Abstract
Recent findings support the hypothesis that the CD34+-cell population in bone marrow and peripheral blood contains hematopoietic and endothelial progenitor and stem cells. In this study, we report that human AC133+ cells from granulocyte colony-stimulating factor–mobilized peripheral blood have the capacity to differentiate into endothelial cells (ECs). When cultured in the presence of vascular endothelial growth factor (VEGF) and the novel cytokine stem cell growth factor (SCGF), AC133+ progenitors generate both adherent and proliferating nonadherent cells. Phenotypic analysis of the cells within the adherent population reveals that the majority display endothelial features, including the expression of KDR, Tie-2, Ulexeuropaeus agglutinin-1, and von Willebrand factor. Electron microscopic studies of these cells show structures compatible with Weibel-Palade bodies that are found exclusively in vascular endothelium. AC133-derived nonadherent cells give rise to both hematopoietic and endothelial colonies in semisolid medium. On transfer to fresh liquid culture with VEGF and SCGF, nonadherent cells again produce an adherent and a nonadherent population. In mice with severe combined immunodeficiency, AC133-derived cells form new blood vessels in vivo when injected subcutaneously together with A549 lung cancer cells. These data indicate that the AC133+-cell population consists of progenitor and stem cells not only with hematopoietic potential but also with the capacity to differentiate into ECs. Whether these hematopoietic and endothelial progenitors develop from a common precursor, the hemangioblast will be studied at the single-cell level.
The formation of new blood vessels can be due to two different processes. The first process, vasculogenesis, implies the in situ differentiation of endothelial cells (ECs) from hemangioblasts and their subsequent organization into a primary capillary plexus.1,2 The second process, termed angiogenesis, is defined as the formation of new vessels by sprouting from preexisting blood vessels.3,4 At present, primary differentiation of ECs from hemangioblasts or angioblasts seems to be a process that is restricted to early embryogenesis, whereas angiogenesis occurs both during development and postnatal life. The hemangioblast has recently been identified and was shown to be a transient cell stage that develops early and is lost quickly during embryonic development.5 It is important to note that these findings are based on in vitro experiments with murine embryoid bodies and may not necessarily reflect developmental steps in humans. The possibility that hemangioblasts or more mature endothelial progenitors persist into adult life whereby they may circulate, differentiate, and contribute to the formation of new blood vessels remains to be determined. In this context, previous studies have demonstrated the existence of mature ECs in the peripheral circulation.6-15 Recently, Asahara et al16 reported the presence of CD34+ endothelial progenitors in human peripheral blood. The investigators showed that the progenitors differentiated into ECs in vitro and were incorporated into sites of neovascularization in vivo. However, no data were provided that definitely proved the CD34+ progenitors to be the source of the generated ECs or the endothelial nature of the cultured cells. Nevertheless, a similar study by Shi et al17 provided strong evidence that CD34+ cells isolated from human bone marrow, umbilical cord blood, and granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood contains endothelial progenitors. In a canine bone marrow transplant model, the investigators could demonstrate that bone marrow-derived CD34+ endothelial progenitors had the capacity to line an implanted vascular prosthesis.
The molecular mechanisms responsible for vasculogenesis and angiogenesis are not completely understood.18 Several growth factors are involved in regulation of endothelial differentiation, proliferation, migration, and formation of functional vessels. Previous studies19-23 have shown that vascular endothelial growth factor (VEGF) is one of the major inducers of vasculogenesis and angiogenesis. VEGF signaling is mediated by two receptor tyrosine kinases, called VEGF-R1 (flt-1) and VEGF-R2 (flk-1/KDR), respectively.20,21 Gene targeting experiments demonstrated that a functional flk-1/KDR receptor is essential for the development of hematopoiesis and vasculogenesis.20
Stem cell growth factor (SCGF), a novel cytokine, exerts its action on primitive hematopoietic progenitor cells.24 In combination with other hematopoietic growth factors, such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and erythropoietin, SCGF stimulates the formation of erythroid and granulocyte/macrophage colonies, whereas SCGF alone cannot induce colony formation.24 Whether this early-acting growth factor has an influence on endothelial progenitors, or even on the common precursor of endothelial and hematopoietic cells, is currently unknown. Here, we describe the influence of SCGF in combination with VEGF on proliferation and differentiation of enriched progenitor cells from G-CSF mobilized peripheral blood.
In this study, we wanted to test whether AC133+ progenitor cells can be induced to differentiate into ECs in vitro. AC133+ cells represent a subset of CD34+ stem and progenitor cells with known hematopoietic potential.25-27 Because anti-AC133 antibody recognizes all of the noncomitted CD34+ cell population whereas it does not bind to mature ECs,25 we suggested that AC133+ cells could be an ideal starting population to generate ECs.
Materials and methods
Patient characteristics and specimens
The study included aliquots of leukapheresis products from 9 patients (3 patients with breast cancer, 1 patient with ovarian cancer, 3 patients with testicular cancer, and 2 patients with sarcoma) undergoing high-dose chemotherapy and autologous hematopoietic progenitor cell support. The median age of the study group was 38 years (range 21-57 years). All patients were enrolled in University Hospital Eppendorf Bone Marrow Transplant protocols, and all patients gave their informed written consent to participate in these treatment protocols and to use part of their leukapheresis products for experimental purposes. Peripheral blood progenitor cells were collected by leukapheresis after mobilization of hematopoietic progenitor cell with recombinant human G-CSF (5 μg/kg/day s.c.) for 7 days. Leukaphereses were performed on days 5, 6, and 7 of this administration course.
Positive selection of AC133+ cells by magnetic cell sorting
Mononuclear cells were isolated by density gradient centrifugation over Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) for 25 minutes at 500g and washed 3 times in phosphate-buffered saline (PBS; Life Technologies, Karlsruhe, Germany) sequentially at 200g, 300g, and 500g to remove platelets. Mononuclear cells were then resuspended in PBS, incubated with AC133 (monoclonal antibody; MoAb) conjugated super paramagnetic microbeads (AC133 Isolation Kit, Miltenyi Biotec, Bergisch-Gladbach, Germany), washed, and processed through a MACS magnetic separation column (Miltenyi Biotec) to obtain purified AC133+ cells. An aliquot of the AC133+ cell fraction was analyzed to assess purity.
Liquid cultures of human AC133+ cells
Isolated AC133+ cells were cultured in fibronectin-coated chamber slides (fibronectin from Life Technologies; chamber slides from Becton Dickinson GmbH, Heidelberg, Germany) at a density of 2 × 106 cells/mL in IMDM supplemented with 10% fetal bovine serum (FBS; Sigma, Deisenhofen, Germany), 10% horse serum (Sigma), and 10-6 mol/L hydrocortisone (Sigma). The following cytokines were added to the media: SCGF (100 ng/mL; TEBU, Frankfurt, Germany) and VEGF (50 ng/mL; TEBU). Cells were incubated for up to 14 days at 37°C in 5% CO2. Additional feeding was performed, depending on cell proliferation. Then supernatant was removed by gentle pipetting and replaced with fresh medium. Proliferating cells in the supernatant were counted, adjusted to 2 × 106 cells/mL, and transferred to fresh culture chambers to further study their developmental potential. Analysis of adherent and nonadherent cells derived from freshly isolated AC133+ cells as well as analysis of corresponding cell populations generated from transferred AC133-derived nonadherent cells was performed separately.
Colony assays for multilineage hematopoietic and endothelial progenitor cells
Purified AC133+ cells as well as cells cultured for 8 and 14 days were plated at 1 × 103-5 × 104 cells/mL in semisolid growth media that consisted of 0.9% methylcellulose in IMDM, 30% FBS, 1% bovine serum albumin (BSA), 10-4 mol/L mercaptoethanol, and 2 mmol/L l-glutamine (complete media from Cell Systems, St. Katharinen, Germany). In parallel experiments, cultures were stimulated either with a combination of hematopoietic growth factors, including stem cell factor (SCF; 50 ng/mL), interleukin 3 (IL-3; 20 ng/mL), IL-6 (20 ng/mL), G-CSF (20 ng/mL), GM-CSF (20 ng/mL), plus erythropoietin (3 U/mL; all from Cell Systems) or with the combination of SCGF (100 ng/mL) and VEGF (50 ng/mL; both from TEBU). All cultures were performed in quadruplicate, incubated at 37°C in 5% CO2 and 95% humidity, and scored after 14 days of culture using an inverted microscope.
Immunostaining of ECs
Freshly isolated AC133+ cells were spun at 500 rpm for 5 minutes onto glass slides by cytocentrifugation. Slides were air-dried for at least 24 hours and immunostained for the expression of the following proteins: AC133 (Miltenyi Biotec); CD34, CD41a, CD41b (all from Pharmingen, Hamburg, Germany); von Willebrand factor (vWF), VE-Cadherin (Immunotech, Marseille, France); KDR (Sigma); P1H12 (MoAb 16 985; Chemicon International, Temecula, CA), and EN4 (Cell Systems). All primary antibodies used were monoclonal. After blocking with 10% AB-serum /PBS (AB-serum from Biotest, Dreieich, Germany) for 20 minutes, cytospins were incubated with the primary antibody for 45 minutes. This step was followed by incubation with a biotinylated rabbit-anti-mouse secondary antibody (DAKO, Hamburg, Germany) for 30 minutes and an additional incubation with a streptavidine-alkaline phosphatase conjugate (DAKO) for 30 minutes, combined with fast blue stain (Sigma) for visualizing positive staining. The substrate reaction was performed in the presence of 1 mmol/L levamisole (Sigma).
Cultured cells in the chamber slides were washed twice with PBS and fixed for 10 minutes with 4% paraformaldehyde. Specimens were then incubated for 1 hour with the primary antibody as previously described.28 29 The following primary antibodies were used: anti-CD34, anti-CD31, anti-CD62E, anti-CD105, anti-CD106, anti-CD1a, anti-CD14 (all from Pharmingen), anti-VE-cadherin, anti-vWF (both from Immunotech), anti-KDR, and anti-Tie-2 (both from Santa Cruz Biotechnology Inc, Heidelberg, Germany). With the exception of anti-Tie-2, all antibodies used were monoclonal. In brief, we used a peroxidase anti-peroxidase complex. The peroxidase activity was visualized by means of the Nickel-glucose oxidase technique. The specimens were counterstained with Calcium Red. Controls included replacement of primary or secondary antibody with PBS, visualization of peroxidase only, and incubation of cells with either normal rabbit serum (for MoAbs; Sigma) or normal swine serum (for polyclonal antibodies; Sigma) in concentrations ranging from 0.1% to 0.01% instead of primary antiserum.
For the staining of putative endothelial colonies, semisolid medium containing the colonies was overlayered with 4% paraformaldehyde for 10 minutes. Dissolved colonies were pipetted onto glass slides, air-dried for 24 hours, and analyzed for the expression of vWF (Immunotech), CD41a, and CD41b (Pharmingen). Immunostaining was performed, using either the fast blue stain or the peroxidase anti-peroxidase complex combined with the Nickel-glucose oxidase technique.
Flow cytometry
The purity of AC133-selected cells was determined for each isolation. After lysis of erythrocytes with hemolytic buffer (0.155 mol/L NH4Cl, 0.012 mol/L NaHCO3, 0.1 mmol/L EDTA, pH 7.2) for 2 minutes, 5 × 105 cells were incubated with phycoerythrin (PE)-conjugated anti-AC133 MoAb (Miltenyi Biotec). For two-color flow cytometry, FITC-anti-CD34 (Pharmingen) was used as described.30 Isotype-matched mouse immunoglobulin served as controls. All incubations were performed at 4°C. After each incubation, cells were washed in PBS containing 0.1% BSA. Cells were incubated with the MoAb for 30 minutes in the presence of normal goat serum. Single- and two-color flow cytometric analyses were performed, using a FACS SCAN flow cytometer (Becton Dickinson) and Cell Quest software (Becton Dickinson). Each analysis included at least 5000 events. The percentage of AC133+ cells present was assessed after correction for the percentage of cells reactive with an isotype control. By using isotype-controls for PE and FITC, gates for phenotypic analysis of CD34+ cells were set so that the lower left panel contained at least 98% of the total cells analyzed.
Transmission electron microscopy
After 14 days of culture, adherent cells in the chamber slides were trypsinized, fixed in 5.5% glutaraldehyde for 16 hours, postfixed for 2 hours in 1% OsO4, and dehydrated in 35% ethanol for 15 minutes. One percent of gelatin solution was then added to the cell sediment, and the mixture of gelatin and cell sediment was placed at -20°C for 4 minutes before dehydration in 50% ethanol. The cells were further prepared for embedding in Epon 812 (Serva, Heidelberg, Germany) as previously described.31 Thin sections were cut, stained with uranyl acetate and lead citrate, and then examined, using a Philips EM 300 (Einthoven, Netherlands) transmission electron microscope.
Culture and preparation of A549 lung cancer cells
The lung cancer cell line A549 used for in vivo studies was obtained from the Institute of Molecular Biology and Cancer Research, University of Marburg, Germany (a gift from K Havemann) and maintained in DMEM with 10% FBS. A549 cells were harvested by trypsinization and washed with PBS before subcutaneous injection into mice.
In vivo studies
After 14 days of liquid culture in the presence of VEGF and SCGF, AC133-derived adherent cells were harvested by trypsinization and mixed with AC133-derived nonadherent cells that were obtained by pipetting the supernatant of the culture. Additionally, cultured tumor cells of the lung cancer cell line A549 were prepared as described above. In parallel experiments, SCID mice (bred at the University Hospital Eppendorf) were injected subcutaneously with either 1 × 106 tumor cells, with 1 × 106 AC133-derived putative ECs, or with a mixture of 1 × 106 tumor cells and 1 × 106 AC133-derived cells. All recipients survived the procedure. Tumor growth in the different groups of mice was measured weekly. At week 5 posttransplantation, all mice were killed for histocytochemical analysis of the grown tumors.
Immunohistocytochemical staining of mouse tumors
Tissue blocks of tumors grown in SCID mice following either subcutaneous injection of 1 × 106 A549 lung cancer cells (10 tissue blocks) or subcutaneous injection of 1 × 106 A549 cells plus 1 × 106AC133-derived putative ECs (10 tissue blocks) were fixed for 24 hours with Bouin fixative at room temperature. After dehydration in ascending alcohol concentrations, tissue blocks were embedded in paraffin. Sections 6 μm in size were mounted onto chrome-gelatin precoated slides. The sections were deparaffined, rehydrated, and further processed for the visualization of CEACAM1 with the MoAb 4D1/C2 (1:200, a gift from C. Wagener). We used an amplification combination of the peroxidase anti-peroxidase and the avidin-biotin-peroxidase complex technique. The peroxidase activity was visualized by use of the Nickel-glucose technique. The specimens were counterstained with Calcium Red.
The following controls were performed: (1) The primary, secondary, and tertiary antibodies were replaced by PBS; (2) only the peroxidase was visualized; and (3) instead of the primary antiserum, sections were incubated with normal rabbit serum (Sigma) in concentrations ranging between 0.1% and 0.01%.
Results
Characterization of AC133+-enriched cell fractions
As determined by flow cytometry, the median purity of positively selected AC133+ cells after magnetic cell sorting was 72% (range 62%-98%). A small portion of enriched AC133+ cells did not coexpress CD34 (median 0.92%, range 0%-17.1%), whereas all CD34+ cells coexpressed AC133. The obtained purities showed a positive correlation with the initial content of AC133+cells in the leukapheresis products.
Immunocytochemical analysis of the AC133+ cells revealed that 1.0% ± 0.3% were KDR-positive. Less than 0.1% of the AC133+ cells were found to express the endothelial markers vWF, VE-Cadherin, and P1H12, respectively. These findings suggest that the AC133+ population did not contain notable numbers of mature ECs.
Development of AC133+ cells in liquid culture
AC133+ cells were grown for 14 days on fibronectin-coated chamber slides in the presence of SCGF and VEGF. Within 1-2 hours of culture under these conditions, cells became adherent. During the first days of culture, adherent cells formed a monolayer, consisting predominantly of small-sized cells. Single, large cells with endothelial morphology were observed. No significant proliferation was noted during this culture period. On day 6-7, a proliferating population of round nonadherent cells occurred. These cells were transferred to fresh chambers in which they again became adherent and started to proliferate. Adherent cells in the initial chambers also continued to expand so that proliferating cells needed to be transferred on average every third day. Growth curves showed an up to 8-fold increase in the cell number after 14 days of culture (Figure1). Morphological analysis of the adherent cells at this time point revealed a heterogeneous cell population, comprising small-sized round cells, large-sized round cells with cytoplasmic granules, and large flat cells with endothelial morphology that formed clusters in some areas of the chamber (Figure2). In a few experiments, cultures were continued for up to 3 months. Following stimulation with SCGF and VEGF for 14 days, proliferation could then be maintained in the presence of VEGF alone (data not shown), whereas VEGF alone could not induce significant expansion of freshly isolated AC133+ cells.
Growth curves of nonadherent cells generated from AC133+ progenitor cells in liquid cultures with stem cell growth factor and vascular endothelial growth factor.
Proliferating nonadherent cells were obtained with the supernatant, and the number of cells per mL was counted. Three independent experiments are shown. Evaluation of the cell count was performed at various time points, depending on the individual growth pattern in each experiment. Results of 3 independent experiments are shown. Abbreviations: Pat. 1-3, patient 1-3.
Growth curves of nonadherent cells generated from AC133+ progenitor cells in liquid cultures with stem cell growth factor and vascular endothelial growth factor.
Proliferating nonadherent cells were obtained with the supernatant, and the number of cells per mL was counted. Three independent experiments are shown. Evaluation of the cell count was performed at various time points, depending on the individual growth pattern in each experiment. Results of 3 independent experiments are shown. Abbreviations: Pat. 1-3, patient 1-3.
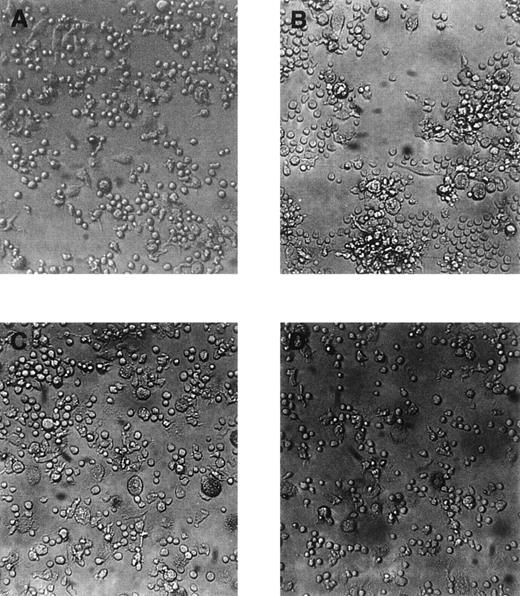
Morphology of adherent cells cultured for 14 days in liquid cultures in the presence of stem cell growth factor and vascular endothelial growth factor.
All photographs are taken from the same experiment. (A) shows an area with predominant small-sized round cells and flat elongated cells, (B) cluster formation, (C) large-sized round cells with cytoplasmic granules, and (D) area with exclusively small-sized round cells. Original magnification ×100.
Morphology of adherent cells cultured for 14 days in liquid cultures in the presence of stem cell growth factor and vascular endothelial growth factor.
All photographs are taken from the same experiment. (A) shows an area with predominant small-sized round cells and flat elongated cells, (B) cluster formation, (C) large-sized round cells with cytoplasmic granules, and (D) area with exclusively small-sized round cells. Original magnification ×100.
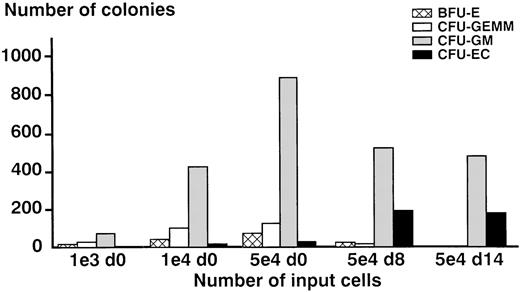
Clonogenic potential of cultured cells
Purified AC133+ cells and proliferating cells were plated in semisolid medium that was supplemented either with a combination of hematopoietic growth factors known to support multilineage colony formation or with SCGF and VEGF to stimulate the formation of endothelial colonies. As shown in Figure3, a linear relationship between input of cells and number of colonies grown could be demonstrated. Interestingly, nonadherent cells cultured for 8 days (d8 cells) and even for 14 days (d14 cells) in the presence of the recombinant growth factors VEGF and SCGF still had clonogenic potential. Furthermore, day 8 cells were capable of inducing the growth of burst-forming unit erythroid under the influence of a combination of hematopoitic growth factors, indicating that this population still contained immature progenitors. In comparison to freshly isolated AC133+cells, day 8 cells as well as day 14 cells produced higher numbers of putative endothelial colonies when stimulated with SCGF and VEGF (Figure 3). Independent of the developmental stage of input cells, colonies grown under the influence of SCGF and VEGF showed a morphology (Figure 4B and D) that was different from the morphology of the hematopoietic colonies (Figure 4A and C). To determine whether these loosely packed colonies composed of very small cells were of the endothelial lineage (so called colony-forming unit endothelial cell), colonies were analyzed by immunocytochemistry (see below).
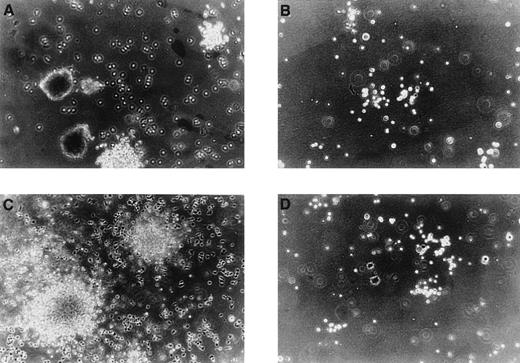
Morphology of colonies generated from AC133-derived cells.
In parallel experiments, cells cultured for 8 days (day 8 cells, A and B) and 14 days (day 14 cells, C and D) in liquid cultures with stem cell growth factor (SCGF) and vascular endothelial growth factor (VEGF) were transferred to methylcellulose and stimulated either with the hematopoietic growth factor combination SCF, interleukin 3 (IL-3), IL-6, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor plus erythropoietin, or with the combination of SCGF and VEGF. In response to the hematopoietic growth factors, day 8 cells formed multilineage colonies, including the formation of burst-forming unit erythroid (A); whereas under the same culture conditions, day 14 cells produced colonies that were restricted to the granulocyte-macrophage lineage (C). Colonies grown from day 8 cells and day 14 cells, respectively, under the influence of SCGF and VEGF displayed a morphology that was different of the hematopoietic colonies and were assumed to represent endothelial colonies. Original magnification ×100.
Morphology of colonies generated from AC133-derived cells.
In parallel experiments, cells cultured for 8 days (day 8 cells, A and B) and 14 days (day 14 cells, C and D) in liquid cultures with stem cell growth factor (SCGF) and vascular endothelial growth factor (VEGF) were transferred to methylcellulose and stimulated either with the hematopoietic growth factor combination SCF, interleukin 3 (IL-3), IL-6, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor plus erythropoietin, or with the combination of SCGF and VEGF. In response to the hematopoietic growth factors, day 8 cells formed multilineage colonies, including the formation of burst-forming unit erythroid (A); whereas under the same culture conditions, day 14 cells produced colonies that were restricted to the granulocyte-macrophage lineage (C). Colonies grown from day 8 cells and day 14 cells, respectively, under the influence of SCGF and VEGF displayed a morphology that was different of the hematopoietic colonies and were assumed to represent endothelial colonies. Original magnification ×100.
Comparison of the clonogenic potential of freshly isolated AC133+ cells (day 0 cells) and AC133-derived proliferating cells cultured for 8 days (day 8 cells) and 14 days (day 14 cells), respectively, in liquid culture with stem cell growth factor (SCGF) and vascular endothelial growth factor (VEGF).
As demonstrated in the 3 left panels of bars, a different number of day 0 input cells were tested to evaluate their capacity to form endothelial colonies (colony-forming unit endothelial cell, CFU-EC). The input of 5 × 104 AC133+ progenitor cells was found to produce reliable numbers of putative CFU-EC and was then compared with the input of 5 × 104 day 8 cells and day 14 cells, respectively. The results of 4 independent experiments are shown, each experiment was performed in quadruplicate. Abbreviations: d0 = day 0 cells, freshly isolated AC133+progenitor cells; d8 = day 8 cells, AC133-derived cells cultured for 8 days in liquid culture; d14 = day 14 cells, AC133-derived cells cultured for 14 days in liquid culture; BFU-E = burst-forming unit erythrocyte; CFU-GEMM, colony-forming unit granulocyte-erythrocyte-macrophage-megakaryocyte; CFU-GM = colony-forming unit granulocyte-macrophage.
Comparison of the clonogenic potential of freshly isolated AC133+ cells (day 0 cells) and AC133-derived proliferating cells cultured for 8 days (day 8 cells) and 14 days (day 14 cells), respectively, in liquid culture with stem cell growth factor (SCGF) and vascular endothelial growth factor (VEGF).
As demonstrated in the 3 left panels of bars, a different number of day 0 input cells were tested to evaluate their capacity to form endothelial colonies (colony-forming unit endothelial cell, CFU-EC). The input of 5 × 104 AC133+ progenitor cells was found to produce reliable numbers of putative CFU-EC and was then compared with the input of 5 × 104 day 8 cells and day 14 cells, respectively. The results of 4 independent experiments are shown, each experiment was performed in quadruplicate. Abbreviations: d0 = day 0 cells, freshly isolated AC133+progenitor cells; d8 = day 8 cells, AC133-derived cells cultured for 8 days in liquid culture; d14 = day 14 cells, AC133-derived cells cultured for 14 days in liquid culture; BFU-E = burst-forming unit erythrocyte; CFU-GEMM, colony-forming unit granulocyte-erythrocyte-macrophage-megakaryocyte; CFU-GM = colony-forming unit granulocyte-macrophage.
Identification of ECs
The percentages and the staining patterns of cells expressing the EC markers CD34, CD31, VE-Cadherin, KDR, Tie-2, Ulex europaeusagglutinin-1, and vWF, respectively, as determined by immunostaining of adherent cells from day 14, are shown in Table1 and Figure 5. All of the cultured cells were positive for KDR, Tie-2, Ulexeuropaeus agglutinin-1, and vWF, whereas CD34, CD31, and VE-Cadherin were expressed by a subset of 30%-50% of the cells. Similar results were observed in 3 independent experiments. Because a close developmental association might exist between the endothelial lineage and macrophages/dendritic cells (data not shown), adherent cells were examined for their reactivity with MoAbs against CD1a (dendritic cells) and CD14 (monocytes). No staining for these markers was observed. In addition, immunocytochemical analysis of the colonies derived from day 8 and day 14 cells in the presence of SCGF and VEGF revealed that all of the stained colony-forming cells expressed vWF (Figure 6). Because the expression of vWF is not specific for cells of the endothelial lineage, but also found on early megakaryocytes, additional immunostaining with anti-CD41a MoAb and anti-CD41b MoAb, targeting megakaryocytic antigens, was performed. Colonies grown in the presence of VEGF and SCGF were found neither to express CD41a nor to be positive for CD41b. The findings suggest that the colonies generated from the AC133-derived nonadherent cell population as well as the AC133-derived adherent cells in liquid cultures are of endothelial lineage.
Percentages of positive cells in the adherent cell fraction derived from AC133+ cells in liquid culture for 14 days in the presence of VEGF and SCGF
| Patient . | Antibodies . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CD34 . | CD31 . | VE-Cadherin . | KDR . | Tie-2 . | Ulex . | vWF . | CD1a . | CD14 . | |
| 1 | 31.0 | 28.5 | 48.0 | 99.5 | 99.5 | 99.5 | 99.5 | 0 | 0 |
| 2 | 30.4 | 15.6 | 52.3 | 99.8 | 99.8 | 99.8 | 99.8 | 0 | 0 |
| 3 | 35.0 | 32.2 | 55.5 | 99.7 | 99.8 | 99.7 | 99.7 | 0 | 0 |
| Patient . | Antibodies . | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CD34 . | CD31 . | VE-Cadherin . | KDR . | Tie-2 . | Ulex . | vWF . | CD1a . | CD14 . | |
| 1 | 31.0 | 28.5 | 48.0 | 99.5 | 99.5 | 99.5 | 99.5 | 0 | 0 |
| 2 | 30.4 | 15.6 | 52.3 | 99.8 | 99.8 | 99.8 | 99.8 | 0 | 0 |
| 3 | 35.0 | 32.2 | 55.5 | 99.7 | 99.8 | 99.7 | 99.7 | 0 | 0 |
The percentage of positive cells was obtained by calculating the number of positive cells per number of cells counted.
Abbreviations: SCGF = stem cell growth factor; Ulex = Ulexeuropaeus agglutinin-1; VEGF = vascular endothelial growth factor.
Immunocytochemical analysis of AC133-derived adherent cells using the nickel-glucose peroxidase anti-peroxidase technique.
(A) Negative control (AC133-derived adherent cells cultured for 14 days in the presence of stem cell growth factor [SCGF] and vascular endothelial growth factor [VEGF]). (B-G) AC133-derived adherent cells after 14 days of culture with SCGF and VEGF and labeled with anti-CD34 monoclonal antibody (MoAb), anti-VE-Cadherin MoAb, anti-KDR MoAb, anti-Tie-2 polyclonal antibody, anti-Ulex europaeusagglutinin-1 MoAb, and anti-von Willebrand factor MoAb, respectively. Original magnification ×100.
Immunocytochemical analysis of AC133-derived adherent cells using the nickel-glucose peroxidase anti-peroxidase technique.
(A) Negative control (AC133-derived adherent cells cultured for 14 days in the presence of stem cell growth factor [SCGF] and vascular endothelial growth factor [VEGF]). (B-G) AC133-derived adherent cells after 14 days of culture with SCGF and VEGF and labeled with anti-CD34 monoclonal antibody (MoAb), anti-VE-Cadherin MoAb, anti-KDR MoAb, anti-Tie-2 polyclonal antibody, anti-Ulex europaeusagglutinin-1 MoAb, and anti-von Willebrand factor MoAb, respectively. Original magnification ×100.
Immunocytochemical analysis of colonies grown from day 8 cells in semisolid media.
Day 8 cells were cultured for 14 days in methycellulose supplemented with stem cell growth factor and vascular endothelial growth factor. Resulting colonies were then dissolved by fixation, transferred to glass slides, and stained with anti-von Willebrand factor monoclonal antibody. Original magnification ×100.
Immunocytochemical analysis of colonies grown from day 8 cells in semisolid media.
Day 8 cells were cultured for 14 days in methycellulose supplemented with stem cell growth factor and vascular endothelial growth factor. Resulting colonies were then dissolved by fixation, transferred to glass slides, and stained with anti-von Willebrand factor monoclonal antibody. Original magnification ×100.
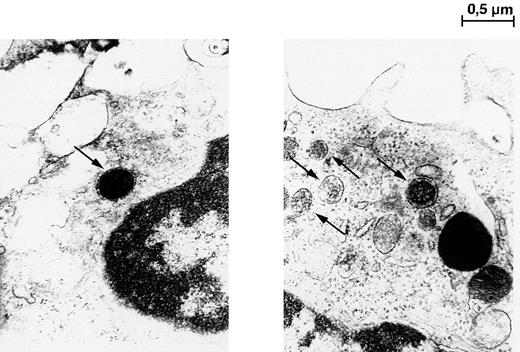
To further confirm these results, transmission electron microscopy was performed. As shown in Figure 7, adherent cells cultured for 14 days displayed organelles morphologically corresponding to Weibel-Palade bodies that are unique for vascular endothelium.32 These findings from immunostaining together with transmission electron microscopy analysis provide strong evidence for the endothelial nature of AC133+-derived cells.
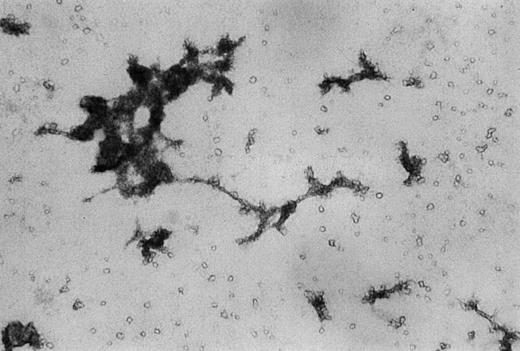
Transmission electron microscopy photomicrographs of adherent cells cultured in liquid cultures with stem cell growth factor and vascular endothelial growth factor for 14 days.
Single-membrane bound structures, identified as Weibel-Palade bodies, are indicated by arrows. Magnification × 30,000.
Transmission electron microscopy photomicrographs of adherent cells cultured in liquid cultures with stem cell growth factor and vascular endothelial growth factor for 14 days.
Single-membrane bound structures, identified as Weibel-Palade bodies, are indicated by arrows. Magnification × 30,000.
In vivo studies
Xenogeneic transplantation studies were performed, using the SCID mouse model. In this set of experiments, SCID mice were injected subcutaneously with either 1 × 106 tumor cells from the lung cancer cell line A549, with 1 × 106AC133-derived putative ECs, or with a mixture of 1 × 106 A549 cells and 1 × 106AC133-derived cells. Beginning at week 2 posttransplantation, a different kinetic of tumor growth could be observed within the 3 groups of mice. Animals injected with AC133-derived putative ECs alone did not develop any tumors. Mice that were inoculated with A549 cells alone developed tumors that reached half the size of tumors initiated with both A549 cells and AC133-derived putative ECs.
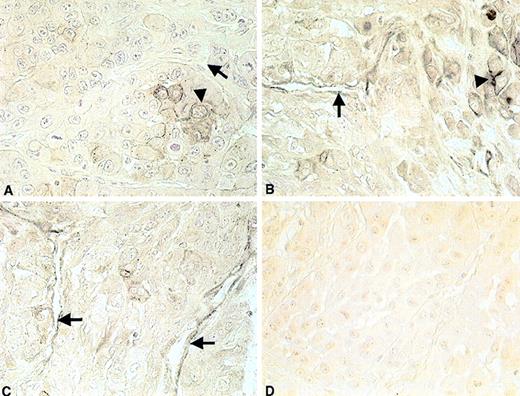
Histochemical staining clearly showed a different morphology of A549-induced tumor cell growth in comparison to the growth pattern of tumors initiated with A549 cells together with AC133-derived putative ECs. Subcutaneous tumors generated from A549 cells showed central necrosis and less blood vessel formation (as quantified by counting the number of blood vessels per tumor tissue section) than tumors grown in the presence of A549 cells combined with AC133-derived cells. In tumors initiated by injection of AC133-derived cells with A549 cells, no areas of necrosis could be observed. Additional histocytochemical analysis, using the human specific MoAb 4D1/C2 (anti-CEACAM1, a gift from C. Wagener), demonstrated positive staining of ECs in tumor blood vessels generated with AC133-derived cells and A549 cells (Figure8B and C), whereas staining of blood vessels in tumors initiated with A549 cells alone was negative (Figure8A).
Histochemical staining of tumor grown in mice with severe combined immunodeficiency with the human-specific anti-CEACAM1 monoclonal antibody 4D1/C2.
Panel (A) shows negative staining with 4D1/C2 in tumors grown after subcutaneous injection of A549 lung cancer cells. Panels (B) and (C) show positive staining of endothelial cells lining blood vessels in tumors initiated with A549 lung cancer cells plus AC133-derived putative endothelial cells. (D) Negative control (tumor tissue of mice injected subcutaneously with A549 lung cancer cells and AC133-derived putative endothelial cells). Tumor cells are indicated by arrowheads. Endothelial cells are indicated by arrows. Original magnification ×100.
Histochemical staining of tumor grown in mice with severe combined immunodeficiency with the human-specific anti-CEACAM1 monoclonal antibody 4D1/C2.
Panel (A) shows negative staining with 4D1/C2 in tumors grown after subcutaneous injection of A549 lung cancer cells. Panels (B) and (C) show positive staining of endothelial cells lining blood vessels in tumors initiated with A549 lung cancer cells plus AC133-derived putative endothelial cells. (D) Negative control (tumor tissue of mice injected subcutaneously with A549 lung cancer cells and AC133-derived putative endothelial cells). Tumor cells are indicated by arrowheads. Endothelial cells are indicated by arrows. Original magnification ×100.
Discussion
This study examined the capacity of AC133+ cells to differentiate into the endothelial lineage. Hypothesizing from previous studies with CD34+ cells that the peripheral blood progenitors may require an early-acting growth factor to induce proliferation and that a lineage-restricted growth factor could then trigger differentiation of proliferating cells into a certain lineage,30 we stimulated the cells with the combination of SCGF and VEGF. It is important to note that the addition of FBS and horse serum was found to be essential for endothelial development in vitro, indicating that serum contains further unidentified endothelial growth stimuli. By using these culture conditions, we demonstrated that enriched AC133+ cells continued to generate ECs. Adherent cells displayed endothelial characteristics, including the expression of CD34, CD31, VE-Cadherin, KDR, Tie-2, Ulexeuropaeus-agglutinin-1, and vWF, respectively, and the presence of Weibel-Palade bodies. Theoretically, endothelial development observed in our study could have resulted from contaminating ECs within the AC133+ population. However, the following considerations argue against this possibility. First, peripheral blood usually contains very low numbers of ECs.11 Second, no AC133-CD34+ cells were found in the isolated population, as revealed by flow cytometric analysis. Additionally, <0.1% AC133+ cells stained positive for markers that are found on mature ECs, such as VE-Cadherin, Tie-2, Ulexeuropaeus-agglutinin-1, and vWF, respectively. Furthermore, it is implausible that contaminating mature ECs give rise to endothelial colonies in semisolid medium.11 12
It has been discussed for a long time whether hematopoietic and ECs arise from a common precursor, the hemangioblast. In a recent study, Choi et al5 identified the hemangioblast within a murine embryonic stem cell-derived precursor population. Although not proven, it can be hypothesized that this finding in general is transferable to the human system. Hence, it is no longer a question whether the hemangioblast exists, but rather whether it can persist into adult life. In our experiments, we show that, depending on the culture conditions used, AC133+ cells from G-CSF-mobilized peripheral blood can be differentiated along the endothelial or the hematopoietic pathway. In our opinion, these AC133+ cells fulfill many criteria of true hemangioblasts. Whether the enriched AC133+-cell population contains separate progenitors for endothelial and hematopoietic cells or a common precursor cannot be answered by the present study. Further investigation of this question will require analysis at the single-cell level, using multiparameter cell sorting. The coexpression of AC133, Flk-1/KDR, and c-kit could serve as an ideal marker combination for the selection of bipotent precursors.
We showed that, in the presence of SCGF and VEGF, progenitor cells could be maintained for at least 14 days in vitro. Even after this extended period of culture, proliferating cells still had the capacity to form hematopoietic and endothelial colonies in semisolid media. Our culture system may have great potential for the ex vivo expansion of endothelial and hematopoietic cells (eg, to provide autologous ECs for in vivo studies). Therefore, the observation that the AC133+-cell population includes endothelial precursors may not only help improve our understanding of the developmental association of the hematopoietic and endothelial lineages in the adult but also be of clinical relevance (eg, in the field of ischemic disorders). In addition, several studies33-35 have shown that tumor growth depends on the formation of new blood vessels in the tumor tissue. It is currently believed that the underlying mechanisms of tumor-induced blood vessel formation is angiogenesis.36However, it cannot be excluded that malignant tumors are capable of incorporating circulating ECs or their precursors for tumor neovascularization. Peripheral blood stem cells that are increasingly used for autologous and allogeneic transplantation following high-dose chemotherapy may, in part, differentiate into ECs in vivo and so may contribute to enhanced tumor growth.
We have studied tumor growth-promoting activity of AC133-derived putative ECs in SCID mice that were injected subcutaneously with and without tumor cells. We could demonstrate enhanced tumor growth when tumor cells were injected together with AC133-derived cells. Furthermore, we could show that injected AC133-derived cells formed the endothelial layer of new blood vessels in these tumors. These findings from the in vivo studies suggest that the AC133-derived population contains ECs.
In summary, we have described the developmental potential of AC133+ precursors from G-CSF-mobilized peripheral blood. We showed that AC133+ cells, when cultured in the presence of SCGF and VEGF, give rise to both hematopoietic and EC lineages. Further identification and characterization of the AC133+-cell subset with endothelial potential provides an unique approach to prove the existence of the hemangioblast in the adult and its role in postnatal blood vessel formation.
Acknowledgments
We thank P. Kühnl and C. Löliger for providing aliquots from leukapheresis products. The authors would also like to thank D. Oestreich for assistance with photography. We are grateful to K. Havemann for providing the A549 lung cancer line.
Supported by grants from the Eppendorfer Krebs-und Leukämiehilfe-Foundation, Hamburg, Germany, and by grant No. Fi 389/4-1 from the Deutsche Forschungsgemeinschaft (DFG).
Reprints:Ursula M. Gehling, Department of Hematology/Oncology, University Hospital Eppendorf, Martinistraße 52, 20 246 Hamburg, Germany; e-mail: gehling@uke.uni-hamburg.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Fig. 5. Immunocytochemical analysis of AC133-derived adherent cells using the nickel-glucose peroxidase anti-peroxidase technique. / (A) Negative control (AC133-derived adherent cells cultured for 14 days in the presence of stem cell growth factor [SCGF] and vascular endothelial growth factor [VEGF]). (B-G) AC133-derived adherent cells after 14 days of culture with SCGF and VEGF and labeled with anti-CD34 monoclonal antibody (MoAb), anti-VE-Cadherin MoAb, anti-KDR MoAb, anti-Tie-2 polyclonal antibody, anti-Ulex europaeusagglutinin-1 MoAb, and anti-von Willebrand factor MoAb, respectively. Original magnification ×100.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/10/10.1182_blood.v95.10.3106/5/m_bloo0100805z.jpeg?Expires=1769112168&Signature=1Hxm2KwvIoec7eu1O3aIB6yC2G8JzxG0ePhpv4ZjkZsErKnIhOXK8FjVSo8pN5T9sbgPTAEo0tra93V3TMTrW7lYgcR1JhdqSCp0i9Rs52dB-UJ80JfVuB1u8vPGYO0hQ7BIxWhmYm1yndHY3rUaJxe49eoIT-RBs-o~0aSAHauqKU4A~em1kHdUIAQv2PYDr2mElSY85caLNbbq-VPaxGzFaMs2TXrMDCa954w0nipFrIMgpfDxjsXWGvu0L8mq8k93GQVSSMlQn78Dflu9rbTUqBqt36eDFvyaAhwrtbZoE1WuBlMeWUXUl6bbAV0Eh94v2Gy9av3SZ9J0GRfcfw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal