate. Since none of these substances stimulated actin response in eosinophils, nonspecific anionic activation was excluded (Dichmann and Norgauer, unpublished data, July 1998). ATP can be released in tissue via nonlytic pathways by different cells, such as neurons, platelets, and epithelial and endothelial cells, following hypoxia and stress.18-20 Furthermore, activated cytotoxic T cells and macrophages are able to secrete high amounts of ATP.23,24 Since intracellular ATP levels usually show mean average concentrations in the 10 mmol/L range, any agents causing plasma membrane damage might provoke accumulation of extracellular ATP in the μmol/L range.26 Extracellular ATP concentrations in tissue are regulated by membrane-bound ectonucleotidases.24 Recent experiments indicated down-regulation of the ATP diphosphohydrolase activity during inflammatory reactions.27 This reaction pattern further supports our assumption about the pathophysiological relevance of ATP in the activation process of eosinophils.
In tissue and cells, ATP can be easily metabolized into various products. To confirm that ATP and not its metabolic products produce these effects, experiments were performed with ATP and its stable analogues ATPγS and met-ATP. In addition, our study determined that ATP induced rapid intracellular Ca++ transients. To date we know of 2 different families of P2 purinergic receptors in the immune system.36 ATP acts either through G protein–coupled 7 membrane-spanning P2Y receptors or channel-forming P2X receptors. Since experiments with the Ca++ chelator EGTA in the extracellular medium did not significantly alter the magnitude of the response at initial time points, mobilization of Ca++ from intracellular stores by ATP can be assumed. Release of sequestered Ca++ into the cytosol might be caused by soluble inositol trisphosphate generated by activation of phospholipase C through G protein–coupled P2Y receptors.35 However, accelerated recovery to initial values in the presence of extracellular EGTA also suggests involvement of influx of extracellular Ca++ in this response. Because oxidized ATP reduced the ATP-dependent intracellular calcium, we can assume that P2X receptors have an increased participation in the activation process of eosinophils by ATP. At present, underlying molecular mechanisms of this response are unknown. ATP could either interact directly with P2X ion channels or indirectly activate transmembranous Ca++ fluxes following G protein activation.36
Intracellular Ca++ transients and actin reorganization have been implicated in many biological regulation mechanisms including the activation of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and translocation of adhesion receptors.8,9Production of reactive oxygen metabolites as well as up-regulation of the CD11b integrin by ATP and its analogues has been demonstrated in this report. These findings implicate proinflammatory activities of ATP in diseases with an eosinophilic infiltrate. Previous studies revealed that various activators of eosinophils, such as C5a, PAF, eotaxin, and RANTES, induced similar effector functions.8,9 14 Our study demonstrated that the effects provoked by ATP were slightly stronger than the responses upon well-characterized eosinophil activators. Therefore, one can speculate that in the inflammatory microenvironment, ATP could act in a tightly regulated and transient manner.
Cell biology studies and cloning of the cDNA of many chemotaxin receptors in eosinophils identified interaction of these proteins with pertussis toxin–sensitive Gi proteins.7 The production of reactive oxygen metabolites and actin polymerization induced by ATP were completely inhibited by the pertussis toxin, which inactivates heterotrimeric Gi protein by adenosine 5′-diphosphate (ADP) ribosylation.10 To exclude effects unrelated to ADP ribosylation, we pretreated the eosinophils with the enzymatically inactive pertussis toxin B oligomer. Since the ATP-induced reactions were not influenced by the pertussis toxin B oligomer, effects unrelated to ADP ribosylation can be excluded. In contrast, pertussis toxin only partially blocked ATP-induced Ca++ transients and CD11b up-regulation. These findings and the fact that the P2X antagonist oxidized the ATP partially blocked calcium response suggest that biologic effects of ATP are mediated by at least 2 different receptors. One receptor belongs to the P2Y receptor family, which couples to Gi proteins; the other receptor may be a member of the P2X receptor family. Currently several different P2Y receptors are known.36 Since in different inflammatory cells P2Y2 specifically interacts with pertussis toxin–sensitive Gi proteins, action of this receptor in eosinophils is conceivable.36
The present results indicate that ATP in eosinophils induced actin polymerization and production of reactive oxygen metabolites via pertussis toxin–sensitive Gi proteins. In addition, ATP is a strong activator of Ca++ transients and stimulates up-regulation of integrin CD11b. These findings characterize the biological activities of ATP in eosinophils and strongly suggest that this nucleotide plays a role in the pathogenesis of eosinophilic inflammation.
Acknowledgments
The authors gratefully acknowledge the assistance of A. Komann and D. Purlis.
S.D. and M.I. contributed equally to this work.
Supported by grant 01 GC 9701 from the Bundesministerium fūr Bildung, Wissenschaft, Forschung und Technolgie.
Reprints:Johannes Norgauer, Department of Dermatology, Hauptstraβe 7, D-79104 Freiburg i. Br., Germany; e-mail:norgauer@haut.ukl.uni-freiburg.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.