After bone marrow transplantation (BMT), there is a rapid regeneration to normal pretransplantation levels in the number of hematopoietic progenitors and mature end cells, whereas hematopoietic stem cell (HSC) numbers recover to only 5% to 10% of normal levels. This suggests that HSC are significantly restricted in their self-renewal behavior and hence in their ability to repopulate the host stem cell compartment. Previously, we have reported that HSC engineered to overexpress the homeobox transcription factor HOXB4 have a large repopulation advantage over untransduced cells as assessed at 4 months in a murine transplantation model (Sauvageau et al, Genes Dev 9:1753, 1995). This phenomenon has now been examined in detail for periods extending to 12 months in cohorts of mice transplanted with various numbers of HOXB4-transduced HSC. In all mice analyzed, HOXB4-transduced HSC were capable of fully reconstituting the HSC compartment, resulting, on average, in some 14-fold greater numbers of HSC than observed when transplanting control, non–HOXB4-transduced bone marrow cells. These data indicate that HOXB4 is a limiting factor in the regeneration of HSC to normal levels after BMT. Furthermore, we show thatHOXB4-transduced HSC did not expand above levels normally observed in unmanipulated mice, indicating that its overexpression does not override the regulatory mechanisms that maintain the HSC pool size within normal limits.
HEMATOPOIESIS IS the process by which mature blood cells are continuously generated throughout adult life from a small number of totipotent hematopoietic stem cells (HSC). The HSCs have the key properties of being able to self-renew and to differentiate into mature cells of both lymphoid and myeloid lineages. Although the genetic mechanisms responsible for the control of self-renewal and differentiation outcomes of HSC divisions remain largely unknown, a number of studies have implicated a variety of transcription factors as key regulatory components of these processes.1
Among such factors are the mammalian Hox homeobox gene family of transcription factors, consisting of 39 members arranged in 4 clusters (A, B, C, and D), initially described as important regulators of pattern formation in a variety of embryonic tissues.2These genes are structurally related by the presence of a 183-bp sequence, the homeobox, that encodes a helix-turn-helix DNA binding motif.3 Apparent stage- and lineage-specific expression of numerous HOXA, B, and C genes has now been demonstrated for both hematopoietic cell lines4 and primary hematopoietic cells.5-7 For example, we have shown that members of the HOXA and HOXB cluster genes are preferentially expressed in the CD34+ fraction of human bone marrow cells that contains most if not all of the hematopoietic progenitor cells.7 Further detailed analysis of Hoxgene expression in functionally distinct subpopulations of CD34+ cells has shown that genes, primarily located at the 3′ end of the clusters (eg, HOXB3 and HOXB4), are preferentially expressed in the subpopulation containing the most primitive hematopoietic cells.7
Using a murine bone marrow transplantation (BMT) model, we previously obtained evidence indicating that retroviral overexpression ofHOXB4 in hematopoietic cells can greatly enhance the regeneration of the HSC compartment after BMT,8 thus implicating HOXB4 as a regulator of self-renewal divisions of HSC. In these initial studies, the effects of HOXB4overexpression were assessed at 20 weeks posttransplantation, at which time the HSC compartment had regenerated to slightly above normal pretransplantation levels or some 47-fold higher than achieved in control transplant recipients. This enhanced regenerative ability was further suggested by a significant expansion ofHOXB4-transduced HSC after transplantation into secondary recipients. From these limited initial studies it was unclear whetherHOXB4 overexpression in steady-state hematopoiesis would lead to continuing expansion of HSC over time, suggesting that it could override the normal processes that control the population size and self-renewal potential of HSC. Also unanswered was whetherHOXB4 could act on a wide spectrum of transduced HSC or, as opposed, on a limited subset, as would be reflected in polyclonal versus monoclonal or oligoclonal expansion, respectively.
To address these questions, the current studies have examined the size and the degree of polyclonality of the regenerated pool of HSC in mice transplanted with HOXB4-transduced bone marrow cells as a function of time for a period extending up to 1 year after transplantation.
MATERIALS AND METHODS
Animals.
Recipients were 7- to 12-week-old male or female (C57Bl/6J × C3H/HeJ)F1 [(B6C3)F1] mice and donors were (C57Bl/6Ly-Pep3b × C3H/HeJ)F1 [(PepC3)F1] mice. (B6C3)F1 and (PepC3)F1 mice are phenotypically distinguishable by their cell surface expression of different allelic forms of the Ly5 locus; (B6C3)F1 are homozygous for the Ly5.2 allotype and (PepC3)F1 are heterozygous for the Ly5.1/Ly5.2 allotypes. These mice were bred from parental strain breeders originally obtained from Jackson Laboratories (Bar Harbor, ME), maintained in microisolator cages, and provided with sterilized food and acidified water in the animal facility of the British Columbia Cancer Research Center.
Retroviral generation and infection of primary bone marrow cells.
Retroviral vectors carrying the HOXB4 cDNA under the control of the viral long terminal repeat and/or a neomycin gene cassette under the control of an internal PGK promoter were constructed, and high-titer viral producers were generated in the GPE-86 packaging line as previously described.8 Bone marrow cells were obtained from (PepC3)F1 (Ly5.1) mice who had received 4 days previously an intravenous injection of 150 mg/kg body weight of 5-fluorouracil (5-FU), prestimulated in Dulbecco’s modified Eagle medium (DMEM) containing 15% fetal calf serum (FCS), 6 ng/mL of murine interleukin-3 (mIL-3), 100 ng/mL murine Steel factor (mSF), and 10 ng/mL human IL-6 (hIL-6) for 48 hours and then cocultivated on irradiated viral producer cells using identical medium with the addition of 6 μg/mL polybrene for an additional 48 hours. mSF, hIL-6, and mIL-3 were used as diluted supernatant from transfected COS cells as prepared in the Terry Fox Laboratory. Loosely adherent and nonadherent bone marrow cells were recovered from the cocultures by repeated washing of dishes and then counted using a hemocytometer. Unless otherwise specified, all media, serum, and growth factors were obtained from StemCell Technologies Inc (Vancouver, British Columbia, Canada).
Transplantation of retrovirally transduced bone marrow.
For bone marrow transplantation procedures, lethally irradiated (950 cGy, 110 cGy/min, 137Cs γ-rays) (B6C3)F1 (Ly5.2) recipients were injected intravenously with 2 × 105 bone marrow cells derived from (PepC3)F1 (Ly5.1/Ly5.2) immediately after their cocultivation with HOXB4- or neo-viral producer cells. Donor-derived repopulation in recipients was assessed using flow cytometry to measure the proportion of leukocytes in bone marrow, thymus, spleen, and peripheral blood that expressed the Ly5.1 surface antigen recognized by the fluorescein isothiocyanate (FITC)-conjugated anti-Ly5.1 monoclonal antibody (kindly provided by Dr G. Spangrude, Salt Lake City, UT).
In vitro clonogenic progenitor assays.
In vitro myeloid and pre-B clonogenic progenitor assays were performed as previously reported.9
Competitive repopulation unit (CRU) assay.
The CRU assay10 was used to evaluate the regeneration of HSC in neo and HOXB4 mice. Briefly, bone marrow cells from neo or HOXB4 mice that were transplanted earlier with transduced cells derived from (PepC3)F1 (Ly5.1/Ly5.2) mice were injected at different dilutions into lethally irradiated (B6C3)F1 (Ly5.2) mice together with a life-sparing dose of 1 × 105 competitor bone marrow cells from (B6C3)F1 (Ly5.2) mice. The level of lymphoid and myeloid repopulation with Ly5.1+ donor-derived cells in these secondary recipients was evaluated more than 13 weeks later by flow cytometry analysis of peripheral blood, as described.11 Recipients with greater than 1% donor-derived peripheral blood lymphoid and myeloid leukocytes as determined by the side scatter distribution of Ly5.1+cells (ie, lymphoid low side scatter; myeloid high side scatter) were considered to be repopulated by at least 1 lympho-myeloid repopulating (CRU) cell. CRU frequency in the test cell population was then calculated by applying Poisson statistics to the proportion of negative recipients at different dilutions, as described previously.10 Secondary recipients were killed 16 weeks after transplantation.
Southern and Northern blot analyses.
DNA was isolated from bone marrow, spleen, and thymus of neoand HOXB4 mice using DNAzol (GIBCO BRL, Burlington, ON, Canada). High molecular weight DNA was digested withKpn I, which cuts in the long terminal repeat region to release the integrated provirus, or with EcoRI or BamHI, which each cut the provirus once to release DNA fragment(s) specific to the proviral integration site(s). DNA fragments were separated on a 0.9% agarose gel, transferred to nylon membrane (Zeta-Probe; Bio-Rad, Hercules, CA), prehybridized, hybridized, and washed as described.8 Total cellular RNA was isolated using TRIzol (GIBCO BRL), separated on a 1% formaldehyde/agarose gel, transferred to nylon membrane (Zeta-Probe), prehybridized, hybridized, and washed as described.8 Probes used were a Xho I/SalI fragment of pMC1neo,12 the full-length HOXB4cDNA, a 2.0-kb Pst I fragment containing the chicken β-actin gene, and the 1.8-kb Kpn I/HindIII genomic fragment of the murine SH2-containing inositol phosphatase (SHIP) gene.13
RESULTS
Retroviral transduction and transplantation of murine bone marrow cells.
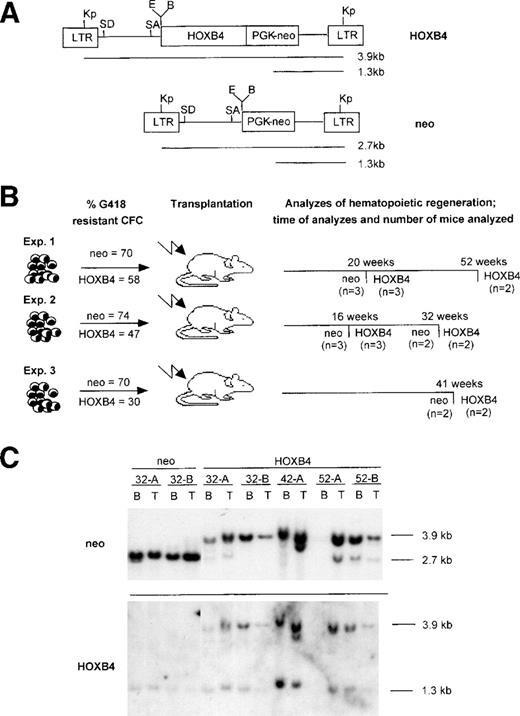
The MSCV recombinant retroviral vector containing the HOXB4cDNA (Fig 1A) was used to transfer and overexpress this gene in mouse bone marrow cells. To assess the long-term effects of HOXB4 overexpression on hematopoietic regeneration, lethally irradiated mice were transplanted withHOXB4- or neo-transduced bone marrow cells. Cohorts of mice from 3 independent transplantation experiments (hereafter calledHOXB4 and neo mice) were assessed for regeneration of various hematopoietic compartments at different times after transplantation, beginning as soon as 16 weeks and as late as 52 weeks after transplantation (Fig 1B). Each recipient was transplanted with an inoculum of 2 × 105 bone marrow cells, as recovered from retroviral infection cultures. This cell dose is estimated to contain approximately 35 HSC, as previously measured for recovered cells under identical infection conditions, using the CRU assay.8 The gene transfer efficiencies to the transplanted bone marrow as assessed by the proportion of G418-resistant clonogenic cells varied between experiments and were 30% to 58% and 70% to 74% for HOXB4- and neo-transduced cells, respectively (Fig1B). Assuming a retroviral infection efficiency of CRU (HSC) no greater than that of clonogenic progenitor cells, each recipient would have received an estimated maximum of 16 to 24 transduced (neo orHOXB4; see Table 3) CRU, plus an approximately equal number of nontransduced CRU. Findings from the 20-week timepoint in experiment no. 1 have been previously reported8; selected summary data from that timepoint are provided in Figs 2and 3 to facilitate comparison with the new data from mice of that same transplant cohort now assessed at 52 weeks posttransplantation.
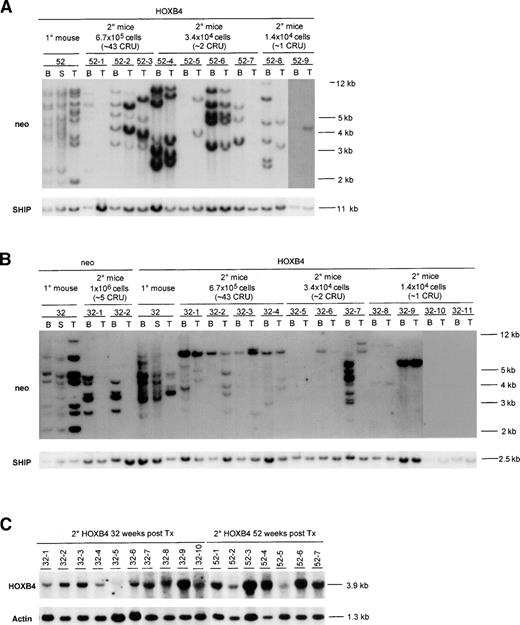
Structure of the HOXB4 and control neoretroviruses and the experimental outline. (A) Diagrammatic representation of the integrated HOXB4 and neoproviruses. Expected size of the full-length viral transcripts and also those initiated from the PGK promoter are shown, as are the sites for the various restriction enzymes used in this study. (B) Experimental outline showing the number of HOXB4 and neo mice from 3 transplantation experiments that were used in this study and the time posttransplantation when they were analyzed. Also shown is the initial gene transfer to the transplanted bone marrow inoculum received byneo and HOXB4 mice in these transplantations. (C) Southern blot analysis of DNA isolated from bone marrow and thymus of some of the neo (killed 32 weeks posttransplantation) andHOXB4 mice (killed 32, 41, and 52 weeks posttransplantation) used in this study to demonstrate the presence of the integrated provirus. DNA was cut with Kpn I, which releases theneo (2.7 kb) and the HOXB4 (3.9 kb) proviruses, and the blot was successively hybridized to probes specific for the neoand HOXB4 genes (full-length HOXB4 cDNA was used as a probe). The endogenous murine HOXB4 is detected at 1.3 kb by the HOXB4 probe and provides a single gene copy control of loading. In some of the HOXB4 mice, in addition to the full-length HOXB4 provirus, a weaker 2.7-kb proviral signal is detected with the neo probe but not with the HOXB4probe. Because this fragment failed to hybridize to the HOXB4probe, it likely represents a rearrangement resulting in the loss of the HOXB4 gene from some of the integrated proviruses. Kp,Kpn I; E, EcoRI; B, BamHI; SD, splice donor; SA, splice acceptor; CFC, colony-forming cells; B, bone marrow; T, thymus.
Structure of the HOXB4 and control neoretroviruses and the experimental outline. (A) Diagrammatic representation of the integrated HOXB4 and neoproviruses. Expected size of the full-length viral transcripts and also those initiated from the PGK promoter are shown, as are the sites for the various restriction enzymes used in this study. (B) Experimental outline showing the number of HOXB4 and neo mice from 3 transplantation experiments that were used in this study and the time posttransplantation when they were analyzed. Also shown is the initial gene transfer to the transplanted bone marrow inoculum received byneo and HOXB4 mice in these transplantations. (C) Southern blot analysis of DNA isolated from bone marrow and thymus of some of the neo (killed 32 weeks posttransplantation) andHOXB4 mice (killed 32, 41, and 52 weeks posttransplantation) used in this study to demonstrate the presence of the integrated provirus. DNA was cut with Kpn I, which releases theneo (2.7 kb) and the HOXB4 (3.9 kb) proviruses, and the blot was successively hybridized to probes specific for the neoand HOXB4 genes (full-length HOXB4 cDNA was used as a probe). The endogenous murine HOXB4 is detected at 1.3 kb by the HOXB4 probe and provides a single gene copy control of loading. In some of the HOXB4 mice, in addition to the full-length HOXB4 provirus, a weaker 2.7-kb proviral signal is detected with the neo probe but not with the HOXB4probe. Because this fragment failed to hybridize to the HOXB4probe, it likely represents a rearrangement resulting in the loss of the HOXB4 gene from some of the integrated proviruses. Kp,Kpn I; E, EcoRI; B, BamHI; SD, splice donor; SA, splice acceptor; CFC, colony-forming cells; B, bone marrow; T, thymus.
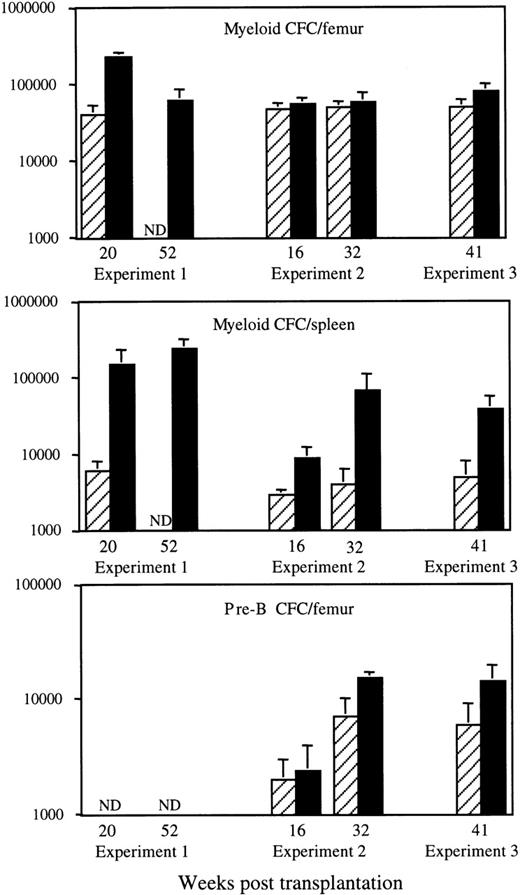
Effects of HOXB4 overexpression on the number of myeloid and pre-B colony-forming cells after BMT. Results shown are the means ± SD of the numbers of in vitro myeloid colony-forming cells in bone marrow (top) and spleen (middle) and of IL-7–responsive B-lymphoid progenitor cells (pre-B CFC) in the bone marrow (bottom) of individual neo (▨) and HOXB4 (▪) mice at various time points after transplantation. The number of neo andHOXB4 mice analyzed at each time point are shown in Fig 1B. Consistent with their preferential derivation fromHOXB4-transduced cells, a major proportion of myeloid and pre-B lymphoid progenitor cells in HOXB4 mice were G418-resistant (HOXB4 mice, 59% ± 9% v neo mice, 37% ± 10% for all 3 experiments).
Effects of HOXB4 overexpression on the number of myeloid and pre-B colony-forming cells after BMT. Results shown are the means ± SD of the numbers of in vitro myeloid colony-forming cells in bone marrow (top) and spleen (middle) and of IL-7–responsive B-lymphoid progenitor cells (pre-B CFC) in the bone marrow (bottom) of individual neo (▨) and HOXB4 (▪) mice at various time points after transplantation. The number of neo andHOXB4 mice analyzed at each time point are shown in Fig 1B. Consistent with their preferential derivation fromHOXB4-transduced cells, a major proportion of myeloid and pre-B lymphoid progenitor cells in HOXB4 mice were G418-resistant (HOXB4 mice, 59% ± 9% v neo mice, 37% ± 10% for all 3 experiments).
Variation in CRU numbers in recipients of neo-andHOXB4-transduced bone marrow cells over 1-year period. The number of CRU in femurs of cohorts of HOXB4 recipients from 3 different experiments ([▪] experiment no. 1, [⧫] experiment no. 2, and [•] experiment no. 3) and neo recipients from 2 different experiments (□ and ◊) were evaluated using the CRU assay. The results shown are expressed as the mean ± 95% confident interval of the CRU numbers in 1 femur of neo and HOXB4 mice at the various time points after transplantation. The number ofneo and HOXB4 mice analyzed at each time point are shown in Fig 1B. The shaded area represents the normal number of CRU measured in the femoral cavity of an unmanipulated (C57Bl/6J × C3H/HeJ)F1 mouse.9
Variation in CRU numbers in recipients of neo-andHOXB4-transduced bone marrow cells over 1-year period. The number of CRU in femurs of cohorts of HOXB4 recipients from 3 different experiments ([▪] experiment no. 1, [⧫] experiment no. 2, and [•] experiment no. 3) and neo recipients from 2 different experiments (□ and ◊) were evaluated using the CRU assay. The results shown are expressed as the mean ± 95% confident interval of the CRU numbers in 1 femur of neo and HOXB4 mice at the various time points after transplantation. The number ofneo and HOXB4 mice analyzed at each time point are shown in Fig 1B. The shaded area represents the normal number of CRU measured in the femoral cavity of an unmanipulated (C57Bl/6J × C3H/HeJ)F1 mouse.9
Effect of HOXB4 overexpression on progenitor and mature populations in vivo.
Hematopoietic regeneration in both neo and HOXB4 mice, from all 3 transplantation experiments, was essentially completely donor-derived, because greater than 85% of bone marrow, spleen, thymic, and peripheral blood leukocytes were of transplant origin (Ly5.1+) at all time points analyzed. A contribution by transduced cells to this reconstitution was evident by Southern blot analysis that readily detected the neo-or theHOXB4-proviruses in the bone marrow and thymuses of these mice (Fig 1C).
In contrast to differences in HSC levels (see below), the bone marrow, spleen, and thymus nucleated cell counts, as well as the peripheral blood white and red blood cell counts, were similar in HOXB4and neo mice (Table 1). Furthermore, fluorescence-activated cell sorting (FACS) analysis showed that the absolute numbers of bone marrow and splenic myeloid (Mac-1+), erythroid (Ter119+), B (ie, proB [B220+CD43+], immature B [B220+IgM+], and mature B [IgM+IgD+]), and CD4 and CD8 thymic T cells subpopulations were all also within normal range in HOXB4 mice (Table 2).
We had previously reported that the bone marrow myeloid progenitor numbers, as assayed in semisolid cultures supplemented with growth factors, were increased by 5-fold in recipients ofHOXB4-transduced cells when compared with neo control mice. This increase, initially detected in recipients analyzed at 20 weeks posttransplantation,8 was not observed inHOXB4 mice from transplantation experiments no. 2 or 3 (Fig 2). However, the number of myeloid progenitors in the spleen were always increased in these mice (Fig 2), with an overall increase of total body myeloid progenitor numbers of less than 2-fold in all mice analyzed. Evaluation of bone marrow IL-7–responsive B-lymphoid progenitor cells at 16, 32, and 41 weeks after transplantation showed that their numbers were slightly increased in the HOXB4 mice compared withneo control mice. However, at none of these time points was this increase statistically significant (Fig 2). Together, these data indicate that long-term overexpression of HOXB4 has mild to moderate effects on the number of clonogenic progenitors and essentially no effect on the number of mature end cells in recipient mice.
Effect of HOXB4 overexpression on long-term repopulating cells.
To determine the frequency of long-term lympho-myeloid repopulating cells (or HSC) in neo and HOXB4 primary mice, the CRU assay was used,10 which combines principles of limiting dilution together with competitive repopulation. Bone marrow cells were harvested from HOXB4 and neo mice at 5 different time points (Fig 1B), spanning as early as 16 weeks to as late as 52 weeks posttransplant. These cells were then transplanted at several different dilutions into secondary recipients that were themselves analyzed 12 to 15 weeks later for donor-derived (ie, Ly5.1+) lympho-myeloid repopulation.
At all time points examined and in all experiments, CRU numbers were markedly higher, on average some 14-fold, in HOXB4 mice when compared with neo control mice, whose CRU numbers achieved values that were approximately 10% of normal untransplanted mice (Fig3). The low recovery of CRU in recipients of neo-transduced bone marrow is consistent with previous studies using nontransduced unmanipulated adult bone marrow.14-17 The results withHOXB4-transduced cells thus stand in sharp contrast with recovery to the normal pretransplantation level at all times posttransplant analyzed.
Interestingly, once normal levels of CRU were reached in theHOXB4 mice, which is at least as early as 16 weeks, the CRU did not expand further or become exhausted, as indicated by the similar number of CRU cells present at 20 versus 52 weeks posttransplantation in experiment no. 1 (solid box in Fig 3) and at 16 versus 32 weeks in experiment no. 2 (solid diamond in Fig 3). Northern blot analysis of primary recipients (data not shown) and secondary mice used for CRU determination (Fig 4C) confirmed continued expression of the transducedHOXB4 gene. Thus, plateauing of CRU numbers was not associated with extinction of HOXB4 gene expression. Taken together, these results clearly indicate that HOXB4 appears to be a limiting factor in the regeneration of CRU numbers to normal levels after BMT and that its overexpression does not override the regulatory mechanisms that maintain the CRU pool size within normal limits and neither does it lead to rapid exhaustion of CRU.
Polyclonal expansion and hematopoietic regeneration by HOXB4-transduced CRU.
To prove that the enhanced regeneration of CRU cells in theHOXB4 mice was indeed caused by preferential expansion ofHOXB4-transduced CRU cells and to analyze the degree of polyclonality of the regenerated pool of CRU cells, we performed Southern blot analyses of proviral integration sites in DNA isolated from various hematopoietic tissues of primary and secondaryHOXB4 and neo mice. Results from representative primary recipients and their corresponding secondary recipients used for CRU assay are shown in Fig 4.
Southern blot analysis of proviral integration patterns in primary and secondary recipients of neo- andHOXB4-transduced bone marrow cells. DNA samples isolated from various hematopoietic organs of primary recipients killed 52 (A) or 32 (B) weeks posttransplantation and their secondary recipients (killed 16 weeks posttransplantation) were first digested with EcoRI and then BamHI, both of which cut the integrated provirus once, generating a DNA fragment specific for each proviral integration site. The number of transduced clones detected with either enzyme were the same; thus, the results for only 1 of the enzymes is shown. The membranes were first hybridized to aneo-specific probe for detection of proviral fragments and subsequently with a probe specific for the SHIP gene to provide a single copy control of loading. Exposure times were 48 hours for theneo and SHIP probes. To demonstrate that the proviral fragments contained the HOXB4 cDNA, the blots were also hybridized with full-length HOXB4 cDNA probe, which generated the same proviral banding pattern as the neo probe (data not shown). Each mouse is identified with a specific number derived from the time that the primary recipient was killed, and indicated above that number are the number of bone marrow cells received by each secondary recipient as well as the estimated number of CRU cells that they received. Expression of the 3.9-kb HOXB4-containing message in the bone marrow of secondary recipients is shown in (C). The percentages of the donor-derived repopulation (ie, Ly5.1+) in the secondary neo mice were as follows: 32-1 (44%) and 32-2 (18%). In secondary HOXB4 mice, the percentages were as follows: 32-1 (60%), 32-2 (76%), 32-3 (83%), 32-4 (52%), 32-5 (4%), 32-6 (23%), 32-7 (18%), 32-8 (8%), 32-9 (51%), 32-10 (5%), 32-11 (0%), 52-1 (20%), 52-2 (69%), 52-3 (73%), 52-4 (47%), 52-5 (5%), 52-6 (36%), 52-7 (47%), 52-8 (30%), and 52-9 (20%). B, bone marrow; S, spleen; T, thymus.
Southern blot analysis of proviral integration patterns in primary and secondary recipients of neo- andHOXB4-transduced bone marrow cells. DNA samples isolated from various hematopoietic organs of primary recipients killed 52 (A) or 32 (B) weeks posttransplantation and their secondary recipients (killed 16 weeks posttransplantation) were first digested with EcoRI and then BamHI, both of which cut the integrated provirus once, generating a DNA fragment specific for each proviral integration site. The number of transduced clones detected with either enzyme were the same; thus, the results for only 1 of the enzymes is shown. The membranes were first hybridized to aneo-specific probe for detection of proviral fragments and subsequently with a probe specific for the SHIP gene to provide a single copy control of loading. Exposure times were 48 hours for theneo and SHIP probes. To demonstrate that the proviral fragments contained the HOXB4 cDNA, the blots were also hybridized with full-length HOXB4 cDNA probe, which generated the same proviral banding pattern as the neo probe (data not shown). Each mouse is identified with a specific number derived from the time that the primary recipient was killed, and indicated above that number are the number of bone marrow cells received by each secondary recipient as well as the estimated number of CRU cells that they received. Expression of the 3.9-kb HOXB4-containing message in the bone marrow of secondary recipients is shown in (C). The percentages of the donor-derived repopulation (ie, Ly5.1+) in the secondary neo mice were as follows: 32-1 (44%) and 32-2 (18%). In secondary HOXB4 mice, the percentages were as follows: 32-1 (60%), 32-2 (76%), 32-3 (83%), 32-4 (52%), 32-5 (4%), 32-6 (23%), 32-7 (18%), 32-8 (8%), 32-9 (51%), 32-10 (5%), 32-11 (0%), 52-1 (20%), 52-2 (69%), 52-3 (73%), 52-4 (47%), 52-5 (5%), 52-6 (36%), 52-7 (47%), 52-8 (30%), and 52-9 (20%). B, bone marrow; S, spleen; T, thymus.
In the bone marrow, spleen, and thymus of primary HOXB4recipients, which were killed 52 and 32 weeks posttransplantation, multiple proviral integrations could be detected (Fig 4A and B). Variations in the proviral signal intensities detected in most of these tissues are a signature for multiple proviral integrations and thus demonstrate polyclonal regeneration by HOXB4-transduced long-term repopulating cells, rather than multiple proviral integrations into the same cell. In contrast, moderate proviral integration complexity was observed in the primary neorecipients, consistent with oligoclonal repopulation byneo-transduced cells (Fig 4B). This difference betweenneo and HOXB4 mice cannot be contributed to higher numbers of transduced stem cells being initially transplanted to theHOXB4 mice, because the neo control and theHOXB4 mice received similar numbers (Table 3), thus underscoring the repopulating advantages of HOXB4-transduced CRU cells over untransduced cells.
Southern blot analysis of proviral integration patterns in bone marrow (myeloid) and thymus (lymphoid) of secondary recipients was used to further analyze the clonality of the regenerated pool of transduced lympho-myeloid repopulating (CRU) cells present in primaryHOXB4 and neo mice at the time of their death (Fig 4A and B). In all of the 9 secondary recipients of the primary donor mouse killed at 52 weeks posttransplantation that were scored positive for donor-derived repopulation by FACS (Ly5.1+), HOXB4proviral integration(s) were detected in their bone marrow and/or thymus (Fig 4A). Furthermore, the intensities of the proviral signals correlated, for most of the mice, with their level of donor derived (Ly5.1+) repopulation (Fig 4A). As can be seen in Fig 4A and summarized in Table 3, a total of at least 15 differentHOXB4-transduced clones could be detected in these secondary mice. In addition, at least 4 of these mice had a common proviral integration pattern in their bone marrow and thymus (mice 52-2, 52-4, 52-6, and 52-9 [bone marrow signal very faint due to low amount of DNA]), indicating the lympho-myeloid repopulating potential of the regenerated CRU cells. The lack of detection of common proviral intergration patterns both in bone marrow and thymus in the other secondary mice can be explained in some cases by the low amount of DNA analyzed (mouse 52-7 in thymus), the lack of bone marrow sample (mouse 52-3), or very low donor-derived repopulation levels (mouse 52-5, Ly5.1+ PBL only 5%). Thus, even as late as 52 weeks posttransplantation, the hematopoietic regeneration in the primaryHOXB4 mice was polyclonal and without apparent dominance of anyHOXB4-transduced clone.
Similarly, analysis of the proviral intergration sites in the secondary recipients receiving bone marrow cells from 1 of the HOXB4recipients killed 32 weeks posttransplantation showed a complete concordance between detection of donor-derived hematopoietic regeneration by FACS analysis and contribution to regeneration byHOXB4-transduced cells, again indicating selective or competitive regeneration of the HOXB4-transduced cells over nontransduced cells (Fig 4B). Of the 10 secondary recipients (32-1 through 32-10) that were positive for lympho-myeloid repopulation by FACS, 8 had detectable HOXB4 proviral integration in their bone marrow and thymus (Fig 4B). In the case of the 2 negative mice (32-5 and 32-10), which both had low donor repopulation (Ly5.1+PBL, 4% and 5%, respectively), the expression of HOXB4 could, however, be detected by Northern blot analysis of their bone marrow (Fig 4C). Several of these secondary recipients, including those that were estimated to receive between 1 and 2 CRU cells (mice 32-6, 32-8, and 32-9), had a common proviral integration in their bone marrow and thymus, thus again confirming the lympho-myeloid repopulating potential of the regenerated HOXB4-transduced CRU cells (Fig 4B). Self-renewal of HOXB4-transduced CRU cells was also demonstrated by detecting a common lympho-myeloid repopulating clone in all of the secondary recipients (32-1 to 32-4) receiving high cell dose (43 CRU/mouse) and in 1 mouse (32-6) receiving fewer CRU cells (2 CRU/mouse). However, interestingly, this clone could not be detected in bone marrow, spleen, or thymus of the primary HOXB4 mouse (Fig4B), indicating that this cell, despite extensive self-renewal division, did not contribute significantly to bone marrow, spleen, or thymic repopulation in this primary HOXB4 recipient at the time of death. The detection of 4 different HOXB4-transduced lympho-myeloid repopulating clones and at least 9 others with either lymphoid or myeloid potential in these secondary HOXB4 mice (Fig 4B and Table 3) strongly suggest that the enhanced CRU regeneration in the primary HOXB4 mouse killed 32 weeks posttransplantation was also a polyclonal event.
In contrast to the HOXB4 mice, those secondary neorecipients that were scored positive for donor-derived lympho-myeloid repopulation (estimated to receive ∼5 CRU cells) were only positive for proviral integrations in their bone marrow (Fig 4B). These data for the neo mice thus stand in sharp contrast to that of theHOXB4 mice, in which transduced cells with lymphoid-myeloid repopulating potential can be detected in secondary mice that received approximately 70 times lower number of bone marrow cells than these secondary neo mice (1.4 × 104 cells for theHOXB4 v 1 × 106 for the neo; Fig 4A and B).
DISCUSSION
Using retroviral gene transfer and the murine BMT model, we have previously obtained evidence that HOXB4 overexpression can markedly enhance CRU regeneration as assessed 5 months posttransplantation of transduced bone marrow cells.8 In this current study, we extend those initial observations to provide significant new insights into the kinetics, control, and magnitude of this phenomenon. Our current findings show that the enhanced regeneration of the CRU compartment by the HOXB4-transduced CRU is evident as early as 16 weeks posttransplantation and persists for at least 1 year after transplantation. This increase in regenerative behavior is such that normal, pretransplantation levels of CRU are achieved and maintained—levels some 14-fold higher than observed with non–HOXB4-transduced stem cells. Moreover, the apparent stabilization in the CRU pool at normal pretransplantation levels suggests that the expansion of HOXB4-transduced CRU in these mice is ultimately subjected to existing in vivo control mechanisms.HOXB4 was also demonstrated to act on multiple CRUs, because the regenerated pool of HOXB4-transduced CRUs in the transplanted mice was highly polyclonal even as late as 1 year after transplantation. Furthermore, detailed analysis of various mature cell populations in these mice strongly suggests that overexpression ofHOXB4 neither alters myeloid or lymphoid differentiation nor leads to dominant outgrowth of any type of hematopoietic cells.
Serial transplantation studies have suggested that the transplantable HSCs may fail to fully regenerate the HSC compartment, because the self-renewal capacity of HSCs may be intrinsically limited or at least subjected to exhaustion.14,15 In contrast to depletion of the HSC pool, recent studies have suggested that the failure to fully regenerate the HSC compartment after transplantation could be the result of negative feedback mechanisms activated when progenitors and mature cells have been regenerated to their normal levels, which prematurely inhibit further HSC expansion.16 17HOXB4 overexpression might thus render HSC less sensitive to this negative feedback mechanism.
Using fibroblasts engineered to overexpress HOXB4, it was recently shown that these cells acquire the capacity to grow in low concentrations of serum.18 An alternative explanation for the HOXB4 effect described in the present studies is thatHOXB4 might alter the sensitivity of HSC to extrinsic factors acting during hematopoietic regeneration, thus allowing for a greater expansion of the HOXB4-transduced HSC compartment.
Several studies have indicated that, in steady-state hematopoiesis, the proliferation of HSC is tightly controlled. In mice, HSC numbers remain relatively constant throughout most of their adult life, although in very old mice (>2 years) their numbers appears to increase, possibly due to accumulation of genetic lesions.19 The HSC population in mice has also been demonstrated to be quiescent (or slowly cycling), because the vast majority of these cells are resistant to cytotoxic agents such as 5-FU or hydroxyurea.20 The stabilization of the CRU pool in HOXB4 mice at normal levels suggests that, although CRU cells overexpressing HOXB4 have enhanced regenerative potential, their ability to respond to this regulatory mechanism is not altered. However, because the cycling status of CRU cells in HOXB4 recipients is currently unknown, other regulatory mechanisms acting to maintain stable levels ofHOXB4-transduced CRU cells cannot be ruled out.
Despite a profound and consistent effect on the expansion of primitive hematopoietic cells, overexpression of HOXB4 did not promote preferential expansion along any hematopoietic lineage or lead to leukemia, despite evidence of persistent HOXB4 expression for at least 52 weeks. These results stand in sharp contrast to our published data for the retroviral overexpression of eitherHOXB3 or HOXA10 in a similar transplantation model.9 21 The different outcomes on hemopoiesis generated by overexpression of these 3 different HOX genes strongly indicate that these proteins activate and/or repress different sets of target genes in hematopoietic cells. The effects of HOXB4overexpression during hematopoietic regeneration can thus be viewed as more restricted than those generated by overexpression of eitherHOXB3 or HOXA10, suggesting that targets open toHOXB4 are restricted to primitive hematopoietic cells.
Together, the results presented in this report document that as few as 10 to 20 HOXB4-transduced HSC are sufficient to regenerate the HSC pool size to pretransplantation levels and, more importantly, once that level is reached, the number of HOXB4-transduced HSC is controlled over time. These findings now suggest that it is possible to engineer HSC that possess repopulating potential 10- to 20-fold higher than that of unmanipulated HSC. In addition, these findings provide important insights into the control and molecular mechanism of stem cell self-renewal and point to potential application in stem cell-mediated therapies such as those where HSC numbers may be limiting.
ACKNOWLEDGMENT
The authors are grateful to Patricia Rosten and Wieslawa Dragowska at the Terry Fox Laboratory for expert technical assistance. We also acknowledge Drs Margaret Hough, Jana Krosl, Jeffrey Lawrence, Corey Largman, Connie Eaves, and Peter Lansdorp for insightful discussions during the course of these studies.
Supported by the National Cancer Institute of Canada with funds from the Canadian Cancer Society and the Terry Fox Foundation; the Medical Research Council of Canada; and the National Institutes of Health (Grant No. DK48642). U.T. was the recipient of a University of British Columbia Graduate Fellowship. G.S. is the recipient of a Clinician Scientist Fellowship of the Medical Research Council of Canada.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to R. Keith Humphries, MD, PhD, Terry Fox Laboratory, 601 W 10th Ave, Vancouver, BC, V5Z IL3, Canada; e-mail:keith@terryfox.ubc.ca.

![Fig. 3. Variation in CRU numbers in recipients of neo-andHOXB4-transduced bone marrow cells over 1-year period. The number of CRU in femurs of cohorts of HOXB4 recipients from 3 different experiments ([▪] experiment no. 1, [⧫] experiment no. 2, and [•] experiment no. 3) and neo recipients from 2 different experiments (□ and ◊) were evaluated using the CRU assay. The results shown are expressed as the mean ± 95% confident interval of the CRU numbers in 1 femur of neo and HOXB4 mice at the various time points after transplantation. The number ofneo and HOXB4 mice analyzed at each time point are shown in Fig 1B. The shaded area represents the normal number of CRU measured in the femoral cavity of an unmanipulated (C57Bl/6J × C3H/HeJ)F1 mouse.9](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/8/10.1182_blood.v94.8.2605.420k19_2605_2612/7/m_blod42019003x.jpeg?Expires=1768135904&Signature=H4M7SPsuy8twD-FPRgO~jcb7jP9vPAptwI2JNTnZJgHtfD6q4wkx9-hcArCM4npp6R59TvPwKJszqIzXGneY6yAYMRNL8NsgwJeW0tEzzsvtpsgKDJHtaB9REAhH1mCIm9vOrrCoAndXebRiyGiDOhamp~G0mHsAMdMTNafJ~ycEE4B-QDwRXeRho86WKW790gTEb2v9XuBNTfi30C5VeyxnD2dr2TDKPP5u9LYvAVaJ6Ur0ol2n6ug4Dhf6PzaUoD28VdWZUoyw-T8mZPGeBXgRIHZ~cHFR6Se53zw6z6PwTaQMjixKu1k6AGV0RBgeE0eOpiJY~kwK3IjdZ4Hw2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)