Abstract
Three distinct classes of epitopes on human CD164 have been identified. Two of these, recognized by the monoclonal antibodies 105A5 and 103B2/9E10, are the CD164 class I and class II functionally defined epitopes, which cooperate to regulate adhesion and proliferation of CD34+ cell subsets. In this article, we demonstrate that these 2 CD164 epitopes are expressed on CD34+ cells throughout ontogeny, in particular on CD34+ cell clusters associated with the ventral floor of the dorsal aorta in the developing embryo and on CD34+ hematopoietic precursor cells in fetal liver, cord blood, and adult bone marrow. While higher levels of expression of these CD164 epitopes occur on the more primitive AC133hiCD34hiCD38lo/− cell population, they also occur on most cord blood Lin−CD34lo/−CD38lo/− cells, which are potential precursors for the AC133hiCD34hiCD38lo/− subset. In direct contrast to these common patterns of expression on hematopoietic precursor cells, notable differences in expression of the CD164 epitopes were observed in postnatal lymphoid and nonhematopoietic tissues, with the class I and class II CD164 epitopes generally exhibiting differential and often reciprocal cellular distribution patterns. This is particularly striking in the colon, where infiltrating lymphoid cells are CD164 class I–positive but class II–negative, while epithelia are weakly CD164 class II–positive. Similarly, in certain lymphoid tissues, high endothelial venules and basal and subcapsular epithelia are CD164 class II–positive, while lymphoid cells are CD164 class I–positive. It therefore seems highly likely that these CD164 class I and II epitopes will mediate reciprocal homing functions in these tissue types.
CD164 is a type 1 integral transmembrane molecule belonging to the sialomucin family and containing in its extracellular region 2 O-glycosylated domains (I and II) linked by a cysteine-rich nonmucin subdomain.1-3 These sialomucins encompass a diverse protein family, exhibiting low levels of amino acid identity to one another.4 They are classified by (a) their high content of proline, threonine, and serine residues that are concentrated in 1 or more regions of the molecule, the so-called PTS domains,4 and (b) O-linked glycosylation of the serine and threonine residues that constitute the mucin domains.4
The mucins were initially categorized into 2 classes on the basis of the cell type from which they were isolated, with the epithelial mucins encompassing MUC-1 to MUC-8 and the leukocyte/endothelial mucins including CD34, CD43, CD45RA, glycosylation-dependent cell adhesion molecule-1 (GlyCAM-1), mucosal addressin (MAd)CAM-1, Tactile (CD96), and P-selectin glycoprotein ligand-1 (PSGL-1) (CD162).4 For these and the more recently cloned mucins, such as kidney injury molecule-1 (KIM-1), podocalyxin-like protein (PCLP), endomucin, CD42bα, CD68, and CD107, this classification is not absolute. For example, MUC-1 can be found on hematopoietic cells,5 while GlyCAM-1 is also produced by epithelial cells in the lactating mammary gland, CD34 by embryonic fibroblasts and bone marrow stromal cells, PSGL-1 by human fallopian tube epithelia and by most mouse tissues, CD107 by both hematopoietic cells and tonsillar epithelium, and CD68 by both hematopoietic and nonhematopoietic cell types.4,6-9Most epithelial mucins are secreted molecules containing variable numbers of tandem repeats within 1 or more mucin domains,4,10,11 which may be flanked or interrupted by cysteine-rich subdomains.12 Some leukocyte/endothelial mucins possess mosaic structures, but they are generally transmembrane molecules that lack the variable number of tandem repeats.4,10,11 Exceptions include molecules that primarily contain mucin domains and lack cysteine-rich extracellular regions (eg, CD43 and PSGL-1) and those that contain tandem repeats (eg, CD43, MAdCAM-1, and PSGL-1).4,10,11,13 Examples of mosaic leukocyte/endothelialmucins that contain cysteine-rich immunoglobulin (Ig) set domains are MAdCAM-113 and Tactile (CD96).14 CD42bα adds to this diversity in that it contains a region of 7 leucine-rich repeats at its N-terminus, followed by a 60–amino acid globular structure containing 4 cysteine residues, before the addition of the mucin domain.15 Alternatively, the mucin domain may precede a cysteine-rich globular region and 3 fibronectin type III domains as occurs in CD45RA,16 or simply a membrane-proximal cysteine-rich globular region as observed for CD34,6 PCLP,17,18 and CD68.19 In CD164, the 2 mucin domains are interrupted by a cysteine-rich subdomain.2 3
While these diverse peptide backbones provide the basic structure that determines their ligand binding capacities, the functions of the mucins in specific cells are dependent on the differential expression of variant isoforms that result from differential splicing or sulfation of specific tyrosine residues or modifications of oligosaccharide side chains.20-22 The latter are dependent on specific enzymes, the glycosyltransferases,20,23-25 or PAPS (3 ′ -phosphoadenosine-5 ′ -phosphosulfate) synthetase,26 within specific cells. By genomic analysis, the leukocyte/endothelial mucins can be subdivided into 2 groups: those in which the coding sequence is contained in a single exon and those encoded by multiple exons. The former do not undergo differential splicing and include CD43, CD42bα, and PSGL-1.4,7 The latter, which can form differentially spliced variants, include GlyCAM-1, CD34, MAdCAM-1, and CD45.4,6,13,16 Variant isoforms can therefore be generated by changes in the basic polypeptide structure and by amino acid or oligosaccharide modifications. These modifications allow molecules such as CD34, PCLP, GlyCAM-1, PSGL-1, and MAdCAM-1 to act as ligands for the selectins on either endothelia, particularly on high endothelial venules in secondary lymphoid tissues, or CD34+ progenitor cells.22
We have identified 3 classes of epitopes on CD164.27 The class I and II epitopes recognized by the 105A5 and 103B2/9E10 monoclonal antibodies (Mabs), respectively, are conformationally independent and located within the N-terminal mucin domain I. The former is associated with long chain sialylated O-linked glycans, while the class II epitope encompasses both N- and O-linked glycans. The class III epitopes, defined by the Mabs N6B6 and 67D2, are conformationally dependent and encompass the cysteine-rich subdomain that links mucin domains I and II. These 3 classes of epitopes are expressed by a subset of CD34+ hematopoietic progenitor cells and stromal reticular cells from adult bone marrow.1-3 We have recently demonstrated that interactions of the class I and class II CD164 epitopes with their surrogate ligands, the 105A5 and 103B2/9E10 Mabs, inhibit cytokine-induced recruitment of CD34+CD38lo/− cells into cycle.3 Furthermore, the 103B2/9E10 Mab partially inhibits the adhesion of CD34+ bone marrow–derived hematopoietic progenitor cells to stroma.3 In this paper, we examine the distribution of the CD164 class I and II epitopes on CD34+cells throughout ontogeny, on cord blood CD34− cells that may be precursors of the CD34+ subset, and in a variety of postnatal tissues, and we compare this to the expression patterns of CD164 class III epitopes. Importantly, we show that the CD164 class I and II epitopes are uniformly expressed on the most primitive hematopoietic progenitor cells. However, they are differentially and often reciprocally expressed in postnatal tissues. This is particularly evident in lymphoid tissues and in colon. These CD164 class I and II epitopes may have distinct functions on different postnatal cell types, perhaps being involved in blood cell homing, trafficking, and recirculation.
Materials and methods
Cell lines and antibodies
The human KG1A, U937, RPMI-1788, Calu-1, HeLa, and 293T cell lines (American Type Culture Collection, Bethesda, MD, or the European Collection of Cell Cultures, Salisbury, Wiltshire, England) were maintained in RPMI-1640 containing 10% to 15% (vol/vol) fetal calf serum.
The murine CD164 Mabs, 103B2/9E10 (mIgG3), 105A5 (mIgM), N6B6 (mIgG2a), 67D2 (mIgG1), and 9C4 (mIgG2b), were generated as previously described.2,3 28 The mouse IgG1 Mabs used for dual- and triple-color immunofluorescence included CD3 (clone 3D4), CD19 (clone HD37), CD31 (clone JC70/A), CD33 (clone WM54), CD66abde (clone Kat 4c), antiglycophorin c (clone Ret40S), antiglycophorin a (clone JC159), antiband III (clone Q1/156), and CD68 (clone KP1), all from Dakopatts (Glostrup, Denmark); the AC133-phycoerythrin (PE) Mab (Amcell Corp, Sunnyvale, CA); and CD38-PE, CD38-allophycocyanin (APC; clone HB7), and CD34-PerCP (clone 8G12), all from Becton Dickinson (Sunnyvale, CA). A rabbit antikeratin polyclonal antibody (Dakopatts) was used to label epithelial cells. The mouse IgG2a Mab NA1/34 (a kind gift from Prof. A.J. McMichael, Institute of Molecular Medicine, Oxford, England) against CD1 T cells and OKT3 (American Type Culture Collection, mIgG2a) against CD3 T cells were used to label cortical thymocytes and mature T cells, respectively. L243 (mIgG2a; Becton Dickinson) was used as a marker for major histocompatibility complex class II molecules. Mouse Mabs used for lineage-negative selection were all fluorescein isothiocyanate (FITC) conjugates and included CD2 (clone MT910; mIgG1), CD3 (clone UCHT-1; mIgG1), CD4 (clone RFT-4; mIgG1), CD8 (clone RFT-8; mIgG1), CD14 (clone Tük4; mIgG2a), CD16 (DJ130c; mIgG1), CD19 (clone HD37; mIgG1), CD41 (clone 5B12; mIgG1), CD56 (NCAM 16.2; mIgG2b), CD66abce (Kat4c; mIgG1), and antiglycophorin A (clone JC159; mIgG1). All of these antibodies were purchased from Dakopatts, except CD56, which was obtained from Becton Dickinson, and RFT-4 and RFT-8, which were kindly provided by Prof G. Janossy of the Royal Free Hospital, London, and N. Willcox of the Institute of Molecular Medicine. For human embryo staining, the CD34 (HPCA-1; Clone My10; mIgG1) and CD45 (2D1; mIgG1) Mabs were purchased from Becton Dickinson. The CD43 (clone MT1; mIgG1) and pan CD44 (clone F10.44.2; mIgG2a) Mabs were obtained from Novocastra, Newcastle-upon-Tyne, England, and from Dr D. Jackson, Institute of Molecular Medicine, respectively. Comparative negative-control irrelevant unconjugated murine antibodies of the mIgG1, mIgG2a, mIgG2b, mIgM isotypes and mIgG2b-FITC (Dakopatts), mIgG3, and mIgG2a-FITC (Southern Biotechnology Associates Inc, Birmingham, AL) or mIgG1-FITC and mIgG1-PerCP (both from Becton Dickinson) were used in place of primary antibodies. Secondary FITC, Texas red, TRITC, or PE antibody conjugates of goat (Fab)2 anti-mIgG1, anti-mIgG3, anti-mIgM, anti-mIgG2a, antimouse IgG, or antirabbit IgG1 were purchased from Southern Biotechnology Associates Inc.
CD34+ cell isolation
Human CD34+ cells were purified (> 90% purity) from fresh cord blood and bone marrow samples using the Miltenyi Biotec (Bergisch Gladbach, Germany) mini-MACS CD34 stem cell isolation kit as described earlier.2 Human fetal liver CD34+ cells isolated using the same MACS system were purchased from Poietic Technologies, Gaithersburg, MD.
Immunofluorescence labeling and flow cytometric analyses
Single-color analysis of cell lines.
All immunofluorescence labeling procedures were carried out as described previously.2
Triple-color flow cytometric analysis of CD34+ cells.
After Fc receptor blockade (FcR blocking agent, Miltenyi Biotec), CD34+ cells were labeled with the CD164 Mabs, 103B2/9E10, 105A5, or N6B6 Mabs, or isotype-matched negative-control Mabs, CD34-PerCP or mIgG1-PerCP and AC-133-PE or mIgG1-PE for 30 minutes at 4°C in phosphate-buffered saline (PBS)–0.2% (wt/vol) bovine serum albumin followed by FITC-conjugated antimouse isotype-specific antibodies to mIgG3, mIgM, or mIgG2a.2
Four-color flow cytometric analysis of CD34+ cells from fetal liver.
CD38-APC or a negative mIgG1-APC Mab were added in addition to the other 3 Mabs above prior to the analysis of fetal liver CD34+ cells.
Flow cytometric analysis of Lin−CD34lo/−CD38lo/−cord blood cells.
After FcR blockade, erythrocyte-depleted cord blood mononuclear cells2 were labeled with a lineage-negative cocktail of FITC-conjugated CD2, CD3, CD14, CD16, CD19, CD41, CD56, CD66abce, antiglycophorin A, CD4, CD8, and CD38-FITC and then with CD34-PerCP or mIgG1-PerCP. Cells were then stained with 103B2/9E10 or 105A5, or isotype-matched irrelevant-control Mabs, prior to counterstaining with PE-conjugated anti-isotype–specific Mab. Cells were analyzed and gated on low side scatter, low to high forward scatter, and lineage-negative CD38lo/−(Lin−CD38lo/−) fluorescein gates according to Bhatia et al.29 The CD34-PerCP and CD164 epitope/PE-conjugated anti-mIgG3 or mIgM fluorescence was determined as above against mIgG1-PerCP and mIgG3 or mIgM-PE–conjugated anti-mIgG3 or mIgM-matched controls.
Labeled cells were analyzed on a FACSCalibur using Cellquest software (both from Becton Dickinson). The FITC, PE, and PerCP fluorescence was excited with an argon ion laser at 488 nm and detected at emission wavelengths of 530 nm, 570 nm, and 670 nm, respectively.2For 4-color analyses, a FACSCalibur equipped with both an argon ion and a helium-neon laser was used to detect the additional APC stain. The APC fluorescence was excited at 630 nm and the emission detected through a 660-nm band pass filter. The fluorescence was calibrated using Calibrite beads (Becton Dickinson) containing unconjugated and FITC-, PE-, and PerCP-conjugated beads, or APC-beads (Flow Cytometry Standards Corp, San Juan, PR). APC signals were compensated by the standard protocol of Becton Dickinson. Fine compensation was achieved by analyzing unstained or CD34-FITC, -PE, -PerCP, and -APC–stained cells. Compensation was set so that the positive signals were orthogonal to the negative signals. Fluorescence gates were set so that more than 95% of cells labeled with the appropriate isotype-matched negative-control Mab fell in the negative region. All cells above this gate were regarded as positive, while those below the gate were negative or weakly reactive with the test Mabs.
Embryonic tissue processing for histology and immunostaining
Human embryos were obtained immediately after voluntary terminations of pregnancy induced using RU 486 with informed consent according to the guidelines and with the approval of both national and institutional ethics committees. The embryos were fixed in 4% (wt/vol) paraformaldehyde (Sigma Chemical Co, St Louis, MO) and processed as described.30 Sections were stained with primary antibody overnight at 4°C, washed in PBS-0.25% (vol/vol) Triton X-100, and incubated for 1 hour at room temperature first with biotinylated rabbit antimouse Ig antibody (Dakopatts) and subsequently with horseradish peroxidase (HRP)-labeled streptavidin (Dakopatts). Peroxidase activity was developed with 0.025% (wt/vol) 3,3-diaminobenzidine (Sigma) in PBS containing 0.015% (wt/vol) hydrogen peroxide. Slides were counterstained with Harris's hematoxylin and mounted in XAM neutral medium.30
Tissue specimens
Tissues were collected with ethical approval and consent from the hospitals concerned. Tonsils were obtained from the Ear, Nose, and Throat Department of the Radcliffe Infirmary or the John Radcliffe Hospital, Oxford, England. Fresh normal adult tissue samples were provided by the Departments of Histopathology and Paediatric Pathology of the John Radcliffe Hospital. Thymus samples were obtained from 3 patients with myasthenia gravis (2 with thymoma and 1 hyperplastic thymus) and 2 normal thymuses from children undergoing cardiothoracic surgery. Fresh tissues were embedded in OCT (Bright, Huntingdon, England), snap-frozen in isopentane (BDH, Poole, England) in liquid nitrogen, and cryostat sections (5-8 μm) were cut, placed on 3-amino propyl-triethoxy-sialane–coated (Sigma) slides, air-dried overnight, fixed in acetone for 10 minutes, and stored at −20°C or −70°C until used.31 32
Immunohistochemical staining of tissue sections
Sections were blocked with 10% (vol/vol) normal human serum, incubated with Mab, and then incubated with peroxidase-conjugated goat-antimouse Ig (Dakopatts) or biotin-conjugated goat-antimouse Ig (Dakopatts) and streptavidin/HRP (Dakopatts). Staining was developed with diaminobenzidine tetrahydrochloride (DAB; Sigma) and hydrogen peroxide. The slides were then counterstained with hematoxylin (Surgipath, Eynesbury, St. Neots, England) and mounted in Apathy's mountant (BDH, Lutterworth, England).31 32
Immunofluorescence staining of tissue sections
Sections were FcR-blocked and then stained with CD164 Mabs and isotype-specific FITC-conjugated goat antimouse antibody, followed by CD1, CD3, CD19, CD31, CD33, CD43, CD68, HLA-DR, anticytokeratin, or isotype-matched negative-control antibodies, prior to adding the appropriate isotype-specific fluorescent-conjugated goat antimouse or antirabbit antibodies.31 Slides were mounted in fluorescent mounting medium (Dakopatts) with or without 2% (wt/vol) 4,6-diamidine-2-phenylindole dihydrochloride (DAPI) and viewed under an Olympus BX-60 (Olympus, London, England) or a Zeiss Axioskop (Zeiss, Welwyn Garden City, England) microscope. Images were captured on a JVC 3-CCD color video camera using the Neotech JVC software (Datacell Ltd, London, England) or on a CCD camera or Leaf micro lumina camera (Scitex Corp Ltd, Herzlia, Israel) using Improvision Openlab software (Improvision, Coventry, England) and analyzed in Adobe Photoshop. The exposure times were the same for the positive- and negative-control experiments.2
Immunoblotting of cell and tissue lysates
The equivalent of 8 × 104 of each exponentially growing cell line was resuspended in 1× nonreducing Laemmli loading buffer3 containing 1× Complete protease inhibitors (Boehringer-Mannheim, Mannheim, Germany) plus 5-mM DTT. A range of human tissues (heart, pancreas, colon, kidney, and spleen) were obtained from Clontech (Palo Alto, CA) and diluted in 1× Laemmli loading buffer containing 5-mM DTT and 1× Complete protease inhibitors (Boehringer-Mannheim). After boiling for 5 minutes, 2- to 10-μg lysate proteins or the equivalent of 8 × 104 were fractionated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidine difluoride immobilon membranes, and probed with the CD164 or isotype-matched negative-control Mabs followed by HRP-conjugated antimouse Ig or antimouse Ig isotype-specific Mabs (Southern Biotechnology Associates Inc). Blots were developed using the ECL system (Amersham International, Amersham, Bucks, England).27
Results
Expression of functional CD164 epitopes on CD34+hematopoietic cells during ontogeny
Because the interaction of class I and II CD164 Mabs 105A5 and 103B2/9E10, with their epitopes on bone marrow CD34+cells,3 inhibits the adhesion of CD34+ cells to stroma or negatively regulates CD34+ cell proliferation, we examined the expression of these CD164 epitopes on CD34+cells throughout ontogeny.
CD164 epitopes are expressed on primitive CD34+ intraaortic cell clusters in human embryos.
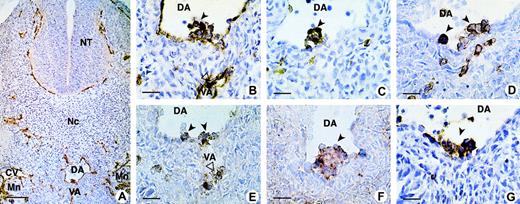
The sialomucin CD34 was used to identify intraaortic cell clusters and their associated ventral endothelium (Figure1A, 1B), with the panleukocyte marker CD45 distinguishing hematopoietic from endothelial cells (Figure 1C) in human embryo sections. We compared the expression of the homing receptor, CD44 (Figure 1G), with that of CD43 (Figure 1D) and the CD164 epitopes 103B2/9E10 and 105A5 (Figure 1E and 1F, respectively). Our results show clusters of positively stained hematopoietic CD34+ cells adhering to the endothelium on the ventral side of the dorsal aorta and vitelline artery, with these cells also expressing CD45, CD43, CD44, and the CD164 epitopes 103B2/9E10 and 105A5.
CD164 epitope expression on aorta-associated hematopoietic cell clusters in the 32-day human embryo.
(A) On a transverse section of the trunk, the CD34 antibody stains the endothelium of all blood vessels: dorsal aorta (DA), vitelline artery (VA), cardinal vein (CV), and capillaries in the mesoderm, around the neural tube (NT), and in the mesonephros (Mn). (B) Higher magnification of the same section shows clusters of hematopoietic CD34+ cells adhering to the endothelium on the ventral side of the DA and VA. These progenitors also express CD45 (C), CD43 (D), CD164 103B2/9E10 (E), CD164 105A5 (F), and CD44 (G). Dark and open arrows point to hematopoietic clusters located inside the DA and VA, respectively. Scale bars: (A) 100 μm; (B-G) 25 μm. All sections were from 32-day embryos, with A-F containing 39 somites and G, 40 somites. Original magnifications: (A) ×70; (B-G) ×240.
CD164 epitope expression on aorta-associated hematopoietic cell clusters in the 32-day human embryo.
(A) On a transverse section of the trunk, the CD34 antibody stains the endothelium of all blood vessels: dorsal aorta (DA), vitelline artery (VA), cardinal vein (CV), and capillaries in the mesoderm, around the neural tube (NT), and in the mesonephros (Mn). (B) Higher magnification of the same section shows clusters of hematopoietic CD34+ cells adhering to the endothelium on the ventral side of the DA and VA. These progenitors also express CD45 (C), CD43 (D), CD164 103B2/9E10 (E), CD164 105A5 (F), and CD44 (G). Dark and open arrows point to hematopoietic clusters located inside the DA and VA, respectively. Scale bars: (A) 100 μm; (B-G) 25 μm. All sections were from 32-day embryos, with A-F containing 39 somites and G, 40 somites. Original magnifications: (A) ×70; (B-G) ×240.
The class I, II, and III CD164 epitopes are expressed on the surface of primitive CD34+ cells from fetal liver, cord blood, and adult bone marrow.
Although most CD34+ cells in fetal liver, cord blood, and adult bone marrow expressed the CD164 functional epitopes, the level of staining varied from high to low (Figure2). In 3 separate CD34+preparations from fetal liver, 70% ± 34%, 93% ± 3%, and 96% ± 1% of cells were stained with 105A5, 103B2/9E10, and N6B6 Mabs, respectively, above background levels (2% ± 0%, 2% ± 0%, and 3% ± 1% for mIgM, mIgG3, and mIgG2a, respectively). In addition, 55% ± 12%, 93% ± 3%, and 89% ± 9% of CD34+ cord blood cells (4 independent experiments) were positive for these same epitopes, respectively. Similarly, 82% ± 10% of CD34+ human bone marrow cells expressed higher than background levels of the N6B6 epitope, whereas 70% ± 26% and 63% ± 26% of these cells reacted with the 103B2/9E10 and 105A5 Mabs, respectively.
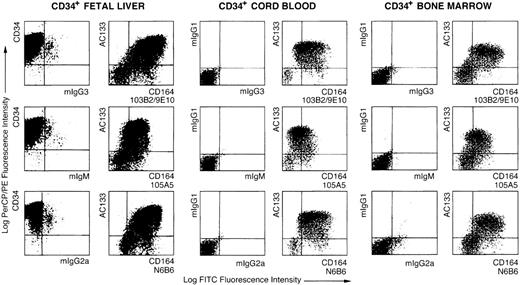
Flow cytometric analyses of AC133+CD34+ fetal liver, cord blood, and adult bone marrow cells with CD164 Mabs.
Dot plots showing MACS-selected CD34+ cells isolated from fetal liver, cord blood, or bone marrow triple-labeled with either CD34-PerCP, AC133-PE, and isotype negative-control Mabs, mIgG3, mIgM, or mIgG2a, plus FITC-conjugated anti-isotype secondary antibodies and the CD164 Mabs 103B2/9E10, 105A5, and N6B6 followed by FITC-conjugated anti-isotype secondary antibodies. Dot plots show displays of CD34-PerCP/PE, AC133-PE, or mIgG1-PE versus the CD164 epitopes, 103B2/9E10, 105A5, and N6B6, or their isotype-matched negative controls on CD34+ cells before or after gating on the CD34+ subsets. Cells were analyzed on a FACSCalibur using Cellquest software.
Flow cytometric analyses of AC133+CD34+ fetal liver, cord blood, and adult bone marrow cells with CD164 Mabs.
Dot plots showing MACS-selected CD34+ cells isolated from fetal liver, cord blood, or bone marrow triple-labeled with either CD34-PerCP, AC133-PE, and isotype negative-control Mabs, mIgG3, mIgM, or mIgG2a, plus FITC-conjugated anti-isotype secondary antibodies and the CD164 Mabs 103B2/9E10, 105A5, and N6B6 followed by FITC-conjugated anti-isotype secondary antibodies. Dot plots show displays of CD34-PerCP/PE, AC133-PE, or mIgG1-PE versus the CD164 epitopes, 103B2/9E10, 105A5, and N6B6, or their isotype-matched negative controls on CD34+ cells before or after gating on the CD34+ subsets. Cells were analyzed on a FACSCalibur using Cellquest software.
The highest cell surface expression of the 3 CD164 epitopes occurs on CD34hiAC133hiCD38lo/−cells.
Using AC133, a marker present on the more primitive subset of CD34+ human bone marrow cells,33 we demonstrated that the 105A5, 103B2/9E10, and N6B6 epitopes are expressed by most AC133+ cells (Figures 2 and 3). On AC133+ cells in bone marrow, cord blood, and fetal liver, the 103B2/9E10 and N6B6 epitopes were expressed at higher levels than the 105A5 epitope (Figure 2). The median fluorescence intensity (MFI) for 103B2/9E10 staining was higher than for the other CD164 epitopes and also higher in the AC133hi (MFI = 259 ± 128) than in the AC133lo (MFI = 133 ± 11) subset (Table1). These observations are consistent with our earlier findings that the 103B2/9E10 epitope of CD164 is expressed on the phenotypically most primitive bone marrow hematopoietic cell subsets.2 3 Four-color analysis of MACS-selected CD34+ fetal liver cells revealed that all of the CD164 epitopes are most intensively expressed on the AC133hiCD34hiCD38lo/−subsets (Tables 1 and 2; Figure 3). An increase of CD38 expression is accompanied by a decrease of AC133 and CD164 epitope expression. This expression terminates on most of the CD34loCD38hi cells.
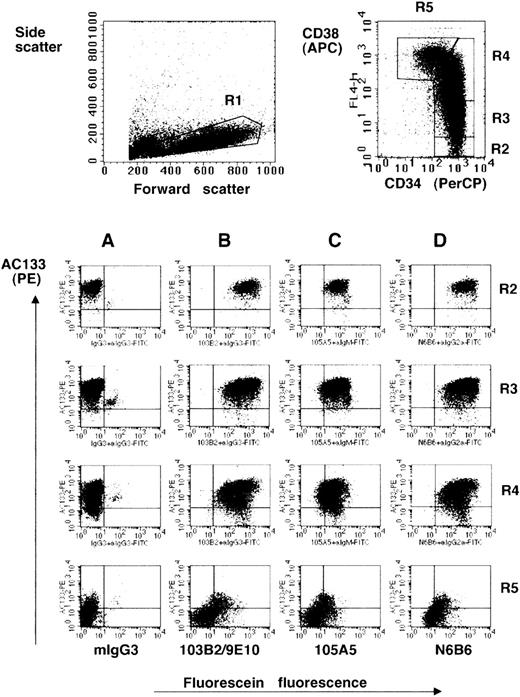
CD164 epitopes are more highly expressed on the AC133hiCD34hiCD38lo/− fetal liver cell subset.
Dot plots show MACS-selected CD34+ cells isolated from fetal liver labeled with CD34-PerCP, CD38-APC, AC133-PE, the CD164 Mabs 103B2/9E10 (B), 105A5 (C), and N6B6 (D) or isotype negative-control Mabs mIgG3 (A), mIgM, or mIgG2a, plus FITC-conjugated anti-isotype secondary antibodies. Isotype matched negative-control mIgG1-PE, mIgG1-APC, and mIgG1-PerCP were used in place of AC133-PE, CD38-APC, and CD34-PerCP (not shown). CD34-PerCP–positive cells with intermediate to high forward scatter and low side angle scatter characteristics (fraction R1) were analyzed on a FACSCalibur and gated on the basis of the relative expression of CD34 and CD38 (fractions R2, R3, R4, R5). These fractions were then analyzed for the expression of AC133 and the CD164 epitopes (Table 1).
CD164 epitopes are more highly expressed on the AC133hiCD34hiCD38lo/− fetal liver cell subset.
Dot plots show MACS-selected CD34+ cells isolated from fetal liver labeled with CD34-PerCP, CD38-APC, AC133-PE, the CD164 Mabs 103B2/9E10 (B), 105A5 (C), and N6B6 (D) or isotype negative-control Mabs mIgG3 (A), mIgM, or mIgG2a, plus FITC-conjugated anti-isotype secondary antibodies. Isotype matched negative-control mIgG1-PE, mIgG1-APC, and mIgG1-PerCP were used in place of AC133-PE, CD38-APC, and CD34-PerCP (not shown). CD34-PerCP–positive cells with intermediate to high forward scatter and low side angle scatter characteristics (fraction R1) were analyzed on a FACSCalibur and gated on the basis of the relative expression of CD34 and CD38 (fractions R2, R3, R4, R5). These fractions were then analyzed for the expression of AC133 and the CD164 epitopes (Table 1).
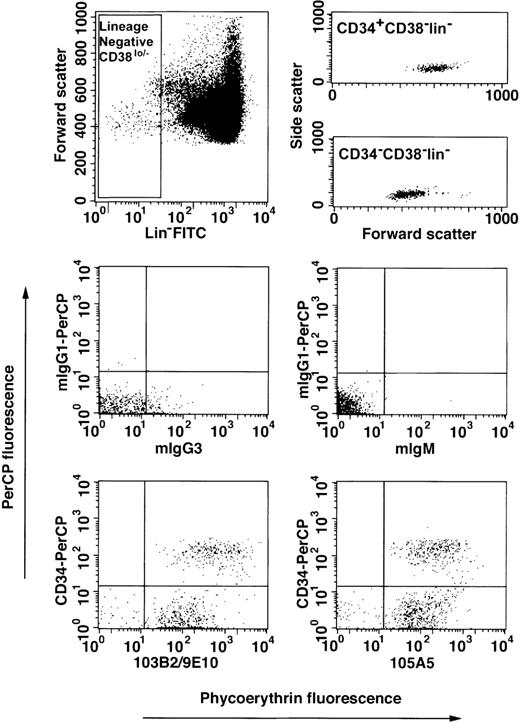
The CD164 class I and II epitopes are expressed on the surface of Lin−CD34hiCD38lo/−and Lin−CD34lo/−CD38lo/−cord blood cell subsets.
Recent studies indicate that primitive hematopoietic progenitor cells may reside in both the CD34+ and CD34lo/− fractions.29 We analyzed the reactivities of the 105A5 and 103B2/9E10 functionally defined CD164 Mabs with the Lin−CD34hiCD38lo/− and Lin−CD34lo/−CD38lo/−subsets in 3 independent experiments on cord blood mononuclear cells. In these experiments, 72% ± 8% and 28% ± 8% of the Lin−CD38lo/− cells were CD34lo/− and CD34+, respectively (Figure4). Of the Lin−CD34lo/−CD38lo/−cells, 82% ± 9% and 75% ± 16% bound the 105A5 and 103B2/9E10 Mabs, respectively. Most of the Lin−CD34hiCD38lo/−cells expressed these CD164 epitopes (97% ± 4% and 92% ± 5%, respectively). CD164 epitope expression was higher on the CD34+ than on the CD34lo/− subset. Interestingly, the cord blood Lin−CD34hiCD38lo/−subset expressed similar levels of the 103B2/9E10 epitope as the corresponding subset in fetal liver (Table2). Furthermore, the Lin−CD34hiCD38lo/− cord blood cells exhibited slightly higher levels of expression of the class I 105A5 epitope compared with the Lin−CD34lo/−CD38lo/−subset, (MFI = 162 ± 78 vs 45 ± 30, respectively). However, the 103B2/9E10 epitope was much more highly expressed on the Lin−CD34hiCD38lo/− than on the Lin−CD34lo/−CD38lo/−cells (MFI = 410 ± 271 vs 52 ± 45, respectively).
Expression of CD164 epitopes on Lin−CD34lo/−CD38lo/− cord blood cells.
Mononuclear cells from pools of up to 3 cord blood samples were collected, subjected to erythrocyte lysis, and Fc receptors blocked as described in “Materials and methods.” Cells were labeled with a lineage-negative cocktail of the following FITC-conjugated Mabs: CD2, CD3, CD4, CD8, CD14, CD16, CD19, CD41, CD56, CD66abce, anti-glycophorin A, with CD38-FITC, with CD34-PerCP or mIgG1-PerCP, and with the 103B2/9E10, 105A5, or isotype-matched irrelevant-control Mabs, mIgG3 or mIgM, prior to counterstaining with PE-conjugated anti-isotype–specific secondary antibodies. Cells were gated on low side scatter, low to high forward scatter, and lineage-negative CD38lo/−(Lin−CD38lo/−) fluorescein gates (upper left dot plot) according to Bhatia et al.29 The CD34-PerCP and CD164 epitope/PE-conjugated anti-mIgG3 or mIgM fluorescence was determined against mIgG1-PerCP and mIgG3 or mIgM/PE-conjugated anti-mIgG3 or mIgM-matched controls. Cells were analyzed on a FACSCalibur and 400 × 103to 500 × 103 events stored as listmode data files per sample. The upper right dot plots show the forward and side scatter distribution of Lin−CD38lo/− cells after CD34+ or CD34− cell gating. The lower dot plots demonstrate the staining of Lin−CD38lo/− cells labeled with isotype-matched control Mabs or with CD164 and CD34 Mabs. The median fluorescence intensity values for mIgG3- and mIgM-labeled Lin−CD34lo/−CD38lo/−cells were 3 ± 1 and 2 ± 1, respectively, and Lin−CD34+CD38lo/− cells were 1 ± 2 and 2 ± 1, respectively.
Expression of CD164 epitopes on Lin−CD34lo/−CD38lo/− cord blood cells.
Mononuclear cells from pools of up to 3 cord blood samples were collected, subjected to erythrocyte lysis, and Fc receptors blocked as described in “Materials and methods.” Cells were labeled with a lineage-negative cocktail of the following FITC-conjugated Mabs: CD2, CD3, CD4, CD8, CD14, CD16, CD19, CD41, CD56, CD66abce, anti-glycophorin A, with CD38-FITC, with CD34-PerCP or mIgG1-PerCP, and with the 103B2/9E10, 105A5, or isotype-matched irrelevant-control Mabs, mIgG3 or mIgM, prior to counterstaining with PE-conjugated anti-isotype–specific secondary antibodies. Cells were gated on low side scatter, low to high forward scatter, and lineage-negative CD38lo/−(Lin−CD38lo/−) fluorescein gates (upper left dot plot) according to Bhatia et al.29 The CD34-PerCP and CD164 epitope/PE-conjugated anti-mIgG3 or mIgM fluorescence was determined against mIgG1-PerCP and mIgG3 or mIgM/PE-conjugated anti-mIgG3 or mIgM-matched controls. Cells were analyzed on a FACSCalibur and 400 × 103to 500 × 103 events stored as listmode data files per sample. The upper right dot plots show the forward and side scatter distribution of Lin−CD38lo/− cells after CD34+ or CD34− cell gating. The lower dot plots demonstrate the staining of Lin−CD38lo/− cells labeled with isotype-matched control Mabs or with CD164 and CD34 Mabs. The median fluorescence intensity values for mIgG3- and mIgM-labeled Lin−CD34lo/−CD38lo/−cells were 3 ± 1 and 2 ± 1, respectively, and Lin−CD34+CD38lo/− cells were 1 ± 2 and 2 ± 1, respectively.
Class I, II, and III CD164 epitopes are differentially expressed in adult tissues in vivo
Although the CD164 epitopes are highly expressed on primitive CD34+AC133+CD38lo/−hematopoietic progenitor cells, we have demonstrated that CD164 expression also occurs on subsets of more mature hematopoietic cells.2 3 We investigated the expression patterns of the class I, II, and III epitopes in a set of normal adult tissues. In contrast to the more uniform expression of CD164 epitopes on hematopoietic progenitor cells, the class I and II epitopes in diverse tissue specimens were often differentially expressed.
Postnatal lymphoid tissues.
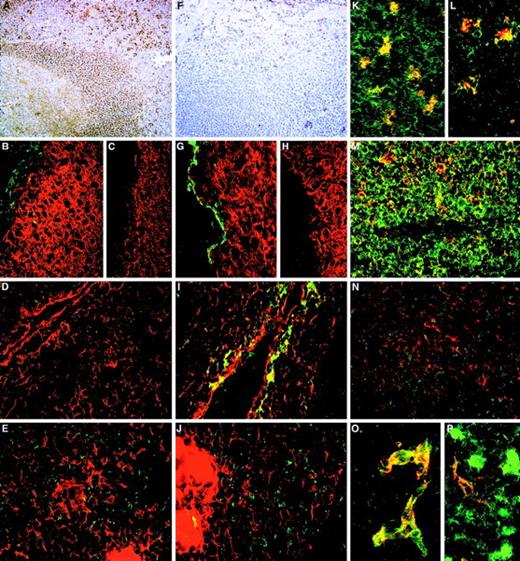
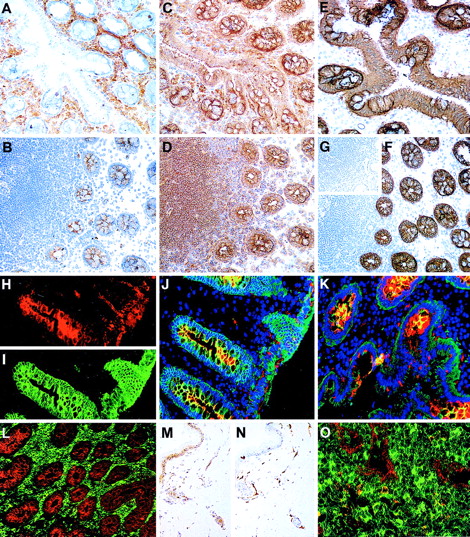
The class I and class II functional epitopes were often distributed on reciprocal cell subsets, whereas the class III epitopes defined by N6B6 and 67D2 Mabs were expressed on both the 105A5+ and 103B2/9E10+ subsets (Table 3and Figure 5). A sub–pan-reactive pattern of staining with strong labeling of T and B lymphocytes and an absence of endothelial labeling was evident for the class I Mab 105A5 in tonsil (Figure 5A), with the macrophage population exhibiting particularly high levels of 105A5 epitope expression (Table 3; Figure 5L and 5K). The class II Mab 103B2/9E10 stained CD31+ endothelium (Figure 5P) in tonsil and thymus, basal-layer epithelium in tonsil (Figure 5G), and subcapsular epithelium in thymus (Figure 5I) but not lymphoid cells (Figures 5F and 6O; Table 3). Most thymic CD1+ cortical lymphoid cells stained very weakly with the class I Mab, with weak coexpression of the 105A5 epitope observed with CD43+ T cells in the thymic cortex and medulla (Figure 5N). 105A5 did not stain thymic epithelial cells (Figure 5D and 5E). Thymic macrophages stained with 105A5 (Figure 5L) and 103B2/9E10 (Figure 5J). The CD31+ thymic blood vessel endothelia was 105A5−103B2/9E10+. In both tonsil and hyperplastic thymic sections (Figure 5O), the 103B2/9E10, but not the 105A5, epitope was expressed on a proportion of MECA-79+high endothelial venules (Table 3).
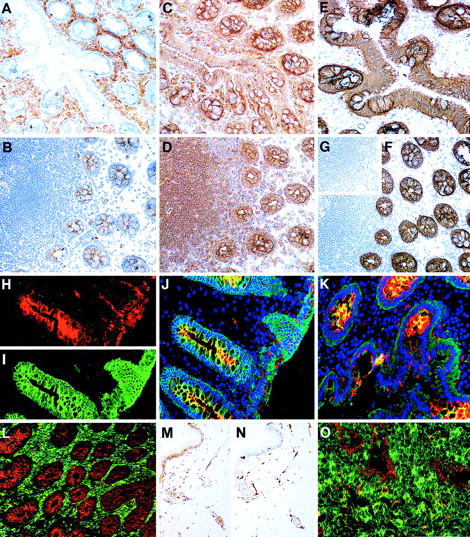
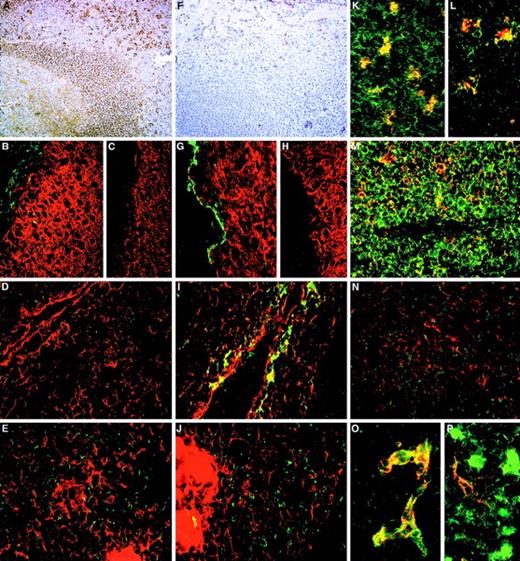
The distribution of CD164 class I and II epitopes in postnatal tissue sections.
105A5 class I Mab staining of frozen sections of human child tonsil (A, B, K, M) and thymus (D, E, L, N) is compared with 103B2/9E10 class II Mab staining of tonsil (F, G) and thymus (I, J, O, P). Staining of tonsil using the immunoperoxidase technique revealed a sub–pan-reactive labeling with 105A5, with lymphoid cells and macrophages positive (A), while with 103B2/9E10 only the endothelia are stained (F). Dual immunofluorescence with the 105A5 (B, D, E), 103B2/9E10 (G, I, J), or isotype-matched mIgM (C) or mIgG3 (H) Mabs (green) and anticytokeratin (red) demonstrates that the basal layer epithelium of tonsil (G) and the subcapsular epithelium of thymus (I) coexpress the 103B2/9E10 epitope and cytokeratin, while medullary epithelium are cytokeratin-positive but 103B2/9E10-negative (J). Cytokeratin-positive epithelia in tonsil (B) and in the thymic subcapsular (D) and medulla (E) were 105A5-negative. 105A5 (red) clearly stained CD68- (green) positive macrophages in tonsil (K) and in the thymus, as illustrated for these cells at the corticomedullary junction (L). Strong double staining of CD43-positive cells (red) with 105A5 (green) and an absence of staining of blood vessel endothelia confirmed that the sub–pan-reactive staining observed with the immunoperoxidase method (A) included T cells but not endothelia (M). This was in contrast to dual labeling of thymic medulla, where cells expressed CD43 strongly but showed weak and often patchy staining with 105A5 (N). These dual-labeled cells were macrophages. In contrast to 105A5 expression, 103B2/9E10 (green) was weakly coexpressed on CD31- (red) positive blood vessel endothelia in thymus, while macrophages stained strongly with 103B2/9E10 but not with CD31 (P). In hyperplastic thymus (O), both CD31 (red) and 103B2/9E10 (green) were coexpressed on high endothelial venules. Original magnifications: (A, F) ×80; (B-E, G-J, M, N) ×240; (K, L) ×500; (O, P) ×600.
The distribution of CD164 class I and II epitopes in postnatal tissue sections.
105A5 class I Mab staining of frozen sections of human child tonsil (A, B, K, M) and thymus (D, E, L, N) is compared with 103B2/9E10 class II Mab staining of tonsil (F, G) and thymus (I, J, O, P). Staining of tonsil using the immunoperoxidase technique revealed a sub–pan-reactive labeling with 105A5, with lymphoid cells and macrophages positive (A), while with 103B2/9E10 only the endothelia are stained (F). Dual immunofluorescence with the 105A5 (B, D, E), 103B2/9E10 (G, I, J), or isotype-matched mIgM (C) or mIgG3 (H) Mabs (green) and anticytokeratin (red) demonstrates that the basal layer epithelium of tonsil (G) and the subcapsular epithelium of thymus (I) coexpress the 103B2/9E10 epitope and cytokeratin, while medullary epithelium are cytokeratin-positive but 103B2/9E10-negative (J). Cytokeratin-positive epithelia in tonsil (B) and in the thymic subcapsular (D) and medulla (E) were 105A5-negative. 105A5 (red) clearly stained CD68- (green) positive macrophages in tonsil (K) and in the thymus, as illustrated for these cells at the corticomedullary junction (L). Strong double staining of CD43-positive cells (red) with 105A5 (green) and an absence of staining of blood vessel endothelia confirmed that the sub–pan-reactive staining observed with the immunoperoxidase method (A) included T cells but not endothelia (M). This was in contrast to dual labeling of thymic medulla, where cells expressed CD43 strongly but showed weak and often patchy staining with 105A5 (N). These dual-labeled cells were macrophages. In contrast to 105A5 expression, 103B2/9E10 (green) was weakly coexpressed on CD31- (red) positive blood vessel endothelia in thymus, while macrophages stained strongly with 103B2/9E10 but not with CD31 (P). In hyperplastic thymus (O), both CD31 (red) and 103B2/9E10 (green) were coexpressed on high endothelial venules. Original magnifications: (A, F) ×80; (B-E, G-J, M, N) ×240; (K, L) ×500; (O, P) ×600.
Adult nonhematopoietic tissues.
The differential patterns of CD164 epitope expression observed in hematopoietic tissues were observed in nonhematopoietic tissues, particularly those with a glandular element, such as salivary gland, thyroid, and colon (Table 3). While all of the Mabs labeled the secretory lobules of salivary gland, the class II 103B2/9E10 Mab labeled the apical membranes of secretory ducts, which were negative for the class I 105A5 epitope. In sections of colon, Mab 105A5 stained the infiltrating CD68+ macrophages, CD3+ T cells, and CD19+ B cells (Table 3) in the laminar propria (Figure 6A). In contrast, 103B2/9E10 labeled the goblet cell vacuolar membranes of the colon and endothelial cells within the laminar propria (Figure 6B, 6H, 6J, 6K, and 6L). This was confirmed by colabeling with 9C4, which stains epithelial cells in many tissues (S.M.W., unpublished data; Figure 6E and 6F). The N6B6 and 67D2 class III Mabs reacted with both the 105A5+ and 103B2/9E10+ cells in the colon sections (Figure 6C and 6D). The basal layer of the skin was 103B2/9E10 class II+(Figure 6M). The splenic sinus endothelia were strongly 103B2/9E10+ while, in brain, the specialized endothelia34 were both class I 105A5+ and class II 103B2/9E10+ (Table 3).
The distribution of CD164 epitopes in adult nonhematopoietic tissue sections.
Frozen sections of colon stained using the immunoperoxidase technique with the 105A5 (A), 103B2/9E10 (B), N6B6 (C), and 67D2 (D) CD164 Mabs revealed that the 105A5 epitope was expressed by infiltrating lymphoid cells and macrophages in the laminar propria (A), the 103B2/9E10 epitope by vacuolar membranes of the goblet cells, with weak expression on the epithelium of the villi and crypts, and the N6B6 and 67D2 epitopes were expressed by both the 105A5- and 103B2/9E10-positive cells. The 9C4 Mab stained the vacuolar membranes of the goblet cells and the epithelium strongly, as is illustrated using the immunoperoxidase (E, F) or immunofluorescence (I) methods, while its isotype-matched control Mab, mIgG2b, was negative (inset G). Sections were triple-labeled with 103B2/9E10 (red), 9C4 (green), and DAPI (blue) for the detection of the cell nucleus using the immunofluorescence technique. Single-color analyses with 103B2/9E10 (H) confirmed that both the vacuolar membranes of goblet cells and endothelium were positive, with weaker labeling of the apical epithelium of the villi and crypts. In contrast, 9C4 did not label endothelia (I). Triple-color analyses (J, K) confirmed the strong coexpression of 103B2/9E10 and 9C4 on goblet cell vacuolar membranes (yellow). A comparison of reciprocal immunofluorescence staining of colon (L) and tonsil (O) sections with the 103B2/9E10 (red) and 105A5 (green) Mabs is shown. Double-stained cells (yellow; O) are macrophages. A skin section incubated with the 103B2/9E10 Mab demonstrates positive immunoperoxidase staining of basal epithelia and endothelia (M), while that stained with CD31 (N) shows endothelial staining only. Original magnifications: (A-G, L) ×90; (H-K) ×240; (M, N) ×70; O ×400.
The distribution of CD164 epitopes in adult nonhematopoietic tissue sections.
Frozen sections of colon stained using the immunoperoxidase technique with the 105A5 (A), 103B2/9E10 (B), N6B6 (C), and 67D2 (D) CD164 Mabs revealed that the 105A5 epitope was expressed by infiltrating lymphoid cells and macrophages in the laminar propria (A), the 103B2/9E10 epitope by vacuolar membranes of the goblet cells, with weak expression on the epithelium of the villi and crypts, and the N6B6 and 67D2 epitopes were expressed by both the 105A5- and 103B2/9E10-positive cells. The 9C4 Mab stained the vacuolar membranes of the goblet cells and the epithelium strongly, as is illustrated using the immunoperoxidase (E, F) or immunofluorescence (I) methods, while its isotype-matched control Mab, mIgG2b, was negative (inset G). Sections were triple-labeled with 103B2/9E10 (red), 9C4 (green), and DAPI (blue) for the detection of the cell nucleus using the immunofluorescence technique. Single-color analyses with 103B2/9E10 (H) confirmed that both the vacuolar membranes of goblet cells and endothelium were positive, with weaker labeling of the apical epithelium of the villi and crypts. In contrast, 9C4 did not label endothelia (I). Triple-color analyses (J, K) confirmed the strong coexpression of 103B2/9E10 and 9C4 on goblet cell vacuolar membranes (yellow). A comparison of reciprocal immunofluorescence staining of colon (L) and tonsil (O) sections with the 103B2/9E10 (red) and 105A5 (green) Mabs is shown. Double-stained cells (yellow; O) are macrophages. A skin section incubated with the 103B2/9E10 Mab demonstrates positive immunoperoxidase staining of basal epithelia and endothelia (M), while that stained with CD31 (N) shows endothelial staining only. Original magnifications: (A-G, L) ×90; (H-K) ×240; (M, N) ×70; O ×400.
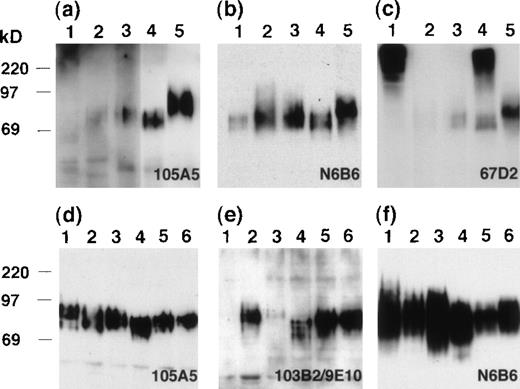
Immunoblot analysis of CD164 epitopes in tissues and cell lines.
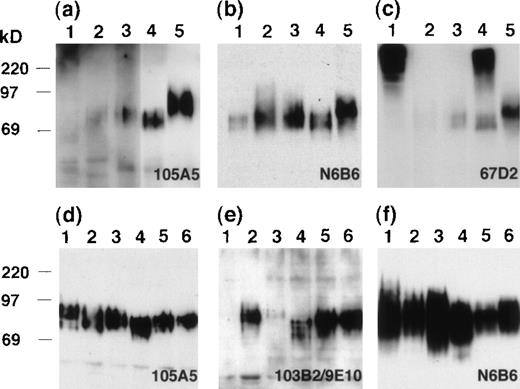
CD164 messenger RNA can be detected in a variety of cell lines and tissues. To confirm that the CD164 Mabs were recognizing molecules of similar apparent molecular weights in these tissues, a set of whole-cell lysates were immunoblotted with each CD164 Mab after SDS-PAGE separation (Figure 7). The Mab 105A5 detected an 80- to 100-kd protein in kidney and spleen on short exposures (Figure 7A) and, on longer exposures, proteins within this molecular weight range were also seen in colon and pancreas. The expression in heart was very weak and only detectable on very long exposures. Equivalent proteins of 80- to 100-kd were detected in each tissue examined using the class III Mab N6B6 (Figure 7B). However, particularly strong reactivity was found with spleen and, as with 105A5 detection, the protein appeared to possess a slightly higher molecular weight than that found in other tissues. The class II Mab, 103B2/9E10, although able to detect this slightly higher–molecular-weight protein in spleen, did not appear to detect any protein bands in the other tissues examined (data not shown), presumably due to the limited cellular expression of this epitope in these tissues. The size and intensities of the bands detected with the different CD164 Mabs are very likely the result of differential/partial glycosylation generated by differential splicing of the CD164 molecule or during processing. The other class III Mab, 67D2, detected the 80- to 100-kd proteins in pancreas, colon, kidney, and spleen as well as much higher–molecular-weight molecules (> 220 kd) in heart and kidney (Figure 7C). On filters of hematopoietic cell lines representing CD34+ progenitor cells (KG1A), monocytic cells (U937), and B cells (RPMI-1788) and of epithelial cell lines derived from the colon (Calu-1), uterus (HeLa), and kidney (293T), the class I Mab 105A5 (Figure 7D), the class II Mab 103B2/9E10 (Figure 7E), and the class III Mab N6B6 (Figure 7F) detected proteins of 80 to 100 kd, while the class III Mab 67D2 detected the 80- to 100-kd and greater than 220-kd protein bands (data not shown). These studies confirm our preliminary observation that the higher–molecular-weight band is found in the SDS solubilized cell and tissue lysates but not in the cell lysates solubilized in Triton X-100, NP-40, or CHAPS detergents. Explanations for these results are that the CD164 molecule may associate with cytoskeletal elements in a complex that is not solubilized by Triton X-100 or that the 67D2 Mab may detect a Triton X-100–insoluble CD164 isoform containing a glycosaminoglycan attachment or CD164 tetramers.27
Immunoblot analyses of tissue and cell lysates.
SDS lysates from tissues (A, B, C) or epithelial and hematopoietic cell lines (D, E, F) were resolved by 10% SDS-PAGE and immunoblotted with the 105A5 (A, D), 103B2/9E10 (E), N6B6 (B, F), or 67D2 (C) Mabs. Tissues analyzed on blots (A, B, C) were heart (lane 1), pancreas (lane 2), colon (lane 3), kidney (lane 4), and spleen (lane 5). Cell lines (D, E, F) were RPMI-1788 (lane 1), HeLa (lane 2), U937 (lane 3), 293T (lane 4), KG1A (lane 5), and Calu-1 (lane 6).
Immunoblot analyses of tissue and cell lysates.
SDS lysates from tissues (A, B, C) or epithelial and hematopoietic cell lines (D, E, F) were resolved by 10% SDS-PAGE and immunoblotted with the 105A5 (A, D), 103B2/9E10 (E), N6B6 (B, F), or 67D2 (C) Mabs. Tissues analyzed on blots (A, B, C) were heart (lane 1), pancreas (lane 2), colon (lane 3), kidney (lane 4), and spleen (lane 5). Cell lines (D, E, F) were RPMI-1788 (lane 1), HeLa (lane 2), U937 (lane 3), 293T (lane 4), KG1A (lane 5), and Calu-1 (lane 6).
Discussion
Hematopoiesis in mammals and birds originates from the extra-embryonic mesoderm of the yolk sac and autonomously from the paraaortic splanchnopleural mesoderm, the presumptive aorta-gonad-mesonephros (AGM) region of the embryo proper.35,36 The observation that hematopoietic and endothelial cells are closely associated during embryonic development, particularly in the blood islands of the yolk sac and in dorsal aorta and vitelline artery, has led to the hypothesis that a common hematopoietic-endothelial precursor, termed the hemangioblast, exists. In this respect, elegant transplantation experiments in birds have suggested the existence of 2 distinct endothelial lineages. The first is derived from the somites and generates the sides and roof of the aorta. The second, which has both endothelial and hematopoietic potentialities, forms the floor of the aorta.37 This may represent a common mechanism for hematopoietic/endothelial development in birds and mammals. In the human 4- to 5-week-old embryo, the largest accumulation of CD45+CD31+CD34+ putative hematopoietic stem cells has been found associated with the ventral endothelium of the aorta and the vitelline artery.30,38-40Here, the endothelial layer is disorganized and replaced by cell clusters that bud into the lumen and express hematopoietic markers such as CD45.32,39 These cells have also recently been shown to coexpress SCL, c-myb, GATA-2, GATA-3, c-kit, flk-1, HCA (CD166), and KG1-kinase.39,40 From murine gene knockout studies, several of these molecules (eg, GATA-2, SCL, and flk-1) appear to be essential for definitive hematopoiesis.36
In the studies presented here, we confirm the existence of these dorsal aorta-associated CD45+CD34+ hematopoietic cell clusters in the human 4- to 5-week-old embryo. Furthermore, we show that these cells express 2 other sialomucins, CD164 and CD43, and the homing receptor CD44. Thus, we demonstrate that at least 3 members of the sialomucin family (CD34, CD43, and CD164) and 5 receptors that have the potential to regulate adhesion, migration, proliferation (CD45, CD43, CD164, CD34, and CD44) are expressed on human hematopoietic precursors at one of the earliest stages of hematopoietic development. Because ligation of the class I (105A5) and II (103B2/9E10) CD164 epitopes has been shown to have functional effects on bone marrow CD34+ cells in vitro, by mediating adhesion to stroma or negatively regulating hematopoietic progenitor cell proliferation,3 we extended these studies to show that both classes of CD164 epitopes are expressed on CD34+hematopoietic cells throughout ontogeny. As well as occurring on the dorsal aorta-associated CD34+ haematopoietic cell clusters, the CD164 epitopes are prominently distributed on the surface of the most primitive CD34+CD38lo/−AC133+hematopoietic cells in fetal liver, cord blood, and adult bone marrow. Analysis of cord blood also suggests that the functional CD164 epitopes are expressed on the surface of most Lin−CD34−CD38lo/−cells. This primitive cell subset has recently been described as a putative precursor for CD34+ cells, although both the CD34+ and CD34− subsets seem to contain long-term repopulating stem cells, which differ in their abilities to be maintained under certain ex vivo culture conditions.29 41 An analysis of the capacity of the CD164+CD34+ and CD164+CD34− cells to engraft and to maintain human hematopoiesis over the long term after in utero transplantation in sheep is currently under investigation.
Several possibilities exist with respect to the functional importance of sialomucins such as CD164 on primitive hematopoietic stem/progenitor cells. It is generally agreed that hematopoietic stem/progenitor cells migrate from their site of origin in the yolk sac or AGM region in the embryo proper to the embryonic liver and, subsequently, to the spleen and bone marrow during embryonic development, with the bone marrow being the major site for hematopoiesis in the adult.35,36The identification of the CD164 epitopes that are expressed on primitive CD34+ cells throughout development and that function, at least, in the regulation of adhesion or proliferation of CD34+ bone marrow progenitor cells provides a first step toward determining their functions on CD34+ cells at different stages of ontogeny. Current knowledge indicates that migration and homing of primitive hematopoietic progenitors is a multistep process involving the coordinated interaction of adhesion, cytokine, or chemokine receptors with their cognate ligands. These processes appear to be controlled by associated crosstalk between cytokine, chemokine, and adhesion receptor signaling pathways, which as a consequence regulates cell proliferation and differentiation.35,36,42-46 Limited studies have been conducted on the functions of other sialomucins in hematopoietic development, although several of these have been shown to mediate functional effects in vitro. For example, CD34, CD45, PSGL-1, PCLP, and CD43 have all been implicated in regulating hematopoietic progenitor cell proliferation, adhesion, or differentiation.18,47-51The most comprehensive in vivo studies have been conducted on the CD34 molecule. CD34-null mice exhibit decreased hematopoietic progenitor cell numbers at hematopoietic sites in the yolk sac blood islands, the fetal liver, fetal bone marrow, adult bone marrow, and spleen, with CD34-deficient progenitors from adult bone marrow having a reduced capacity to expand ex vivo in response to certain cytokines.52 Such effects are reversed by ectopic expression of CD34 with or without the full-length cytoplasmic tail.53 These studies suggest that the extracellular mucin-like domains of CD34 are involved in hematopoietic progenitor cell proliferation and maintenance. Because several sialomucins, namely CD34, CD162 or PSGL-1, and PCLP, have been shown to act as ligands for the selectins and because α1,3-fucosyl transferase (Fuc-TVII) is required for selectin ligand biosynthesis,20sialomucin functions may be deduced from studies on selectin or Fuc-TVII–null mice. For example, elevated levels of hematopoietic progenitor cells, leukocytosis, or a hematopoietic stem cell homing defect to adult bone marrow are found in double CD62P- and CD62E-deficient or in Fuc-TVII–deficient mice.20,25,54,55 It is possible that CD164 may contribute to these effects. Like cytokine receptors, adhesion receptors are likely to display overlapping functions (often referred to inappropriately as “redundancy”) or at least are able to act in concert to finely tune the migration, homing, and proliferative functions of hematopoietic stem/progenitor cells. Thus, as is evidenced by studies on CD34- or CD43-null mice,52,56 mice singly deficient in 1 adhesion, cytokine, or chemokine receptor may exhibit minimal disturbance of hematopoiesis. A second role for sialomucins may lie in their ability to endow the hematopoietic stem/progenitor cell with the specificity to home into and lodge in defined and appropriate hematopoietic microenvironments. Recently, Verfaillie41 has suggested that this specificity may be more a function of sialomucins than of integrins, because the latter are widely distributed on many cell types and generally do not undergo posttranslation modifications. In this respect, our studies show that, like CD34, PSGL-1, and PCLP, CD164 undergoes posttranslational modification, either by the regulated expression of the class I and II glycosylation-dependent CD164 functional epitopes or by the ability of CD164 to undergo alternative splicing, a process that would alter the degree of glycosylation or introduce a potential GAG attachment site into the molecule. Finally, because ligation of the CD164 class I and II epitopes with their surrogate ligands in vitro modulates the recruitment of primitive hematopoietic progenitor cells into cycle even in the presence of multiple cytokines,3 CD164 may function as a signaling molecule to control hematopoietic stem/progenitor cell proliferation. Similar effects on cell survival, proliferation, and differentiation have been proposed for integrin, selectin, and other sialomucin receptors.
The simultaneous expression of all CD164 epitope classes on CD34+ cells is in direct contrast to the differential patterns of expression of the 3 classes of CD164 epitopes in postnatal hematopoietic and nonhematopoietic tissues. Thus, while the CD164 class III epitopes are widely expressed on both hematopoietic and nonhematopoietic cell types, there are significant variations in the expression of class I and II epitopes with their restriction to specific cell types within specific tissues. Notably, the 103B2/9E10 epitope is displayed on most vascular endothelium, on some high endothelial venules in lymphoid tissues, on the venous sinuses in spleen,34 and on the subcapsular and basal epithelia of the thymus, tonsil, and skin. The specialized brain endothelia are both CD164 class I– and II–positive. In contrast, the 105A5 epitope is arrayed on CD164 expressed by lymphoid cells in tissues that are involved in lymphoid/blood cell recirculation, such as in the tonsil, spleen, and gut. Interestingly, the thymic subcapsular epithelium, which is associated with pre-T lymphoid cell trafficking,57 58 expresses the class II CD164 epitope. This suggests that on different cell types the class I and II CD164 epitopes may function in human lymphoid or blood cell homing and trafficking as well as regulating CD34+ cell homing and proliferation. Indeed, other sialomucins, such as CD34, PCLP, MAdCAM-1, PSGL-1, and GlyCAM-1, on high endothelial venules or on hematopoietic cells have been implicated as major players in lymphoid/hematopoietic cell migration or recruitment, an observation initially related to studies on their protein distribution in vivo. Thus, adhesion, cytokine, and chemokine receptors all appear to play a role in locating stem/progenitor cells in an environmental niche where their survival, proliferation, and differentiation are regulated. The studies presented here provide a first step toward determining the in vivo relevance of functional CD164 epitopes in these processes.
Acknowledgments
The authors thank Drs K. Micklem, R. Roberts-Gant, M. Jones, R. Bicknell, and Professors K. Gatter and N. Willcox for their advice on the section staining, and Professors D. J. Weatherall, L. Kanz, and M. J. Watt for their support.
Supported by the Leukaemia Research Fund and the Medical Research Council UK, INTAS/RFBR and EU Biotechnology grants and Smith-Kline Beecham (S.M.W., L.H.B., I.R., R.D., J.Y.-H.C.), Deutsche Forschungsgemeinschaft (SFB 510; project A1; H.-J.B.), National Health and Medical Research Council of Australia (P.J.S., A.C.W.Z., J.-P.L.), State Scholarships Foundation of Greece (I.R.) and, in part, the European Commission (M.T., B.P.).
B.P. and I.R. are joint senior authors of this paper.
Reprints:Suzanne M. Watt, MRC Molecular Haematology Unit, Institute of Molecular Medicine, John Radcliffe Hospital, Headington, Oxford, England; e-mail:swatt@enterprise.molbiol.ox.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.